Tacrolimus and mycophenolate mofetil as second-line treatment in autoimmune hepatitis: Is the evidence of sufficient quality to develop recommendations?
Mohammadreza Abdollahi, Neda Khalilian Ekrami, Morteza Ghojazadeh, H Marike Boezen,Mohammadhossein Somi, Behrooz Z Alizadeh
Abstract
Key Words: Autoimmune hepatitis; Efficacy; Grading of Recommendations Assessment, Development and Evaluation approach; Systematic review; Meta-analysis; Second-line
INTRODUCTION
Autoimmune hepatitis (AIH) is a rare, chronic, inflammatory liver disease, characterized by elevated transaminase and immunoglobulin G levels, positive autoantibodies and interface hepatitis at liver histology[1,2]. It affects people of all ages and can lead to cirrhosis, hepatic failure, liver transplantation, and death[3]. Despite the availability of effective treatment and an evident good response to therapy, patients have a poor prognosis if the disease is left untreated or is treated suboptimally[4,5].
Standard first-line treatment of AIH is based on prednisolone, either given alone or in combination with azathioprine (AZA)[6]; these treatments lead to remission in 80% of patients[7,8]. However, about 20% of patients are refractory to standard treatment; this could be a result of suboptimal response, including treatment failure or incomplete response, or because patients are intolerant to standard treatment due to side-effects[8]. Thus, several second-line treatment modalities have been introduced for refractory AIH patients, including tacrolimus, mycophenolate mofetil (MMF), cyclosporine and budesonide[6-9]. Tacrolimus is a calcineurin inhibitor, which exerts more potent immunosuppressive effects on CD4+ T helper cells with fewer cosmetic side-effects. MMF is a purine antagonist similar to AZA, but it has more potent immunosuppressive properties and is better tolerated than AZA[10,11]. Tacrolimus and MMF have empirically been used the most, as alternative medications based on the American Association for the Study of Liver Diseases (AASLD) practice[8]. They are the most used drugs based on expert opinions[12]. Nevertheless, the efficacy and superiority of these interventions compared to using high-dose prednisolone and AZA has not been reported[8]. Furthermore, there is a lack of primary consensus on overall drug side-effects and disease mortality, at least for tacrolimus.
The accumulating but still sparse data indicate that refractory AIH patients do respond to these alternative treatments[13]. However, there is no firm evidence of their effectiveness. First, there has been no randomized clinical trial (RCT) directly comparing these two medications to each other. Second, the available data are mainly based on small series of patients or case reports, from only a few centers, and little quality assessment has been performed. Thus, the translation of these findings to a guideline is questionable[14]. Third, two recent meta-analyses, which summarized the effect of the two medications in AIH, had a number of shortcomings. One study focused on improvement of aminotransferases rather than biochemical remission[15], while the second study included some (n= 12), but not all of the previously published studies related to MMF, and had analytical issues, such as reporting incorrect heterogeneity, ignoring existing publication bias, and reporting an incorrect overall mortality rate[16]. Therefore, their conclusions did not appear to be well supported by their results.
Furthermore, there is no systematic review or meta-analysis to compare tacrolimus with MMF as a second-line treatment in refractory AIH, or to evaluate the adverse effects, safety profile, and mortality rate of tacrolimus as a second-line treatment in refractory AIH. Recently two studies reported on the efficacy of tacrolimus[17]and MMF[18]as second-line treatment in AIH; these both need to be added to the overall assessment of the efficacy of these two medications in refractory AIH. In the absence of classical RCTs, and the shortcomings of previous investigations, there were still no comprehensive studies to evaluate the superiority of these two drugs for refractory AIH patients. The main question is whether there is sufficient high-quality scientific evidence to adapt the clinical guidelines.
Therefore, to study whether tacrolimus and MMF are superior alternative treatments, we firstly performed a systematic review and meta-analysis on the efficacy and safety of these treatments as second-line treatment in AIH patients. We also critically checked whether the quality of evidence, assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach[19,20], was sufficient to support adapting the clinical guidelines for routine practice.
MATERIALS AND METHODS
Protocol and registration
This study was conducted according to the guidelines in Meta-analysis of Observational Studies in Epidemiology (MOOSE)[21].
Information sources and search strategies
We reviewed the literature, focusing on our aim to identify, appraise, select and synthesize all the high-quality evidence available. To identify published articles and ongoing studies, we conducted a comprehensive search of electronic databases, including PubMed, EMBASE and Cochrane Central Register, with additional searching of the clinical trials website at www.clinicaltrials.gov. All databases were searched from their inception through October 2019, with no language restrictions. The search strategy was designed with the help of an experienced medical librarian and with input from investigators. Search terms were selected using Medical Subject Headings (Mesh) terms, including (but not limited to) "Hepatitis, Autoimmune", "Tacrolimus", "Mycophenolic Acid” and "Drug Resistance". In addition, to minimize the chance of missing any study, the reference lists of the included articles were searched individually for additional studies.
