Acute gastrointestinal injury in critically ill patients with COVID-19 in Wuhan, China
Jia-Kui Sun, Ying Liu, Lei Zou, Wen-Hao Zhang, Jing-Jing Li, Yu Wang, Xi ao-Hua
Kan, Jiu-Dong Chen, Qian-Kun Shi, Shou-Tao Yuan
Abstract
Key Words: Gastrointestinal injury; Organ dysfunction; Septic shock; Critically ill; COVID-19
INTRODUCTION
In December 2019, clusters of acute pneumonia cases of unclear etiology were identified in Wuhan, the capital of Hubei province in China[1-3]. The pathogen was reported to be a novel coronavirus that was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronavirus disease 2019 (COVID-19) was characterized by the World Health Organization (WHO) as a pandemic due to the rapid spread of the disease around the world[4]. As of May 16, 2020, a total of 82947 cases (4634 deaths) were confirmed in China, including 50339 cases (3869 deaths) in Wuhan city[5].
The National Health Commission of China issued a series of diagnosis and treatment recommendations and suggested classifying the disease into four grades: Mild, moderate, severe and critical[5]. Recent studies have reported the clinical characteristics and prognosis of COVID-19 with varied severity[1,2,6-8]. Most critically ill patients had organ injury, including acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), cardiac injury, or liver dysfunction[9]. During our clinical work against the epidemic of COVID-19 in Wuhan, we observed that numerous patients had gastrointestinal symptoms during the course of disease development. It is known that gastrointestinal dysfunction is closely related to adverse outcomes in critically ill patients. However, few studies on acute gastrointestinal injury (AGI) have been reported in critically ill patients with COVID-19. In this study, we investigated the prevalence and outcomes of AGI in critically ill patients with COVID-19 who were admitted to Guanggu District of Wuhan Tongji Hospital.
MATERIALS AND METHODS
Patients
From February 10 to March 10 2020, adult patients (age ≥ 18 years) with confirmed critical COVID-19 admitted to our specialized isolation units and intensive care unit (ICU), Guanggu district of Wuhan Tongji Hospital were enrolled in this retrospective study. Patients with chronic organ dysfunction (e.g., hepatic or renal dysfunction), immunodeficiency, terminal cancer, and patients with a history of long-term use of corticosteroids were excluded. Written informed consent was waived by our institutional review board as this was a retrospective study for emerging infectious disease. The diagnosis of COVID-19 was according to the WHO interim guidance and recommendations of the National Health Commission of China[4,5], and identified by the detection of SARS-CoV-2 RNA in the clinical laboratory of Tongji Hospital.
Definitions
An identified case of COVID-19 was defined as a positive finding on real-time reversetranscriptase–polymerase-chain-reaction (RT-PCR) assay of nasal and pharyngeal swab specimens[4,5,7]. Only laboratory-confirmed cases were enrolled in the analysis. The diagnosis of critical COVID-19 was in accordance with the Chinese recommendations[5]: Meeting any of the following: I, respiratory failure with mechanical ventilation (MV); II, shock; III, multiple organ failure requiring ICU treatment. AGI was defined as a malfunction of the gastrointestinal tract due to acute illness and was categorized into four grades according to its severity[10]. This AGI grading system was based mainly on gastrointestinal symptoms, intra-abdominal pressure, and the presence/absence of feeding tolerance. AGI grade I was defined as an increased risk of developing gastrointestinal dysfunction or failure (a self-limiting condition); AGI grade II was defined as gastrointestinal dysfunction (a condition that requires interventions); AGI grade III was defined as gastrointestinal failure (GI function cannot be restored with interventions); AGI grade IV was defined as marked gastrointestinal failure (a condition that is immediately life-threatening)[10]. Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, septic shock was defined as a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with a higher risk of mortality[11]. The diagnostic criteria for ARDS were in accordance with the Berlin definitions[12]. The definition of AKI was based on the 2012 Kidney Disease: Improving Global Outcomes guidelines[13]. Cardiac injury was defined as serum levels of cardiac biomarkers (e.g., troponin I) above the 99thpercentile reference upper limit or new abnormalities on electrocardiography and echocardiography[2]. Liver injury was defined as serum levels of hepatic biomarkers (e.g., alanine aminotransferase) more than twice the reference upper limit or a disproportionate elevation of alanine aminotransferase and aspartate aminotransferase levels compared with alkaline phosphatase levels[14]. Multiple organ dysfunction syndrome (MODS) was defined as the combined dysfunction of two or more organs.
