小偃麦衍生品系的赤霉病抗性评价
张晓军 肖 进 王海燕 乔麟轶 李 欣 郭慧娟 常利芳 张树伟 阎晓涛 畅志坚,* 武宗信
小偃麦衍生品系的赤霉病抗性评价
张晓军1,2肖 进3王海燕3乔麟轶1李 欣1郭慧娟1常利芳1张树伟1阎晓涛1畅志坚1,2,*武宗信4,*
1山西省农业科学院作物科学研究所 / 作物遗传与分子改良山西省重点实验室, 山西太原 030031;2农业部黄土高原作物基因资源与种质创制重点实验室, 山西太原 030031;3南京农业大学作物遗传与种质创新国家重点实验室, 江苏南京 210095;4山西省农业科学院棉花研究所, 山西运城 044000
由镰孢属()真菌侵染引起的赤霉病是严重威胁小麦生产的重要病害之一, 但小麦育种中可直接利用的抗源非常有限。采用单花滴注法接种赤霉菌株F0609, 对来源于中间偃麦草或长穗偃麦草的119份小偃麦衍生品系进行3年6个环境的抗病鉴定, 发现平均病小穗率<10%的材料有13份, 抗性评价为抗病(R); 平均病小穗率介于10%~25%之间的材料有61份, 抗性评价为中抗(MR); 其余45份材料的平均病小穗率介于25%~50%或>50%, 抗性评价为中感或高感(MS和S)。在13份高抗赤霉病材料中, CH16387的抗性显著优于苏麦3号和望水白, CH16371和CH16379的抗性显著优于望水白, 其余10个品系与抗病对照苏麦3号和望水白的抗性水平相当。这13份材料分别来自小麦−中间偃麦草部分双二倍体TAI8045和小麦−长穗偃麦草部分双二倍体TAP8430与普通小麦的杂交组合, TAI8045抗性显著优于对照品种望水白, TAP8430与苏麦3号和望水白的抗性相当, 而杂交组合中的小麦亲本对赤霉病表现感病, 推测这些材料的抗性可能来自TAI8045和TAP8430。这些抗病材料为小麦抗赤霉病育种提供了新的种质资源。
小麦; 赤霉病; 偃麦草; 遗传改良; 种质资源
由镰孢属()真菌侵染引起的小麦赤霉病不仅会导致小麦大幅减产, 而且, 病原菌产生的脱氧雪腐镰孢菌烯醇(deoxynivalenol, DON)等多种毒素污染还会危害人畜健康[1]。近些年随着全球气候变暖加剧, 以及轮作倒茬、秸秆还田等耕作制度的改变, 小麦赤霉病的高发区已由长江中下游、淮河流域冬麦区和东北春麦区向西南冬麦区、黄淮冬麦区迅速扩展, 直接威胁小麦主产区的安全生产[2]。因此, 开展抗赤霉病育种, 从根本上控制赤霉病的流行与危害对确保国家粮食安全具有重大意义。
小麦赤霉病的研究和防治一直备受关注[3-5]。但由于抗源严重匮乏, 加之抗性表现形式的多样性和遗传方式的复杂性, 使得抗赤霉病育种进展缓慢。我国黄淮、西南、北部冬麦区及东北春麦区的主栽品种对赤霉病的抗性普遍较弱, 可利用的抗源也非常有限。迄今为止, 已从普通小麦及其近缘种属中鉴定出200多个抗赤霉病QTL[6], 分布于小麦各染色体上, 但仅有7个主效抗病基因得到精确定位, 分别是来自“苏麦3号”3BS上的[7]和6BS上的[8]、“望水白”4B上的[9]和5A上的[10], 以及分别从大赖草7Lr#1S、披碱草1Ets#1S和长穗偃麦草7E上转移的[11]、[12]和[13]。因此, 广泛发掘并筛选新的抗赤霉病种质资源, 特别是主效基因控制的抗病材料, 对提高栽培品种的赤霉病抗性, 拓展抗源的遗传基础, 满足小麦育种及生产需求十分必要。
2014—2015年度我们在山西省运城市播种了1100余份小偃麦衍生品系, 恰逢赤霉病严重发生, 从中筛选出119份未感病或感病较轻的品系。2016— 2018年分别在江苏南京、山西太原和四川成都通过单花滴注法对119份小偃麦衍生品系进行了赤霉病抗性鉴定与评价, 以期发掘小麦抗赤霉病优异种质资源。
1 材料与方法
1.1 供试材料
供试的119份小偃麦衍生品系(附表1)分别由TAI8045、TAI8335、TAI8505和TAP8430与普通小麦品种冀麦26、中8701、晋春5号、太原768、晋麦33和京繁309杂交1~2次后自交选育而来。TAI8045、TAI8335和TAI8505是普通小麦与中间偃麦草杂交选育的部分双二倍体小偃麦, TAP8430是普通小麦与十倍体长穗偃麦草杂交选育的部分双二倍体小偃麦。TAI8045、TAI8505、TAI8335、TAP8430以及普通小麦品种太原768、晋春5号和冀麦26均来自山西省农业科学院作物科学研究所; 普通小麦中8701来自中国农业科学院种质资源库(编号为ZM20831)。苏麦3号和望水白为抗病对照品种, 周麦27和Alondra’s为感病对照品种。其中, 苏麦3号、望水白和Alondra’s由南京农业大学作物遗传与种质创新国家重点实验室提供, 周麦27由河南省农业科学院小麦研究所提供。
1.2 赤霉病鉴定地点及接种方法
2016、2017和2018年在江苏省南京市南京农业大学江浦基地日光温室, 2017年和2018年在山西省太原市山西省农业科学院东阳基地日光温室, 2018年在四川省成都市四川省农业科学院新都基地田间分别进行接种鉴定。接种赤霉菌菌株为F0609, 由南京农业大学作物遗传与种质创新国家重点实验室王秀娥博士惠赠。