Eligibility criteria
We included studies which met the following criteria: Randomized or nonrandomized controlled trials, case series of any duration, cohort studies, and reports that provided data on patients over 18 years with AIH who failed or were unable to tolerate first-line therapy prior to liver transplantation. Studies that used tacrolimus and/or MMF had to report on the disease outcomes studied. We excluded studies that reported data for AIH in children or adolescents aged ≤ 18 years, because they have a more aggressive disease, often with a more acute presentation[22]and they therefore need a different management[23]; or that reported data on patients who had had a liver transplantation.
Outcomes
Biochemical remission was the primary outcome for our study. This was defined as the disappearance of symptoms, normal serum bilirubin, γ-globulin and serum aminotransferase levels[8]. Secondary outcomes were the occurrence of adverse events and mortality.We evaluated the available data for these outcomes per individual in the studies included in our analysis. For example, if γ-globulin levels were not described in a certain study, we used the variables available, such as clinical and biochemical variables, to reach the most likely definition of remission for that study.
Some studies reported biochemical remission depending on the reason for using second-line therapy (intolerancevsnon-response). Thus, we performed a subgroup analysis in non-responders and those intolerant to standard therapy to compare the effect of tacrolimus and MMF in the two different groups that used the drug as second-line therapy.
Study selection and data extraction
After excluding duplicated reports, the reports included in our analysis were reviewed on the basis of their title and abstract by two independent reviewers (MA, MG). Thereafter, the full texts of selected reports were retrieved and independently assessed by both reviewers to identify which studies satisfied our inclusion criteria. Discrepancies between the two reviewers regarding eligibility were solved by jointly looking at the study in question. If no consensus was reached, a third reviewer (BZA) was consulted. Agreement was measured using inter-rater reliability (Cohen's Kappa).Next, we extracted data on each study: Study characteristics (including the surname of the first author and year of publication), patients’ characteristics (including mean age and number of patients), intervention characteristics (including duration of applied therapy and length of follow-up), as well as data on our primary and secondary outcome measures.
Methodological quality
The two reviewers independently rated the methodological quality of each study using the GRADE tool for study-level assessments of risk of bias[19,20]. Specifically, the domains assessed for risk of bias included: Failure to develop and apply appropriate eligibility criteria (inclusion of control population), flawed measurement of either intervention or outcome, failure to adequately control confounding, and incomplete follow-up.
GRADE quality assessment
The GRADE approach was used to assess the quality of evidence for primary and secondary outcomes[20,21]. It provides guidance for rating the quality of evidence and grading the strength of recommendations by asking a clear question for each outcome. The level of evidence was graded as high or moderate when it was derived from RCTs, and as low or very low if it was derived from observational studies. The level of evidence could be upgraded or downgraded depending on the quality of the study. The criteria provided for possibly downgrading the quality of evidence include: Risk of bias, publication bias, indirectness, imprecision and inconsistency. The three main reasons for upgrading the quality of evidence include: Large effect size, dose response gradient, and all plausible residual confounders increase confidence in the estimated effect. Each study can be given up to two points for every domain that begins with a high rating that is later downgraded one level (judged to have serious concerns) and two levels (if concerns are very serious). These evaluations result in one of four quality ratings – high, moderate, low and very low – that reveal the degree of confidence one can have in the available evidence correctly reflecting the theoretical true effect of the intervention. GRADE ratings are given as described by Balshemet al[24], and are adjudicated as high (we are very confident that the true effect lies close to that of the estimate of the effect), moderate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it could be substantially different), low (our confidence in the effect estimate is limited,i.e., the true effect may be substantially different from the estimate of the effect), or very low (we have very little confidence in the effect estimate,i.e., the true effect is likely to be substantially different from the estimate of effect).
Statistical analysis
Several studies reported the median, minimum and maximum values. Hence, in order to be able to combine the results, we deducted the sample mean and standard deviation (SD) using Wanet al’s[25]method for those studies. We estimated pooled event rates with corresponding 95% confidence intervals (CI) using the inverse variance method per analyzed outcome. We applied random effects models whenever there was significant heterogeneity between studies.
Qtest, the associatedPvalue (P< 0.10) and also theI2test were used to assess heterogeneity among the included studies. According to Higginset al[26]I2< 40% indicates low heterogeneity, 30%
RESULTS
Study selection
The initial database search yielded 243 valid hits, of which 71 were duplicates and removed (Figure 1). The titles and abstracts of the remaining 172 citations were screened, leading to exclusion of a further 87 citations. The remaining 85 articles were assessed for relevance to our outcomes of interest. A full text review led to 64 articles being excluded. Overall, 21 studies met our eligibility criteria. Cohen's Kappa for interrater reliability between the two reviewers was 0.88. Thus, 21 unique observational studies[17,18,29-47]were eligible to be included in the meta-analysis and to be evaluated on the scientific quality of data using GRADE. The search results are summarized in Figure 1.