Data collection
The baseline clinical characteristics, including sex, age, days from onset to admission, initial symptoms or signs, and body mass index (BMI) were collected from electronic medical and nursing records, and all laboratory tests were performed according to the clinical needs of patients. The acute physiology and chronic health evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score, serum levels of C-reactive protein (CRP), D-dimer, white blood cell (WBC) count, lymphocyte count, procalcitonin (PCT), and blood lactate within 24 h of admission were recorded. The RT-PCR assay of viral RNA was performed using a commercial kit (Tianlong, Xi’an, China) according to the manufacturer’s instructions. All laboratory parameters were detected by the clinical laboratory of Tongji Hospital. Moreover, the numbers of patients with AGI (grades), ARDS, AKI, cardiac injury, liver injury, septic shock, MODS, and patients receiving MV or continuous renal replacement therapy (CRRT) during hospital stay were also recorded. The primary endpoints were the incidence of AGI and 28-d mortality. The secondary endpoints were the incidence of MODS and septic shock.
Statistical analysis
The Kolmogorov-Smirnov test was first performed to test the normal distribution of the data. Normally distributed data were expressed as the means ± standard deviation and were compared byttests. Non-normally distributed data were expressed as the medians (interquartile ranges, IQR) and were compared by the Mann-WhitneyUtest or the Kruskal-Wallis test. Categorical variables were presented as absolute numbers or percentages and were analyzed using theχ2test or Fisher’s exact test. To take into account the repeated nature of the variables, analysis of variance (ANOVA) for repeated measurements of the general linear model was implemented. Pearson’s test was used to analyze the correlation between two variables. To determine the risk factors associated with AGI grade II and above, we performed a series of several univariate logistic regression analyses using the above-mentioned variables. Variables withP< 0.1 in univariate analyses were tested in further multivariate logistic regression analyses. Receiver operating characteristic (ROC) curves were used to evaluate the associations between AGI and MODS, septic shock, and 28-d mortality. Survival curves for up to 28 d after admission and 60 d from disease onset were generated using the Kaplan–Meier method and were compared by the log-rank test. IBM Statistical Package for the Social Sciences (SPSS, version 22.0, NY, United States) software was used for statistical analysis, and two-sidedP< 0.05 was considered statistically significant. The statistical methods used in this study were reviewed by Liu Q, a biostatistician from the Center for Disease Control and Prevention of Jiangsu Province in China.
RESULTS
As shown in Figure 1, a total of 83 critically ill patients with confirmed COVID-19 were enrolled in this retrospective study. The median age was 70 (IQR, 60-79) years, and most patients were male 59 (71.1%). Fever (33/83, 39.8%) and cough (18/83, 21.7%) were the main initial symptoms. Seventy-two (86.7%) patients had AGI during hospital stay, of them, 30 had AGI grade I, 35 had AGI grade II, 5 had AGI grade III, and 2 had AGI grade IV. The incidence of AGI grade II and above was 50.6% (42/83). The detailed clinical data of the patients are presented in Table 1. Forty (48.2%) patients died within 28 d of admission, their median hospital stay was 12.0 (IQR, 8.0-17.8) d, ranging from 3 d to 27 d. The median duration from disease onset to death was 22.0 (IQR, 15.3-33.0) d, ranging from 8 d to 44 d. ARDS developed in most patients (77/83, 92.8%), and 5 patients received extracorporeal membrane oxygenation. MODS developed in 58 (69.9%) patients, and septic shock in 16 (19.3%) patients.