播种鉴定材料于温室或田间, 行长1.5~2.0 m, 每行播种30粒, 行距0.25 m。在小麦完全抽穗后开始扬花时选取中部小穗采用单花滴注法[14]接种赤霉病, 接种剂量为10 μL (约50~80个孢子 μL–1), 每行材料接种10个穗子, 接种后用保鲜膜包裹保湿3~5 d以使发病, 颖壳变褐色时揭开保鲜膜, 每日喷雾保湿4次以上, 以保证发病环境的湿度。
1.3 赤霉病抗性调查与记载
接种后27 d开始调查发病小穗数, 调查每个材料10个单穗, 抗病调查和记载标准按照《中华人民共和国农业行业标准NY/T 1443.4-2007: 小麦抗赤霉病评价技术规范》[15]。小麦赤霉病严重度按病小穗率(percentage of diseased spikelet, PDS)分5级: 免疫(I), 平均严重度为0, 接种小穗无可见发病症状; 高抗(R), 0<平均严重度<2.0, 仅接种小穗发病, 或相邻的个别小穗发病, 但病斑不扩展到穗轴; 中抗(MR), 2.0≤平均严重度<3.0, 穗轴发病, 发病小穗占总小穗数的1/4以下; 中感(MS), 3.0≤平均严重度<3.5, 穗轴发病, 发病小穗占总小穗数的1/4~1/2; 高感(S), 平均严重度≥3.5, 穗轴发病, 发病小穗占总小穗数的1/2以上。
1.4 染色体数目鉴定
采用Han等[16]描述的方法鉴定染色体数目, 滴片后在Olympus BX53相差显微镜下观察, 每个材料5~10个根尖, 选择分裂相完整的细胞进行染色体计数。
1.5 统计分析
利用SAS (Statistical Analysis System) v9.2对不同环境小麦赤霉病病小穗率进行基本数据统计和相关分析。利用Microsoft Excel 2013进行测验分析。
2 结果与分析
2.1 亲本材料抗赤霉病鉴定与评价
来源于中间偃麦草的TAI8045表现抗病, 病小穗率显著低于抗病对照望水白(<0.05); TAI8505表现中抗, 病小穗率与望水白无显著差异, 但与苏麦3号有显著差异; TAI8335表现中感, 病小穗率与2个抗病对照存在极显著差异(<0.01); 来源于长穗偃麦草的TAP8430表现抗病, 病小穗率与苏麦3号和望水白均无显著差异; 其他普通小麦亲本冀麦26、中8701、晋春5号和太原768均表现感病, 严重度3~4级(表1)。未鉴定普通小麦亲本晋麦33和京繁309。

表1 小偃麦衍生品系亲本的病小穗率及抗性评价
R: 抗病; MR: 中抗; MS: 中感; S: 感病。*、**分别表示在< 0.05,< 0.01水平上与抗病对照有显著差异;a: 仅与望水白在< 0.05水平上显著, 与苏麦3号不显著;b: 仅与苏麦3号在< 0.01水平上差异显著, 与望水白差异不显著。
R: resistant; MR: moderately resistant; MS: moderately susceptible; S: susceptible.*,**: significantly different from the resistant control at< 0.05,< 0.01, respectively.a: significantly different from Wangshuibai at< 0.05, but not from Sumai 3;b: significantly different from Sumai 3 at< 0.01, but not from Wangshuibai.

图1 小偃麦衍生品系亲本对赤霉病的抗性反应(山西太原, 2018)
2.2 119份小偃麦衍生品系赤霉病抗性鉴定
2016—2018年3年6点的抗病鉴定中, 抗病对照苏麦3号和望水白的病害严重度均为1级, 表现高抗或中抗, 二者病小穗率无显著差异。感病对照Alondra’s和周麦27的病害严重度均为4级, 都表现高感。119份小偃麦衍生系接种后均有侵染反应, 未发现病小穗率为0、赤霉病抗性免疫(I)的品系(附表1)。平均病小穗率<10%的材料有13份, 抗性分级为抗病(R), 占鉴定材料的10.9%; 平均病小穗率介于10%~25%之间的材料有61份, 抗性评价为中抗(MR), 占51.3%; 平均病小穗率介于25%~50%之间的材料有41份, 表现中感(MS), 占34.5%; 平均病小穗率大于50%的材料有4份, 表现感病(S)(表2和表3)。鉴定材料分别来自9个杂交组合(表2), 其中以小麦–长穗偃麦草部分双二倍体TAP8430为亲本的3个组合共有73份材料, 4份表现抗病(R), 36份表现中抗(MR), 占组合材料总数的54.8%; 以小麦–中间偃麦草部分双二倍体TAI8045为亲本的3个组合共有37份材料, 9份表现抗病(R), 21份表现中抗(MR), 占组合材料总数的81.1%; 以小麦–中间偃麦草部分双二倍体TAI8335为亲本的2个组合共6份材料, 1份表现中抗(MR), 其余5份为中感(MS); 以小麦–中间偃麦草部分双二倍体TAI8505为亲本的3份材料均表现中抗(MR)。

表2 119份小偃麦衍生品系的赤霉病抗性评价
R: 抗病; MR: 中抗; MS: 中感; S: 感病。
R: resistant; MR: moderately resistant; MS: moderately susceptible; S: susceptible.