Study characteristics
Nine studies reported data on tacrolimus and 16 on MMF. Their geographical distribution was diverse, with studies carried out in the United States, Canada, United Kingdom, Denmark, Germany, the Netherlands, Belgium, Sweden, China, India and Australia. Most studies included middle-aged subjects who were predominantly female. In the tacrolimus studies, the average follow-up time was longer than in the MMF studies (51 movs38 mo), patients were younger (mean age 37.8 yearsvs42.8 years) and more females (73.3%vs71.2%) were observed. Applied dosages varied between 2.0 and 6.0 mg daily of tacrolimus, and between 0.5 g and 2.0 g daily of MMF. The basic characteristics of the studies are presented in Table 1.
Methodological quality of the studies
For each of the 21 included studies, the methodological quality (risk of bias) was assessed and downgraded for the presence of bias using the GRADE risk of bias tool on a scale of 0 to -2. Four studies were rated as having no risk of bias (scored as 0), 16 studies had a serious risk of bias (scored as -1) and the remaining two had a very serious risk of bias (scored as -2). The details of the assessment of the methodological quality of the studies is reported in Table 2 and Figure 2-4.
Pooled prevalence of biochemical remission
In total, we included 584 patients with AIH who were unable to tolerate or respond to first-line therapy. In 157 patients treated with tacrolimus, the overall pooled prevalence of biochemical remission was 68.9% (95%CI: 60.4-76.2), and in 427 patients with MMF 59.6% (95%CI: 54.8-64.2) (Figure 3 and 4).
Significant moderate heterogeneity was found among the 21 studies in the metaanalysis for tacrolimus and MMF (Q= 16.25vs29.72, df = 8vs15,P< 0.0001), respectively. According toI2values for the tacrolimus and MMF groups, approximately 50.8%vs49.5% respectively, the variability in effect estimates was due to the heterogeneity between the studies rather than a sampling error or chance. Overall quality assessments using GRADE were very low for biochemical remission in both intervention groups (Table 2).
Egger’s regression test for tacrolimus (intercept = 0.02, 95%CI: -2.14 to 2.18;P= 0.98) and for MMF (intercept = -0.36, 95%CI: -1.77 to 1.06;P= 0.59) did not show statistically significant asymmetry of the funnel plots, suggesting that publication bias was unlikely in both intervention groups (Figure 5).