AGI grades and clinical variables
We divided the patients into four groups based on the AGI grades: No AGI (n= 11), AGI grade I (n= 30), AGI grade II (n= 35), and AGI grade III to IV (n= 7). As shown in Table 2, significant differences in APACHEII scores, SOFA scores, WBC counts, and Ddimer levels were found among the four groups (P< 0.05). Statistical differences in CRP (P= 0.024) and PCT (P= 0.033) were only found between group AGI grade I and grade III to IV. Significant differences in lactate levels were found between group no AGI and AGI grade II (P= 0.027) or grade III to IV (P= 0.009). Statistical differences in lymphocyte counts were found between group no AGI and AGI grade I (P= 0.028) or grade II (P= 0.007). No differences in BMI were found among the four groups (P> 0.05).
Patients without AGI had longer hospital stay than those with AGI grade I (P= 0.002), II (P= 0.022), and III to IV (P= 0.012). Patients with AGI grade III to IV had longer days of MV and CRRT than those without AGI (P= 0.011, 0.013) and with AGI grade I (P= 0.009, 0.007). No differences in days from onset to admission were found among the four groups (P> 0.05).
Correlation analysis showed that the AGI grades were positively correlated with MV days (r= 0.377,P< 0.001), APACHEII (r= 0.590,P< 0.001) and SOFA scores (r= 0.662,P< 0.001), WBC counts (r= 0.433,P< 0.001), CRP (r= 0.261,P= 0.017) and Ddimer levels (r= 0.425,P< 0.001).
AGI grades and clinical outcomes