2.3 13份优良品系的抗赤霉病分析
来源于部分双二倍体TAP8430和TAI8045的5个组合中, 分别有4份和9份材料在6个环境下均表现出优良的赤霉病抗性, 平均病小穗率均小于10.0%, 病害严重度均为1级, 均表现抗病(R)(表2、表3和图2)。测验表明, CH16387的病小穗率显著低于苏麦3号和望水白(<0.05), CH16371和CH16379的病小穗率分别显著低于望水白(<0.05、<0.01), 其余10个品系的病小穗率与苏麦3号和望水白无显著差异(表3)。
从不同环境病小穗率的变化幅度来看, 除CH16371和CH16388的极差分别为13.1%和12.5%外, 其他11份材料的极差均低于苏麦3号的13.2%, 在试验的各个年度及地点间表现较为稳定的抗病性。尤其是CH16387, 在6个点的试验中, 病小穗率最高仅为8.7%, 最低为1.3%, 极差为7.4%, 低于望水白的7.8%。此外, 不同环境间相关分析表明2018年成都与2018年太原、2017年太原与2017年和2016年南京、2016年与2017年南京之间分别存在一定的相关性, 但相关性均较弱(<0.5), 其余环境间并无显著相关性(表4)。其原因可能是赤霉病发病易受温、湿度等气候因素影响, 病小穗率在不同环境间变异较大, 但在相同环境下与抗、感病对照相比, 仍具有显著抗病性, 品种抗病性鉴定结果相对稳定, 筛选的抗源材料可用于抗赤霉病的遗传和育种研究。

表3 13份小偃麦衍生品系不同环境下的抗病性
*和**分别表示在< 0.05,< 0.01水平上与抗病对照有显著差异。a: 与抗病对照苏麦3号有显著差异(< 0.05),与望水白极显著差异(< 0.01);b: 仅与抗病对照望水白在< 0.01水平上差异显著, 与苏麦3号无显著差异。R: 抗病; MR: 中抗; MS: 中感; S: 感病; -: 未调查或数据缺失。
*,**: significantly different from the resistant control at< 0.05,< 0.01, respectively.a: significantly different from Sumai 3 at< 0.05, and from Wangshuibai at< 0.01;b: significantly different from Wangshuibai at< 0.01, but not from Sumai 3. R: resistant; MR: moderately resistant; MS: moderately susceptible; S: susceptible; -: no results or missing data. PDS: percentage of diseased spikelet.

图2 抗病材料和感病材料对赤霉病的反应类型(山西太原, 2018)
a: 13个抗病反应为R的材料; b: 抗病反应为S的部分材料。
a: resistant wheat lines; b: susceptible wheat lines.

表4 13个抗病品系在不同环境条件下赤霉病病小穗率的相关系数
*和**分别表示在< 0.05和< 0.01水平上差异显著。
*,**: significant difference at< 0.05 and< 0.01, respectively.
3 讨论
近年来, 小麦赤霉病呈爆发速度快、流行范围广、发生面积大和危害损失重的严重态势, 直接威胁着小麦的安全生产[2]。抗病种质资源筛选是抗病育种的基础, 许多小麦近缘种对赤霉病具有较高抗病性, 是小麦抗赤霉病基因的重要来源。从这些近缘种中发掘抗病基因, 对于提高小麦抗赤霉病能力具有重要意义[17]。20世纪80年代以来, 一些研究利用近缘种属创造小麦抗赤霉病新材料, 将纤毛鹅观草[18]、大赖草[19]、鹅观草[20]、华山新麦草[21]、黑麦草、长穗偃麦草[22]、簇毛麦等物种的抗赤霉病基因通过外源染色体附加、代换、易位和双二倍体的方式导入小麦背景中, 育成了许多含有外源染色体或染色体片段的抗赤霉病种质[23]。
偃麦草属植物对赤霉病具有很强的抗性[24], 通过远缘杂交将其抗病基因导入普通小麦, 是改良小麦赤霉病抗性的重要途径。经过研究已从中间偃麦草和长穗偃麦草中鉴定出多个抗赤霉病双二倍体[25-27]、附加系[28]、代换系[27]以及易位系[22, 29-30]。其中易位系在导入抗赤霉病基因的同时, 尽量地减少了不利基因的影响, 而且能够保证小麦染色体构成的稳定性, 因而是利用外源基因的最佳材料。本研究通过多个环境鉴定筛选出的13份高抗赤霉病材料, 有9份来源于小麦–中间偃麦草部分双二倍体TAI8045与普通小麦的杂交组合, 4份来源于小麦–十倍体长穗偃麦草部分双二倍体TAP8430与普通小麦的杂交组合(表2), 其普通小麦亲本对赤霉病都表现中感或感病(表1和图1), 而八倍体亲本TAI8045和TAP8430则高抗赤霉病。因此推测这些材料的赤霉病抗性可能来源于部分双二倍体TAI8045和TAP8430。利用细胞学和分子标记技术可以进一步确定这些材料的赤霉病抗性是否来自偃麦草。根据多年的田间表现, 这些材料的穗型(图2)、株高、株型、籽粒、结实性等农艺性状以及染色体数目都与普通小麦完全一致,根据系谱及抗病性分析, 推测它们不含或仅含很小的外源片段, 可直接用于小麦抗病育种和QTL/基因定位。
小麦赤霉病是由镰孢属真菌侵染引起的一种土传真菌病害, 引起小麦赤霉病的镰孢菌至少有20种, 发病机制复杂, 不同菌株间的致病力存在显著差异[31-32], 因此致病菌株的选择是否恰当直接影响着抗病鉴定结果的准确性。本研究使用赤霉菌菌株F0609进行接种鉴定, 经过抗感对照的发病情况对比, 发现苏麦3号和望水白对F0609菌株均表现抗病, 周麦27和Alondra’s则表现感病(表2和图2)。在3年6个环境的接种鉴定中, F0609对周麦27和Alondra’s的致病性表现一致。在鉴定的119份材料中, 表现抗或中抗的品系占总数的62.2%, 感病品系相对较少, 其原因可能是这些材料是经过2015年赤霉病高发期的田间抗病鉴定筛选出的抗病品系, 但由于田间自然发病不均匀, 因而仍有45份材料人工接种鉴定时表现中感或感病(MS或S)。
本研究筛选出的13份抗病品系在测试的6个环境中, 病小穗率变化幅度不大, 在不同环境条件下表现出较好的抗病性。但部分材料在不同年度和地点间表现出较大的差异, 如CH16376的最高病小穗率为42.7%, 最低仅为3.0%, CH16379最高为50.0%, 最低3.0% (附表1)。究其原因, 既有可能是人为接种造成的差异, 也有可能是赤霉病发病易受环境影响所致[33-34]。因此, 要获得具有良好、稳定抗性的抗赤霉病材料, 需要连续多年在不同环境下进行抗病性鉴定[35], 并且设置适宜的抗感病对照来评价材料的抗性, 以保证获得的抗病材料具有可靠抗性。13份抗病品系中CH16387、CH16371和CH16379的抗性显著优于苏麦3号或望水白, 其余10份与抗病对照苏麦3号和望水白的抗性水平相当; CH16352、CH16388、CH16427和CH16432则表现出矮秆、早熟、结实性好、籽粒饱满、株型紧凑等优良农艺性状, 可为小麦抗病遗传育种提供有价值的亲本材料。
4 结论
采用单花滴注法对来源于中间偃麦草和长穗偃麦草的119份小偃麦衍生品系进行多年多点表型鉴定, 发现有13份材料在多个环境下与高抗对照品种苏麦3号和望水白抗性水平相当, 在不同环境条件下均表现出较好的抗病性; 61份材料表现中抗, 41份材料表现中感, 4份材料表现高感。这些抗赤霉病材料为小麦抗病遗传育种提供有效抗源。