Overall, 15 studies specified response rates according to the reason for using tacrolimus or MMF. In patients with intolerance to standard therapy, the pooled biochemical remission rate for tacrolimus was 56.6% (CI: 43.4-56.6), and 73.5% (CI: 58.1-84.7) for MMF. Among non-responders 59.1% (CI: 48.7-68.8) did respond to tacrolimus, while 40.8% (CI: 32.3-50.0) responded to MMF.
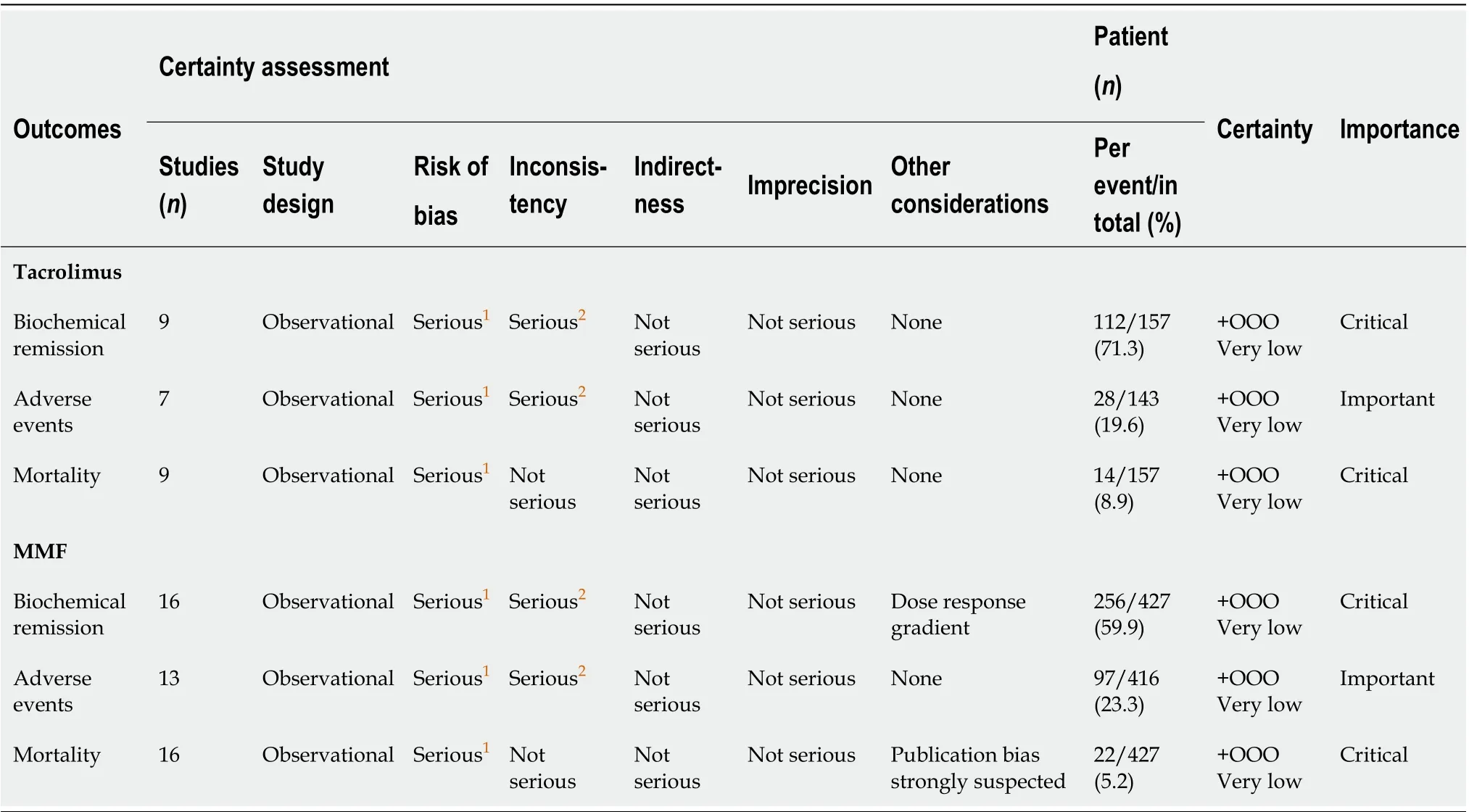
Table 2 Summary of findings and quality assessment of evidence per our outcomes of interest using the Grading of Recommendations Assessment, Development and Evaluation approach
Pooled event rate of adverse events
Frequencies and percentages of reported adverse events were not adequately mentioned in five studies (two studies in tacrolimus[29,33]and three studies in MMF[29,33,39]). Patients given tacrolimus had a number of adverse events, in which neurologic symptoms and gastrointestinal side-effects were the most common; 22 patients had to discontinue the drug due to adverse events. The most common adverse events associated with MMF were gastrointestinal side-effects and leukopenia, which led to 46 patients discontinuing the drug.
The pooled adverse event rate for tacrolimus was 25.5% (95%CI: 12.4-45.3) and for MMF was 24.1% (95%CI: 15.4-35.7) (Supplementary Figures 1 and 5). There was substantial significant heterogeneity among the studies in both intervention groups yield anI2= 66.73% (Phet= 0.006) for tacrolimus and 74.24% (Phet= 0.001) for MMF. Overall quality assessments using GRADE were very low for adverse events in both intervention groups (Table 2).
Egger’s regression test for tacrolimus (intercept = 0.87, 95%CI: -3.35 to 5.09;P= 0.62) and for MMF (intercept = -0.36, 95%CI: -2.93 to 2.22,P= 0.77) did not show statistically significant asymmetry of the funnel plot, suggesting that publication bias was unlikely (Supplementary Figures 2 and 6).
Pooled mortality rate
The studies reported 14 deaths occurring in a total of 157 patients (8.9%) treated with tacrolimus and 22 deaths in 427 patients (5.2%) treated with MMF. The pooled mortality rate was 11.5% (95%CI: 7.1-18.1) in the tacrolimus group and 9% (95%CI: 6.2-12.8) in the MMF group (Supplementary Figures 3 and 7). There was no significant heterogeneity between studies for all-cause mortality in either intervention group, yielding anI2= 0% (Phet= 0.71) for tacrolimus and 12.46% (Phet= 0.31) for MMF. Overall quality assessments using GRADE were very low for mortality in both intervention groups (Table 2).