As shown in Table 2, patients with AGI grade III to IV had a higher incidence of septic shock than those without AGI (P= 0.002) and with AGI grade I (P= 0.001) and II (P= 0.031). Significant differences in 28-d mortality were found among the four groups (P< 0.05) except for group AGI grade I and II (P= 0.540). No differences in the incidence of MODS were found among the four groups (P> 0.05). Non-survivors were accompanied by a higher incidence of AGI grade III to IV than survivors (17.5%vs0.0%,P= 0.004) (Table 3), whereas survivors had a higher incidence of no AGI than non-survivors (25.6%vs0.0%,P< 0.001) (Table 3).
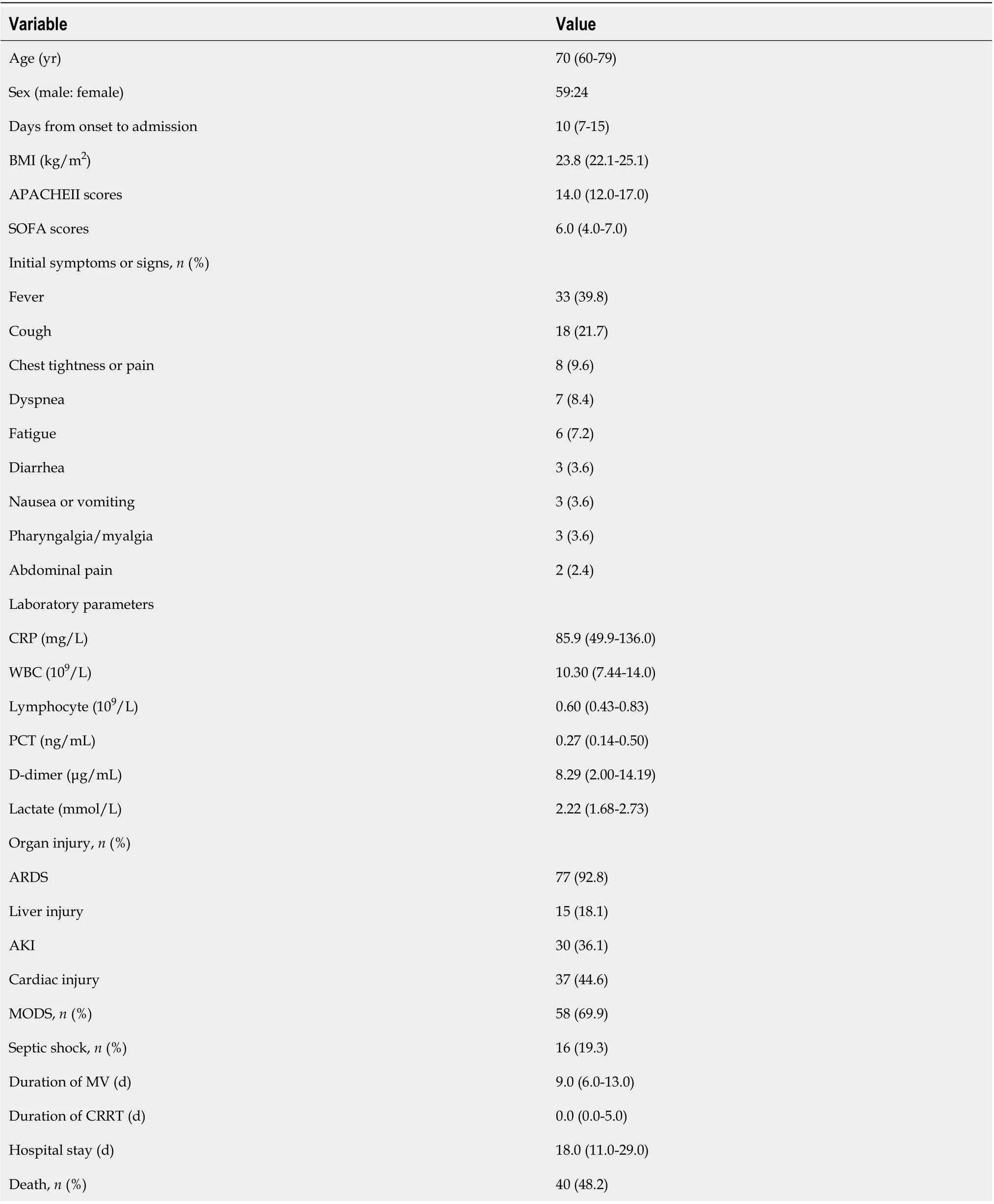
Table 1 Demographic data and clinical parameters
To determine the risk factors associated with AGI grade II and above, univariate logistic regression was performed using the above-mentioned variables (sex, age, days from onset to admission, BMI, APACHEII scores, SOFA scores, CRP, D-dimer, WBC counts, lymphocyte counts, PCT, blood lactate, MV days, CRRT days, and hospital stay). Variables withP< 0.1 in univariate analyses were tested in further multivariatelogistic regression analyses. As shown in Table 4, three variables (SOFA scores, WBC counts, MV days) were established as independent risk factors for the development of AGI grade II and above.
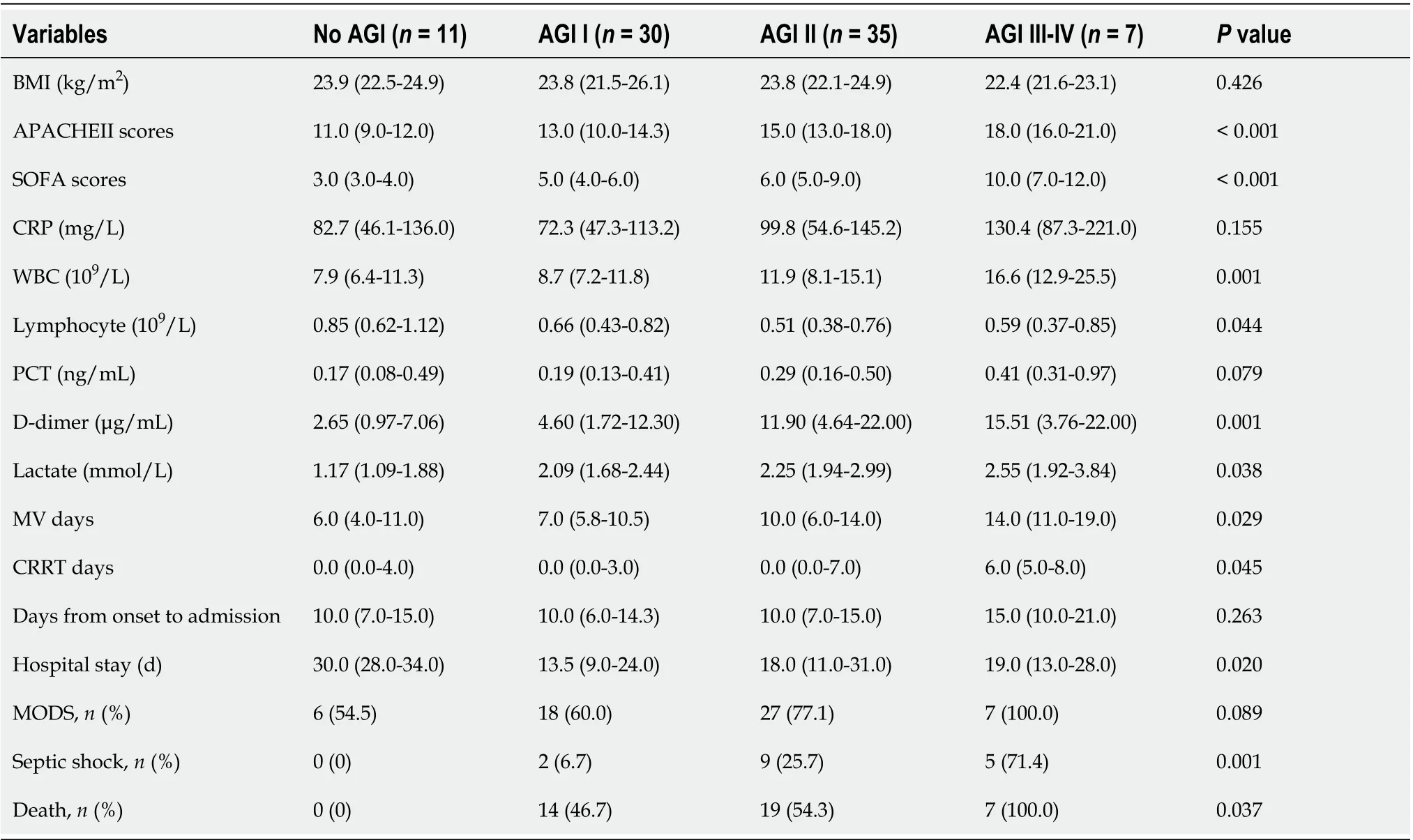
Table 2 Acute gastrointestinal injury grades and clinical variables

Table 3 The incidence of different acute gastrointestinal injury grades in non-survivors and survivors, n (%)