致谢: 对南京农业大学作物遗传与种质创新国家重点实验室王秀娥博士、刘玉博士, 四川农业大学罗培高博士、黄强兰博士, 四川省农业科学院作物所杨恩年博士在种质抗病鉴定方面给予的帮助; 电子科技大学杨足君博士、李光蓉博士, 江苏里下河地区农业科学研究所臧淑江老师在接种与鉴定方法给予的宝贵指导与帮助, 谨致谢忱。
附表1 119份小偃麦衍生品系不同环境下的赤霉病抗性
Supplementary table 1 Responses of the 119 wheat lines derived fromtohead blight under different environments
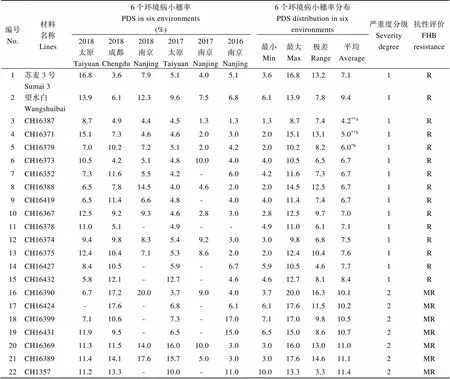
编号No.材料名称Lines6个环境病小穗率PDS in six environments (%)6个环境病小穗率分布PDS distribution in six environments严重度分级Severity degree抗性评价FHB resistance 2018太原Taiyuan2018成都Chengdu2018南京Nanjing2017太原Taiyuan2017南京Nanjing2016南京Nanjing最小Min最大Max极差Range平均Average 1苏麦3号Sumai 316.83.67.95.14.05.13.616.813.27.11R 2望水白Wangshuibai13.96.112.39.67.56.86.113.97.89.41R 3CH163878.74.94.44.51.31.31.38.77.44.2**a1R 4CH1637115.17.34.64.62.03.02.015.113.15.0**b1R 5CH163797.010.27.25.12.04.22.010.28.26.0*b1R 6CH1637310.54.25.14.810.04.04.010.56.56.71R 7CH163527.311.65.54.2-6.04.211.67.36.71R 8CH163886.57.814.54.04.62.02.014.512.56.71R 9CH164196.511.46.64.8-4.04.011.47.46.71R 10CH1636712.59.29.34.62.83.02.812.59.77.01R 11CH1637811.05.1-4.9--4.911.06.17.11R 12CH163749.49.88.35.49.23.03.09.86.87.51R 13CH1637512.410.47.15.38.62.02.012.410.47.61R 14CH164278.410.5-5.9-6.75.910.54.67.71R 15CH164325.812.1-12.7-4.64.612.78.18.41R 16CH163906.717.220.03.79.04.03.720.016.310.12MR 17CH16424-17.6-6.8-6.16.117.611.510.22MR 18CH163997.110.6-7.3-17.07.117.09.810.52MR 19CH1643111.99.5-6.5-15.06.515.08.610.72MR 20CH1636911.311.514.016.010.03.03.016.013.011.02MR 21CH1638911.414.117.615.75.03.03.017.614.611.12MR 22CH135711.213.3-10.0-11.010.013.33.311.42MR
(续附表1)

编号No.材料名称Lines6个环境病小穗率PDS in six environments (%)6个环境病小穗率分布PDS distribution in six environments严重度分级Severity degree抗性评价FHB resistance 2018太原Taiyuan2018成都Chengdu2018南京Nanjing2017太原Taiyuan2017南京Nanjing2016南京Nanjing最小Min最大Max极差Range平均Average 23CH164235.012.312.011.123.06.05.023.018.011.62MR 24CH1639216.914.9-10.0-4.64.616.912.211.62MR 25CH1634624.38.1-4.1-10.04.124.320.211.62MR 26CH1638115.810.224.08.68.05.05.024.019.011.92MR 27CH161188.520.4-8.1-12.18.120.412.312.32MR 28CH1637223.719.314.09.15.03.03.023.720.712.32MR 29CH1636827.411.020.04.812.02.02.027.425.412.92MR 30CH1642624.012.0-7.1-8.87.124.016.912.92MR 31CH1636013.47.1-4.7-27.04.727.022.313.02MR 32CH1638010.815.829.018.41.04.01.029.028.013.22MR 33CH1640917.610.2-19.5-6.06.019.513.513.32MR 34CH1635922.618.58.019.112.02.02.022.620.613.72MR 35CH1641710.812.3-12.0-20.410.820.49.613.92MR 36CH164085.028.5-13.3-8.85.028.523.513.92MR 37CH1637642.76.211.05.615.03.03.042.739.713.92MR 38CH134924.57.5-6.8-17.06.824.517.714.02MR 39CH155624.513.3-6.3-12.16.324.518.214.02MR 40CH1636611.48.08.023.928.06.06.028.022.014.22MR 41CH163828.718.226.022.811.05.05.026.021.015.32MR 42CH1639111.313.129.06.829.03.03.029.026.015.42MR 43CH1640323.120.9-7.1-11.07.123.116.115.52MR 44CH1637714.016.9-6.2-26.06.226.019.815.82MR 45CH1644023.612.6-11.0-17.611.023.612.616.2*2MR 46CH1643927.815.4-13.1-9.59.527.818.316.52MR 47CH1640420.213.714.925.421.04.04.025.421.416.5*2MR 48CH1634229.723.711.09.617.010.09.629.720.116.8*c2MR 49CH1639317.56.7-30.8-13.36.730.824.117.12MR 50CH1642521.820.5-6.5-20.26.521.815.317.2*2MR 51CH1638613.319.521.926.415.08.08.026.418.417.3*2MR 