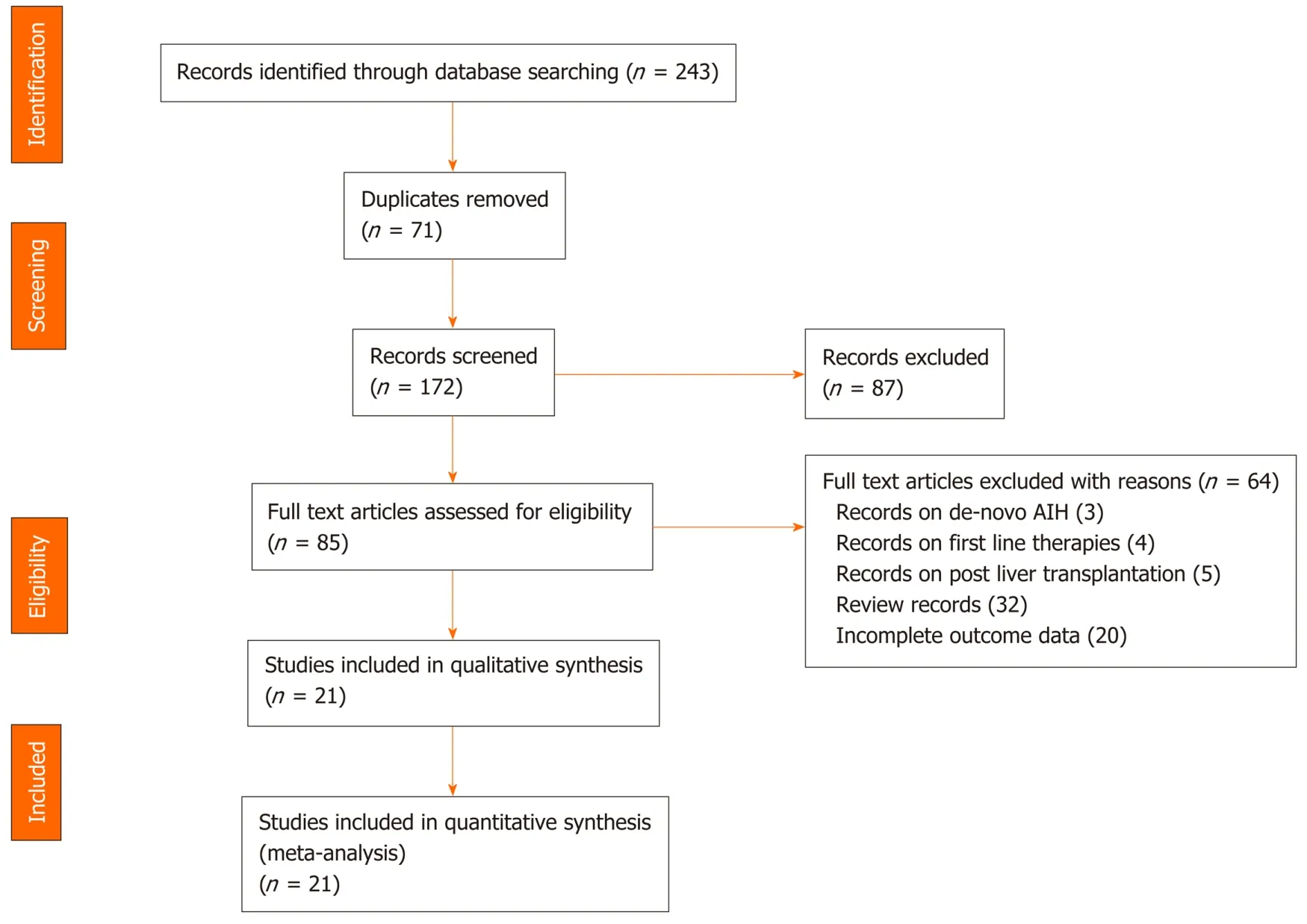
Figure 1 PRISMA flow diagram of the articles retrieved by systematic literature search. AIH: Autoimmune hepatitis.
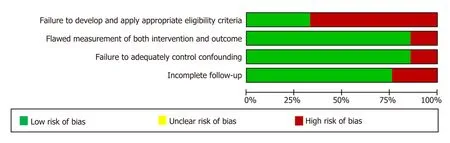
Figure 2 Assessment of methodological quality (risk of bias) of articles identified in the systematic literature search and included in our meta-analysis.
Egger’s regression test for tacrolimus (intercept = -0.35, 95%CI: -1.63 to 0.93,P= 0.54) did not show statistically significant asymmetry of the funnel plot, suggesting that publication bias was unlikely. In the MMF group (intercept = -1.08, 95%CI: -1.86 to -0.30,P= 0.01) there was statistically significant asymmetry of the funnel plot, suggesting there was a publication bias here (Supplementary Figures 4 and 8).
GRADE quality assessment of evidence
The GRADE quality scoring across the studies per outcome is summarized in Table 2. We rated the overall quality of the evidence to be very low for biochemical remission, due to inconsistency (statistically significant heterogeneity) and study limitations (risk of bias in 17 studies); as very low for adverse events due to inconsistency (statistically significant heterogeneity) and study limitations (risk of bias in 14 studies); and as very low for mortality owing to study limitations (risk of bias in 17 studies).