ROC curves were performed to evaluate the associations between AGI and clinical outcome variables. As shown in Figure 2, the area under the curves of MODS (Figure 2A), septic shock (Figure 2B), and 28-d mortality (Figure 2C) were 0.659 (P= 0.022), 0.793 (P< 0.001), and 0.716 (P= 0.001), respectively. Significant differences in 28-d mortality after admission (P= 0.002) and 60-day mortality after disease onset (P= 0.003) were found between group no AGI (n= 11) and AGI (n= 72). As shown in Figure 3, statistical differences in 28-d mortality (P= 0.037) (Figure 3A) and 60-d mortality (P= 0.049) (Figure 3B) were also found between group AGI grade I/no AGI (n= 41) and AGI grade II to IV (n= 42).
DISCUSSION
This retrospective study investigated the prevalence and outcomes of AGI in critically ill patients with COVID-19. 86.7% of the patients had AGI, and 50.6% had AGI grade IIand above during hospital stay. We found that patients with worse AGI grades had worse clinical severity variables, a higher incidence of septic shock, higher 28-d mortality after admission and 60-d mortality after disease onset. SOFA scores, WBC counts, and duration of MV were risk factors for the development of AGI grade II and above. The 28-d mortality and incidence of MODS and septic shock in critically ill patients were 48.2%, 69.9%, and 19.3%, respectively. Non-survivors had a higher incidence of AGI grade III to IV than survivors.

Table 4 Independent factors associated with acute gastrointestinal injury grade II and above in multivariate logistic regression analysis

Figure 1 The flow diagram of participants. AGI: Acute gastrointestinal injury.