52CH163978.817.450.08.718.03.03.050.047.017.72MR 53CH1640620.433.8-4.6-12.04.633.829.217.72MR 54CH1638423.910.413.019.630.010.010.030.020.017.8*2MR 55CH1641823.031.0-6.1-12.06.131.024.918.02MR 56CH1639431.914.7-13.1-12.512.531.919.518.12MR 57CH1610429.718.7-18.4-10.010.029.719.719.2*2MR 58CH16365-26.214.04.046.07.04.046.042.019.42MR 59CH1642218.831.926.06.030.05.05.031.926.919.6*c2MR 60CH1641214.437.3-14.9-14.014.037.323.320.1*c2MR 61CH1643027.117.6-28.4-11.011.028.417.421.0*2MR 62CH1644230.914.5-21.4-19.514.530.916.421.6**2MR 63CH1637037.535.37.036.911.03.03.037.534.521.8*2MR
(续附表1)

编号No.材料名称Lines6个环境病小穗率PDS in six environments (%)6个环境病小穗率分布PDS distribution in six environments严重度分级Severity degree抗性评价FHB resistance 2018太原Taiyuan2018成都Chengdu2018南京Nanjing2017太原Taiyuan2017南京Nanjing2016南京Nanjing最小Min最大Max极差Range平均Average 64CH1641649.98.3-7.9--7.949.942.022.0*2MR 65CH1644526.026.2-18.8-17.617.626.28.622.2**2MR 66CH1642113.451.1-12.1-12.012.051.139.122.2*2MR 67CH1642011.140.6-26.1-11.011.040.629.622.2*c2MR 68CH1635132.012.1-35.9-9.49.435.926.622.4*2MR 69CH1634123.916.0-18.4-32.016.032.016.022.6**2MR 70CH1644327.916.3-23.9--16.327.911.622.7**2MR 71CH16449-25.3-20.2-23.620.225.35.123.0**2MR 72CH1611230.120.3-15.0-27.015.030.115.123.1**2MR 73CH1638330.614.0-15.7-33.014.033.019.023.3**a2MR 74CH1641440.220.3-6.7-27.16.740.233.423.6*2MR 75CH1640117.18.385.011.417.03.03.085.082.023.6*2MR 76CH1641521.56.7-21.4-49.06.749.042.324.6*2MR 77CH1641015.038.6-21.8--15.038.623.625.1*3MS 78CH1643525.3--19.5-31.019.531.011.525.2**3MS 79CH1636238.133.9-13.0-18.013.038.125.025.8*3MS 80CH1643816.931.9-15.4-39.715.439.724.326.0**3MS 81CH1634832.141.4-18.8-13.013.041.428.426.3*3MS 82CH1636418.853.613.08.159.06.06.059.053.026.4*3MS 83CH136436.521.5-31.1-17.017.036.519.526.6**3MS 84CH1640233.321.5-27.4-25.021.533.311.926.8**3MS 85CH1639612.234.135.340.0-13.012.240.027.926.9**3MS 86CH1638530.034.2-23.7-20.220.234.214.027.0**3MS 87CH1634331.160.69.028.2-8.08.060.652.627.4*3MS 88CH1643318.647.4-18.8-26.218.647.428.927.8*3MS 89CH1634028.832.3-31.1-19.119.132.313.227.8**3MS 90CH1643739.720.5-21.8-31.920.539.719.128.5**3MS 91CH1640738.243.3-18.9-14.114.143.329.228.6*3MS 92CH1640526.411.485.09.531.09.09.085.076.028.7*3MS 93CH1635452.712.9-30.5-19.312.952.739.828.8*3MS 94CH1644438.123.1-31.0-29.023.138.115.030.3**3MS 95CH1639516.852.391.06.014.02.02.091.089.030.4*3MS 96CH1642833.440.2-11.3-38.411.340.228.930.8**3MS 97CH1642931.831.8-27.8-31.927.831.94.130.8**3MS 98CH1641323.244.4-24.4-31.823.244.421.231.0**3MS 99CH1634438.623.1-36.0-30.023.138.615.531.9**3MS 100CH1635835.646.8-14.0-34.214.046.832.932.6**3MS 101CH1644152.219.2-31.0-30.019.252.233.033.1**3MS 102CH1639830.149.8-16.5-37.516.549.833.333.5**3MS 103CH164486.157.5-38.5-31.86.157.551.533.5*3MS 104CH1644731.737.7-40.2-27.127.140.213.034.2**3MS
(续附表1)