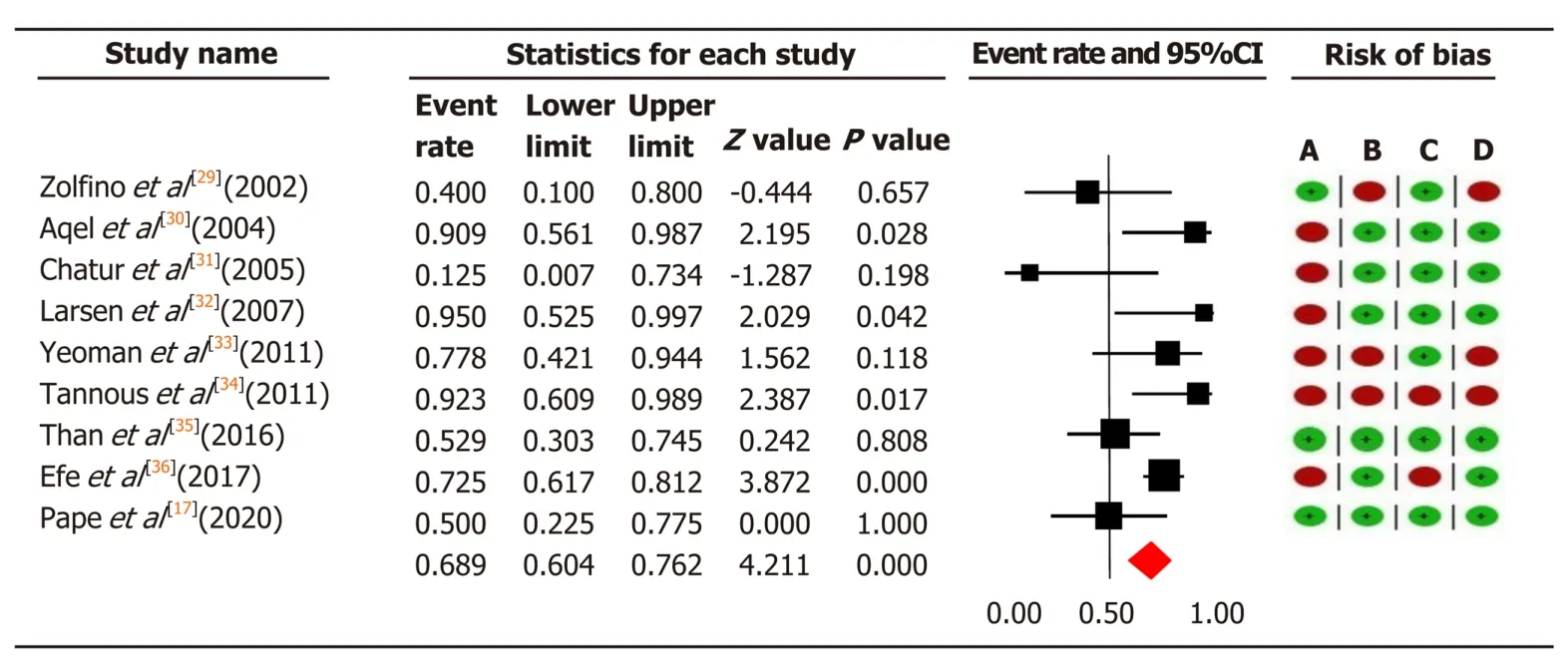
Figure 3 The pooled event rate of biochemical remission in the tacrolimus group with risk of bias assessment per study. Heterogeneity: Q = 16.25, degree of freedom = 8 (P = 0.039); I2 = 50.76%. Test for overall effect: Z = 4.21 (P < 0.0001). A: Failure to develop and apply appropriate eligibility criteria (inclusion of control population); B: Flawed measurement of both exposure and outcome; C: Failure to adequately control confounding; D: Incomplete follow-up. CI: Confidence interval.
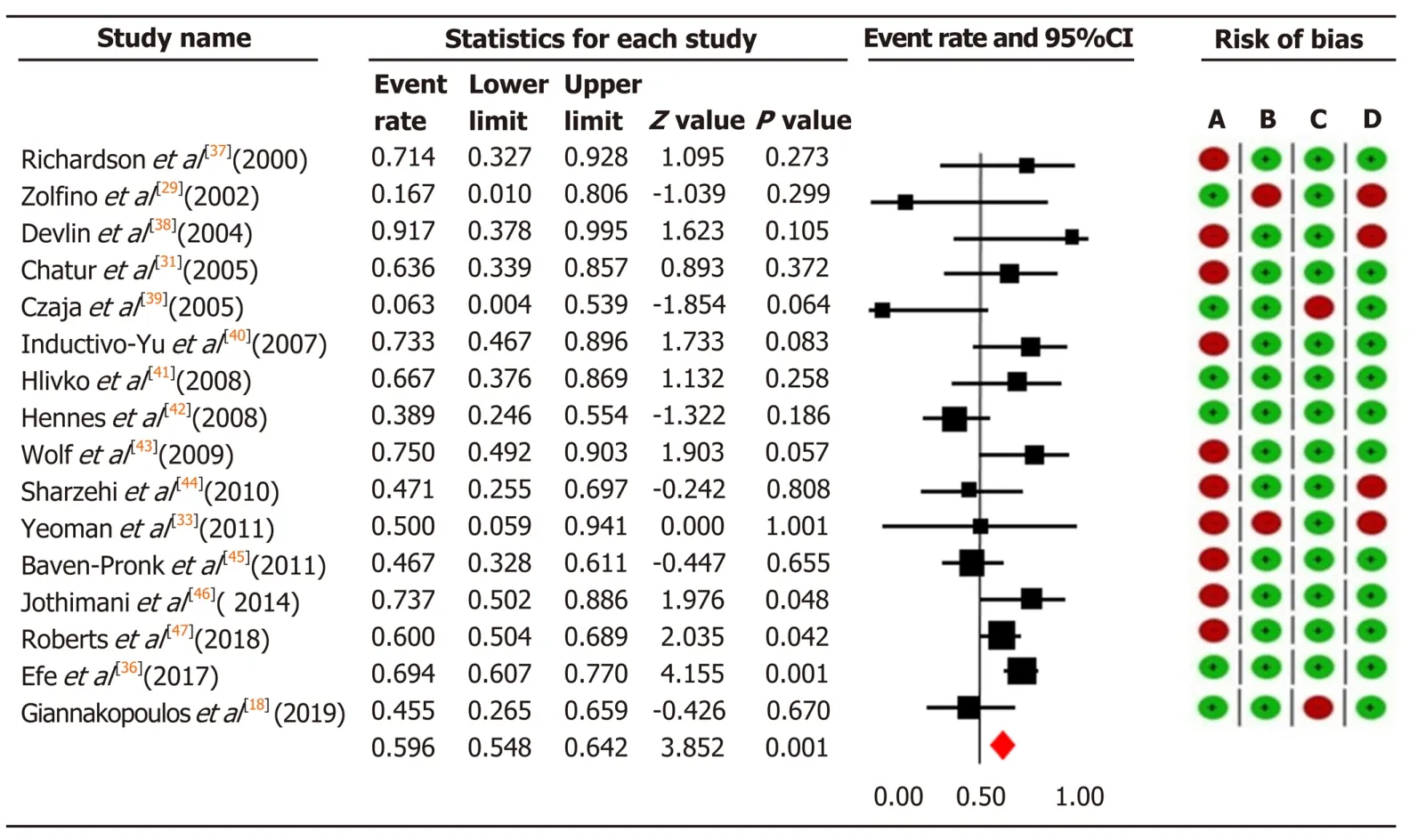
Figure 4 The pooled event rate of biochemical remission in the mycophenolate mofetil group with risk of bias assessment per study. Heterogeneity: Q = 29.72, degree of freedom = 15 (P = 0.013); I2 = 49.52%. Test for overall effect: Z = 3.85 (P = 0 < 0.0001). A: Failure to develop and apply appropriate eligibility criteria (inclusion of control population); B: Flawed measurement of both exposure and outcome; C: Failure to adequately control confounding; D: Incomplete follow-up. CI: Confidence interval.
DISCUSSION
In our comprehensive analysis of 21 observational studies, comprising a total of 584 patients, we first evaluated the efficacy and safety of tacrolimus and MMF as a secondline treatment for patients with AIH. Two of our key findings are that tacrolimus is efficient in treating patients who did not respond to first-line treatments, yielding a biochemical remission rate of 59.1%, while MMF is considered effective for patients who are intolerant to the first-line therapy, yielding a biochemical remission rate of 73.5%.
In our critical assessment of the quality of the evidence using the GRADE approach, and in the absence of RCTs, we graded the quality of the observational studies as poor both for tacrolimus and MMF. Using the current evidence to develop therapeutic guidelines for these two medicines is therefore questionable. The three major strengths of our study are: The comprehensive search performed to trace all the eligible studies, our use of the rigorous methods given by the Cochrane Collaboration for data extraction, analysis and synthesis, and our assessment of risk of bias and the quality of evidence using the GRADE approach.