Most of critically ill patients with COVID-19 had organ injury, including ARDS and AKI[9]. However, few studies on gastrointestinal injury have been reported in patients with COVID-19. Gastrointestinal dysfunction is common and closely related to adverse outcomes in critically ill patients[10,15-17]. In 2012, the Working Group on Abdominal Problems of the European Society of Intensive Care Medicine developed the definitions and a grading system for AGI in intensive care patients[10]. This expert opinion-based AGI grading system had been proven to be a predictor of all-cause mortality[16]. To our knowledge, this is the first study to investigate AGI in critically ill patients infected by SARS-CoV-2. Our results showed that the incidence of AGI was very high in critically ill patients with COVID-19. AGI was also correlated with clinical severity and outcomes of this novel disease. A recent meta-analysis showed that the incidence of AGI was about 40% and mortality was 33% in critically ill patients[15]. The corresponding data in this study were higher than those in previous reports. This indicated that SARS-CoV-2 was also very virulent in the gastrointestinal tract. However, the underlying mechanisms of SARS-CoV-2 causing organ dysfunction are unknown.
Gastrointestinal injury is often caused by an inflammatory reaction, infection or sepsis, severe trauma, shock, pancreatitis, and other critical diseases[10,15,16]. The receptor for SARS-CoV, which is angiotensin-converting enzyme 2 (ACE2) has also been suggested to be the receptor for SARS-CoV-2[18]. ACE2 is expressed in endothelial cells and smooth muscle cells of almost all organs, especially in lung alveolar cells[18]. That is why COVID-19 patients are susceptible to ARDS and even MODS. Our findings also showed that the incidence of ARDS was very high (92.8%), and AGI grades were significantly positively correlated with MV days. Lianget al[19]reported that ACE2 is highly expressed in the small intestine, especially in proximal and distal enterocytes. ACE2 expression in epithelial cells is required for maintaining antimicrobial peptide expression, amino acid homeostasis, and the ecology of gut microbiome in the intestine[20]. Therefore, gastrointestinal symptoms were also reported in previous studies on COVID-19[7,8]. We believe that these gastrointestinal symptoms were the early manifestations of AGI and should be taken seriously in clinical treatment.
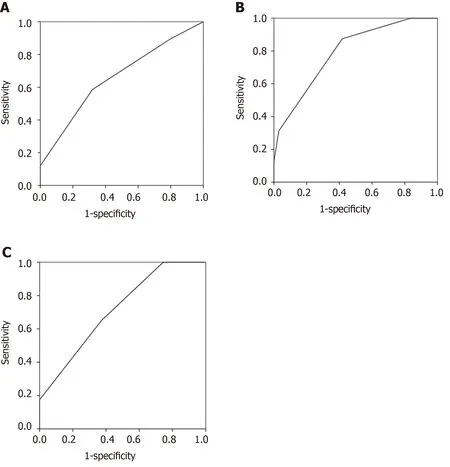
Figure 2 The areas under the receiver operating characteristic curves. A: Multiple organ dysfunction syndrome (0.659, P = 0.022); B: Septic shock (0.793, P < 0.001); C: 28-d mortality (0.716, P = 0.001).

Figure 3 Cumulative survival. Significant differences in 28-d mortality after admission and 60-d mortality after disease onset were found between the group with acute gastrointestinal injury (AGI) grade I/no AGI (n = 41) and the group with acute gastrointestinal injury grade II to IV (n = 42). A: 28-d mortality after admission (P = 0.037); B: 60-d mortality after disease onset (P = 0.049). AGI: Acute gastrointestinal injury.
In this study, we found that AGI grades were correlated with APACHEII and SOFA scores, WBC counts, CRP and D-dimer levels. Moreover, SOFA scores, WBC counts, and duration of MV were risk factors for the development of AGI grade II and above. These results indicated that patients with worse AGI grades had a more serious virus infection and severe inflammatory response, which may lead to a vicious circle between systemic infection and intestinal barrier damage. D-dimer, a fibrin degradation product, is also considered to be associated with adverse outcomes in COVID-19 patients[21]. The abnormal elevation of D-dimer indicated microcirculation disturbance, including microthrombosis formation in intestinal mucosa[21]. During our clinical work against the epidemic of COVID-19 in Wuhan, we observed that gastrointestinal hemorrhage developed in several severe patients. We speculated that stress ulcer and intestinal microcirculation disturbance may be causes of the disorder.