编号No.材料名称Lines6个环境病小穗率PDS in six environments (%)6个环境病小穗率分布PDS distribution in six environments严重度分级Severity degree抗性评价FHB resistance 2018太原Taiyuan2018成都Chengdu2018南京Nanjing2017太原Taiyuan2017南京Nanjing2016南京Nanjing最小Min最大Max极差Range平均Average 105CH1636351.779.729.024.620.02.02.079.777.734.5*3MS 106CH1634753.937.2-30.1-24.424.453.929.536.4**3MS 107CH1644646.041.0-28.2-34.128.246.017.837.3**3MS 108CH1636162.742.8-9.0--9.062.753.738.2*3MS 109CH167350.8-57.032.1-16.016.057.041.039.0**3MS 110CH1643640.846.2-23.1-51.123.151.128.040.3**3MS 111CH1641117.075.451.124.8-39.717.075.458.441.6**3MS 112CH1635547.964.0-23.2-34.123.264.040.842.3**3MS 113CH1634533.473.8-21.5-46.021.573.852.243.7**3MS 114CH1640047.480.274.920.6-15.015.080.265.247.6**3MS 115CH1635740.254.562.727.8-55.027.862.734.948.0**3MS 116CH1635636.358.6-37.5-60.736.360.724.548.3**3MS 117CH1635353.282.1-43.1-21.121.182.161.049.9**3MS 118CH1643438.496.3-31.0-35.331.096.365.250.2**4S 119CH1610678.461.4-40.2-41.440.278.438.355.3**4S 120CH1635063.893.9-53.6-55.653.693.940.466.7**4S 121CH1634995.0100.0-33.6-46.033.6100.066.468.6**4S 122周麦27Zhoumai 2764.357.063.058.638.653.938.664.325.855.9**4S 123Alondra’s53.165.563.851.748.161.448.165.517.457.3**4S
R: 抗病; MR: 中抗; MS: 中感; S: 感病; “-”: 未调查或数据缺失。苏麦3号, 望水白: 抗病对照; 周麦27, Alondra’s: 感病对照。a: 与抗病对照苏麦3号有显著差异(< 0.05), 与望水白极显著差异(< 0.01);b: 仅与抗病对照望水白差异显著(< 0.05), 与苏麦3号无显著差异;c: 仅与苏麦3号有显著差异(< 0.05), 与望水白无显著差异。*、**分别表示在< 0.05、< 0.01水平上与抗病对照有显著差异。
R: resistant; MR: moderately resistant; MS: moderately susceptible; S: susceptible;“-”: no results or missing data. PDS: percentage of diseased spikelet. Sumai 3, Wangshuibai: resistant control; Zhoumai 27, Alondra’s: susceptible control.a: significantly different from Sumai 3 at< 0.05, and from Wangshuibai at< 0.01.b: significantly different from Wangshuibai at< 0.05, but not from Sumai 3.c: significantly different from Sumai 3 at< 0.05, but not from Wangshuibai.*,**: significantly different from the resistant control at< 0.05,< 0.01, respectively.
[1] 程顺和, 张勇, 别同德, 高德荣, 张伯桥. 中国小麦赤霉病的危害及抗性遗传改良. 江苏农业学报, 2012, 28: 938–942.Cheng S H, Zhang Y, Bie T D, Gao D R, Zhang B Q. Damage of wheathead blight (FHB) epidemics and genetic improvement of wheat for scab resistance in China., 2012, 28: 938–942 (in Chinese with English abstract).
[2] 曾娟, 姜玉英. 2012年我国小麦赤霉病暴发原因分析及持续监控与治理对策. 中国植保导刊, 2013, 33(4): 37–41. Zeng J, Jiang Y Y. Analysis and continuous monitoring and control countermeasures of wheat FHB disease outbreak in China in 2012., 2013, 33(4): 37–41 (in Chinese with English abstract).
[3] Bai G H, Shaner G. Management and resistance in wheat and barley tohead blight., 2004, 42: 135–161.
[4] Stepient L, Chelkowski J.head blight of wheat: pathogenic species and their mycotoxins., 2010, 3: 107–119.
[5] Gilbert J, Haber S. Overview of some recent research developments inhead blight of wheat., 2013, 35: 149–174.
[6] 张爱民, 阳文龙, 李欣, 孙家柱. 小麦抗赤霉病研究现状与展望. 遗传, 2018, 40: 858–873. Zhang A M, Yang W L, Li X, Sun J Z. Current status and perspective on research againsthead blight in wheat.(Beijing), 2018, 40: 858–873 (in Chinese with English abstract).
[7] Cuthbert P A, Somers D J, Thomas J, Cloutier S, Brule-Babel A. Fine mapping, a major gene controllinghead blight resistance in bread wheat (L.)., 2006, 112: 1465–147.
[8] Cuthbert P A, Somers D J, Brule-Babel A. Mapping ofon chromosome 6BS: a gene controllinghead blight field resistance in bread wheat (L.)., 2007, 114: 429–437.
[9] Xue S L, Li G Q, Jia H Y, Xu F, Lin F, Tang M Z, Wang Y, An X, Xu H B, Zhang L X, Kong Z X, Ma Z Q. Fine mapping, a major QTLs conditioning resistance toinfection in bread wheat (L.)., 2010, 121: 147–156.