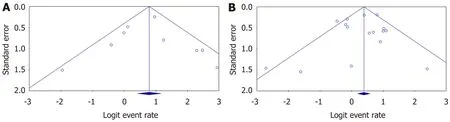
Figure 5 The publication bias of included studies for biochemical remission in (A) tacrolimus and (B) mycophenolate mofetil groups.
One of the important aspects of an unbiased meta-analysis should be the performance of a comprehensive search for published studies. Previous metaanalyses[15,16]had several methodological problems, such as including studies on the treatment of naive AIH patients[15], not including all previously published studies[16], statistical errors such as reporting incorrect heterogeneity, not reporting on publication bias, and incorrect mortality rate[16]. Thus, their estimates on the efficacy of tacrolimus and MMF as second-line treatment modalities for AIH may not be accurate, and their conclusions on the superiority of these drugs may not be truly supported by their results. In the light of these shortcomings, we decided to perform a systematic review and meta-analysis of all the reported studies to date.
Biochemical remission
We found tacrolimus and MMF to be efficient interventions in treating patients who were non-responders or intolerant to first-line treatment. In our meta-analysis, we found a 59.1% biochemical remission rate in patients who were non-responsive to firstline therapy. The results from previous studies varied widely: Some found tacrolimus to be completely effective on biochemical remission[30,32,34], whereas others reported low remission rates[29,31]. In three studies, tacrolimus had a biochemical remission rate of more than 90% in refractory AIH patients[30,32,34], while another study reported a remission rate of 77% in refractory AIH patients[33]. More recently, Thanet al[35]reported that 53% of refractory AIH patients responded to tacrolimus.