Yanget al[9]reported that ARDS developed in 67%, AKI in 29%, cardiac injury in 23%, and liver dysfunction in 29% of critically ill patients with SARS-CoV-2 pneumonia. The study by Zhouet al[21]showed that septic shock developed in 20%, ARDS in 31%, AKI in 15%, and cardiac injury in 17% of the total number of patients with COVID-19. Our results showed that ARDS developed in 92.8%, AKI in 36.1%, cardiac injury in 44.6%, and liver injury in 18.1% of critically ill patients with COVID-19. The incidence of organ injury in this study was higher than that in previous studies, which may suggest that patients with AGI have worse clinical outcomes. The high incidence of MODS (69.9%) and hospital mortality (48.2%) in critically ill patients in this study also confirmed this conclusion. Moreover, we found that hospital duration in patients without AGI was significantly longer than that in patients with AGI. This could be explained by the high 28-d mortality in patients with AGI, as the median hospital stay of non-survivors was only 12.0 (IQR, 8.0-17.8) days, ranging from 3 d to 27 d.
This study had some limitations. Due to the single-center retrospective design and small sample size, the results might be inconclusive, and the accuracy should be confirmed by large-scale clinical prospective studies. Moreover, because the study was not based on pathophysiological models, the results were hypothesis generating, the exact mechanisms of AGI in COVID-19 should be tested by more basic experiments. In addition, patients were sometimes transferred to our hospital late in their illness. Lack of effective antivirals and inadequate adherence to standard supportive therapy may have contributed to the poor clinical outcomes in some patients.
CONCLUSION
To our knowledge, this is the first study to investigate AGI in critically ill patients with COVID-19. The incidence of AGI was 86.7%, and hospital mortality was 48.2% in critically ill patients. SOFA scores, WBC counts, and duration of MV were risk factors for the development of AGI grade II and above. Patients with worse AGI grades had worse clinical severity variables, a higher incidence of septic shock, and higher hospital mortality.
ARTICLE HIGHLIGHTS

Research conclusions
Patients with worse AGI grades had worse clinical severity variables, a higher incidence of septic shock, and higher hospital mortality.
Research perspectives
To our knowledge, this is the first study to investigate AGI in critically ill patients with COVID-19. The incidence of AGI was 86.7%, and hospital mortality was 48.2% in critically ill patients. Sequential organ failure assessment scores, WBC counts, and duration of mechanical ventilation were risk factors for the development of AGI grade II and above. Patients with worse AGI grades had worse clinical severity variables, a higher incidence of septic shock, and higher hospital mortality.
ACKNOWLEDGEMENTS
The authors thank Liu Q for her assistance in the statistical analysis of this study. The authors also thank Li H, Zou J, Dong K, and Jin CC of Tongji Hospital for their contributions to this study. In addition, Sun JK and his family especially thank Sun XP for her meticulous care and support during the past ten years.
 World Journal of Gastroenterology2020年39期
World Journal of Gastroenterology2020年39期
- World Journal of Gastroenterology的其它文章
- Herbal cake-partitioned moxibustion inhibits colonic autophagy in Crohn’s disease via signaling involving distinct classes of phosphatidylinositol 3-kinases
- Identification of differentially expressed genes in ulcerative colitis and verification in a colitis mouse model by bioinformatics analyses
- Artificial intelligence technique in detection of early esophageal cancer
- Polyethylene glycol 35 ameliorates pancreatic inflammatory response in cerulein-induced acute pancreatitis in rats
- Impact of cap-assisted colonoscopy during transendoscopic enteral tubing: A randomized controlled trial
- Single access laparoscopic total colectomy for severe refractory ulcerative colitis