[10] Xue S L, Xu F, Tang M Z, Zhou Y, Li G Q, An X, Lin F, Xu H B, Jia H Y, Zhang L X, Kong Z X, Ma Z Q. Precise mapping, a major QTLs conditioning resistance toinfection in bread wheat (L.)., 123: 1055–1063.
[11] Qi L L, Pumphrey M O, Friebe B, Chen P D, Gill B S. Molecular cytogenetic characterization of alien introgressions with genefor resistance tohead blight disease of wheat., 2008, 117: 1155–1166.
[12] Cainong J C, Bockus W W, Feng Y G, Chen P D, Qi L L, Sehgal S K, Danilova T V, Koo D H, Friebe B, Gill B S. Chromosome engineering, mapping, and transferring of resistance tohead blight disease frominto wheat., 2015, 128: 1019–1027.
[13] Guo J, Zhang X L, Hou Y L, Cai J J, Shen X R, Zhou T T, Xu H H, Ohm H W, Wang H W, Li A F, Han F P, Wang H G, Kong L R. High-density mapping of the major FHB resistance genederived fromand its pyramiding withby marker-assisted selection., 2015, 128: 2301–2316.
[14] Bai G H, Kolb F L, Shaner G, Domier L L. Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling scab resistance in wheat., 1999, 89: 343–348.
[15] 中华人民共和国农业行业标准NY/T 1443.4-2007, 小麦病虫性评价技术规范, 第4部分: 小麦抗赤霉病评价技术规范.Agricultural Standard of the People’s Republic of China, NY/T 1443.4-2007. Rules for Resistance Evaluation of Wheat to Diseases and Insect Pests, Part 4. Rule for Resistance Evaluation to Wheat Scab (in Chinese).
[16] Han F P, Fedak G, Benabdelmouna A, Armstrong K C, Ouellet T. Characterization of six wheat ×derivativesby GISH, RFLP and multicolor GISH., 2003, 46:490–495.
[17] Zhang L, Luo P G, Ren Z L, Zhang H Y. Controlling fusarium head blight of wheat (L.) with genetics., 2011, 2: 263–270.
[18] Wang X E, Chen P D, Liu D J, Zhang P, Zhou B, Friebe B, Gill B S. Molecular cytogenetic characterization ofchromosome additions in common wheat., 2001, 102: 651–657.
[19] 王林生, 张雅莉, 南广慧. 普通小麦–大赖草易位系T5AS-7Lr.7LrS分子细胞遗传学鉴定. 作物学报, 2018, 44: 1442–1447. Wang L S, Zhang Y L, Nan G H. Molecular and cytogenetic identification of–translocation line T5AS-7Lr.7LrS., 2018, 44: 1442–1447 (in Chinese with English abstract).
[20] 丁春邦, 周永红. 小麦族拟鹅观草属研究进展. 四川农业大学学报, 2004, 22: 269–273. Ding C B, Zhou Y H. Advances in research onin the tribe Triticeae., 2004, 22: 269–273 (in Chinese with English abstract).
[21] 王益, 康厚扬, 原红军, 蒋云, 张海琴, 周永红. 普通小麦与华山新麦草衍生后代的农艺性状和细胞遗传学研究. 四川农业大学学报, 2008, 26: 405–410. Wang Y, Kang H Y, Yuan H J, Jiang Y, Zhang H Q, Zhou Y H. Cytogenetic and morphological studies on the derivative progenies of×., 2008, 26: 405–410 (in Chinese with English abstract).
[22] 张璐璐, 陈士强, 李海凤, 刘慧萍, 戴毅, 高勇, 陈建民. 小麦–长穗偃麦草7E抗赤霉病易位系培育. 中国农业科学, 2016, 49: 3477–3488. Zhang L L, Chen S Q, Li H F, Liu H P, Dai Y, Gao Y, Chen J M. Development of wheat–translocation lines resistant tohead blight., 2016, 49: 3477–3488 (in Chinese with English abstract).
[23] 丛雯雯, 郭长虹. 小麦近缘野生植物的赤霉病抗源筛选及其利用. 分子植物育种, 2010, 8: 1043–1049. Cong W W, Guo C H. Selection and utilization of resistance sources tohead blight in wheat wild relatives., 2010, 8: 1043–1049 (in Chinese with English abstract).
[24] Li H J, Wang X M.and: the promising source of resistance to fungal and viral diseases of wheat., 2009, 36: 557–565.
[25] Oliver R E, Cai X, Xu S S, Chen X, Stack R W. Wheat-alien species derivatives: a novel source of resistance tohead blight in wheat., 2005, 45: 1353–1360.
[26] Zeng J, Cao W, Fedak G, Sun S, Mccallum B, Fetch T, Xue A, Zhou Y. Molecular cytological characterization of two novel durum–partial amphiploids with resistance to leaf rust, stem rust andhead blight., 2013, 150: 10–16.
[27] Turner M K, De Haan L R, Jin Y, Anderson J. Wheatgrass–wheat partial amphiploids as a novel source of stem rust andhead blight resistance., 2013, 53: 1994–2005.
[28] Fu S, Lv Z, Qi B, Guo X, Li J, Liu B, Han F. Molecular cytogenetic characterization of wheat–addition, substitution and translocation lines with a novel source of resistance to wheathead blight., 2013, 39: 103–110.
[29] Liu Z H, Xu M, Xian Z P, Li X, Chen W Q, Luo P G. Registration of the novel wheat lines L658, L693, L696, and L699, with resistance tohead blight, stripe rust, and powdery mildew., 2015, 9: 121–124.
[30] Zhang X L, Shen X R, Hao Y F, Cai J J, Ohm H W, Kong LR. A genetic map ofchromosome 7E, harboring resistance genes tohead blight and leaf rust., 2011, 122: 263–270.
[31] 史文琦, 杨立军, 冯洁, 张旭, 曾凡松, 向礼波, 汪华, 喻大昭. 小麦赤霉病流行区镰刀菌致病种及毒素化学型分析. 植物病理学报, 2011, 41: 486–494. Shi W Q, Yang L J, Feng J, Zhang X, Zeng F S, Xiang L B, Wang H, Yu D Z. Analysis on the population structure ofspp. and its mycotoxin chemotypes inhead blight epidemic region., 2011, 41: 486–494 (in Chinese with English abstract).
[32] 俞刚, 陈利锋, 谢卫平, 柴一秋. 禾谷镰孢的产毒与致病性. 南京农业大学学报, 2001, 24(4): 19–23. Yu G, Chen L F, Xie W P, Chai Y Q. Correlation of trichothecene producing potential to pathogenicity ofon wheat., 2001, 24(4): 19–23 (in Chinese with English abstract).
[33] 刘易科, 佟汉文, 朱展望, 陈泠, 邹娟, 张宇庆, 焦春海, 高春保. 小麦赤霉病抗性改良研究进展. 麦类作物学报, 2016, 36: 51–57. Liu Y K, Tong H W, Zhu Z W, Chen L, Zou J, Zhang Y Q, Jiao C H, Gao C B. Review on improvement ofhead blight resistance in wheat., 2016, 36: 51–57 (in Chinese with English abstract).
[34] 唐洪, 彭恒, 刘明龙, 杨建华, 吴吉林. 小麦赤霉病田间病情与抽穗扬花期气象条件和病粒率关系. 中国植保导刊, 2012, 32(7): 10–12.Tang H, Peng H, Liu M L, Yang J H, Wu J L. Relationships among field condition of wheat scab and climate and percentage ofinfected kernels at the heading and flowering stage., 2012, 32: 10–12 (in Chinese with English abstract).
[35] Mesterhazy A. Types and components of resistance tohead blight of wheat., 1995, 114: 377–386.
Evaluation of resistance tohead blight in-derived wheat lines
ZHANG Xiao-Jun1,2, XIAO Jin3, WANG Hai-Yan3, QIAO Lin-Yi1, LI Xin1, GUO Hui-juan1, CHANG Li-Fang1, ZHANG Shu-Wei1, YAN Xiao-Tao1, CHANG Zhi-Jian1,2,*, and WU Zong-Xin4,*
1Institute of Crop Science, Shanxi Academy of Agricultural Sciences / Shanxi Key Laboratory of Crop Genetics and Molecular Improvement, Taiyuan 030006, Shanxi, China;2Key Laboratory of Crop Gene Resources and Germplasm Enhancement on Loess Plateau of Ministry of Agriculture, Taiyuan 030006, Shanxi, China;3State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University, Nanjing 210095, Jiangsu, China;4Institute of Cotton, Shanxi Academy of Agricultural Sciences, Yuncheng 044000, Shanxi, China
head blight (FHB) caused byis one of the most destructive fungal diseases in wheat production; however, only limited sources of resistance are available in wheat. In this study, we evaluated 119 lines derived from the crosses between wheat and wheat−partial amphiploids for their resistance toisolate F0609 over six environments during 2016 to 2018 cropping seasons using single floret inoculation method. Among the wheat–lines tested, 45 were moderately or highly susceptible, with 25%–50% or >50% of the average percentage of diseased spikelets (PDS), 61 were moderately resistant (MR) with 10%–25% of the average PDS, and 13 lines were identified as resistant (R), with the average PDS less than 10%. For the FHB resistance of the 13 resistant lines, CH16387 was superior to ‘Sumai 3’ and ‘Wangshuibai’, the most widely used source of resistance to FHB, CH16371 and CH16379 were superior to ‘Wangshuibai’, and the remaining ten lines were comparable to ‘Wangshuibai’ or ‘Sumai 3’, in terms of number of infected spikelets per spike and percentage of infected spikelets. Furthermore, the average PDS in these resistant lines over the six environments showed a similar distribution, suggesting a relatively stable FHB resistance. The donor parents, wheat-alien partial amphiploids, involved in development of these resistant derivatives, included wheat–partial amphiploid TAI8045 and wheat–partial amphiploid TAP8430. As both TAI8045 and TAP8430 were resistant, but all the wheat parents were susceptible, it was likely that the resistance to FHB in these lines identified originated from TAI8045 and TAP8430. These derivatives can serve as novel sources to enhance resistance of wheat to FHB.
wheat;head blight;; genetic improvement; germplasm resources
2019-02-16;
2019-08-09;
(网络出版日期): 2019-09-03.
10.3724/SP.J.1006.2020.91015
Corresponding authors): 畅志坚, E-mail: wrczj@126.com; 武宗信, E-mail: mhdwzx@126.com
联系方式: E-mail: zxjemail@163.com
本研究由国家重点研发计划项目(2017YFD0100600), 山西省重点研发计划项目(201803D221018-5, 201703D211007, 201803D421020), 山西省农业科学院项目(YGG17123, YCX2018D2YS01)和山西省重点科技创新平台(201605D151002)资助。
This study was supported by the National Key R&D Program of China (2017YFD0100600), the Key R&D Program of Shanxi Province (201803D221018-5, 201703D211007, 201803D421020), Shanxi Academy of Agricultural Sciences (YGG17123, YCX2018D2YS01), and Shanxi Key Scientific and Technological Innovation Platform (201605D151002)
URL: http://kns.cnki.net/kcms/detail/11.1809.S.20190902.1719.008.html