Similarly, previous meta-analysis has focused on the improvement of aminotransferases rather than biochemical remission, and reported an average rate of improvement of 78.7%[15]. Our study suggests tacrolimus can improve biochemical remission in 68.9% of patients with refractory AIH who responded to tacrolimus as second-line therapy.
However, current data concerning the efficacy of MMF in patients intolerant to firstline therapy are inconclusive. We found a 73.5% biochemical remission rate for MMF taken by patients intolerant to first-line treatment. Our findings agree with those of previous studies[38,44,45]that state that biochemical remission is significantly more common in intolerant AIH patients compared to those who were non-responsive. Some studies found MMF to be effective in intolerant patients, whereas other studies reported biochemical remission rates of less than 25% in non-responders[39,42,44-46]. Likewise, a recent meta-analysis[16], although it had various limitations, found a pooled remission rate much greater (82%) in patients intolerant to standard therapy compared to non-responders (32%). The difference in MMF biochemical remission rates in patients intolerant to first-line treatment in our study compared to a previous metaanalysis[16](73.5%vs82%) is likely due to their incomplete or selective inclusion of published studies. Given these data, MMF does seem to be a useful alternative therapy for AIH patients who are intolerant to first-line treatment.
Safety and side-effects
Tacrolimus:There are few reports of side-effects of tacrolimus in AIH patients. To date, our study is the first to evaluate the pooled adverse event rate and safety profile of tacrolimus as a second-line treatment for refractory AIH. The pooled adverse event rate for tacrolimus was 25.5% in our study. Overall, neurotoxicity and gastrointestinal issues are the most common side-effects, while diabetes mellitus, nephrotoxicity, pruritus and alopecia may also occur[48]. Previous studies showed that the high serum tacrolimus levels may have led to the rise in creatinine level and subsequent nephrotoxicity. The latest studies suggest using a lower dose of tacrolimus to maintain blood levels below 6 ng/dL, to prevent probable renal complications or significant changes in creatinine level[32]. Monitoring the drug level to maintain a satisfactory dose and prevent nephrotoxicity is a crucial aspect. Thus, physicians must remain cautious when prescribing tacrolimus at a high dosage. Other reasons for withdrawing tacrolimus treatment include hemolytic uremic syndrome, development of squamous cell carcinoma, intense abdominal pain, non-compliance, overlap with primary sclerosing cholangitis and/or primary biliary cirrhosis, and orthotopic liver transplantation. To date, tacrolimus seems to be a reasonable alternative drug for nonresponders, but there is no uniform guideline to explain the dosing schedule and an acceptable safety profile, nor an established monitoring protocol for AIH.
MMF:The use of MMF for AIH is safe in most patients except during pregnancy. It is, however, associated with a large number of side-effects, which vary from mild, to tolerable to toxicity severe enough to necessitate discontinuation of treatment[6]. We found a 24.1% pooled adverse event rate for MMF. Previous meta-analysis estimated a much lower pooled adverse event rate at 14%[16]. Similar to our findings, the most common side-effects of MMF in AIH patients include leukopenia, which can be relieved by reducing the dose, and gastrointestinal issues, in the form of nausea, vomiting and diarrhea[34,37,40-43]. Other, less commonly reported side-effects include severe neutropenia, sepsis, myalgia, pancreatitis, headache, hair loss, and sore gums/sensitive teeth, as well as facial and upper extremity paresthesia[38,40-43]. MMF has major disadvantages in that it is more expensive than AZA and, most importantly, it is teratogenic, which is a major concern in female patients of reproductive age (since AIH affects mainly young females). MMF is contra-indicated during pregnancy, as the United State Food and Drug Administration (FDA) labels it as pregnancy category D. The safety of MMF during pregnancy was not addressed by any of the studies in our meta-analysis.
A note of caution should be added on the retrospective nature of the included studies, which may over- or underestimate the safety profiles of tacrolimus and MMF. Given the long follow-up periods reported and the observed data, both agents appear to be relatively safe alternatives for treating refractory AIH.
Mortality
Despite a good response to first-line treatment, the long-term mortality rate of AIH patients is greater than that of the general population. Untreated AIH can lead to a mortality rate as high as 40% within 6 mo[8,49]. We found a pooled mortality rate of 11.5% for tacrolimus and of 9% for MMF. So far, our study is the first to evaluate the rate for tacrolimus as a second-line therapy in refractory AIH patients. Most of the included studies reported no deaths or only one dead during tacrolimus or MMF therapy. A previous meta-analysis underestimated the pooled mortality rate, which was reported as 7.2% for MMF[15]. Similar to our findings, the largest cohort to date related to second-line treatments in AIH[36]evaluated both tacrolimus and MMF; they reported the highest mortality rate of 11.2% for tacrolimus and 12.4% for MMF. Variation in mortality rates across individual studies may be due to a pre-selection of patients by referral to tertiary centers or to the exclusion of some high-risk patient categories[50,51]. Cohorts showing higher mortality rates have been larger, from multiple centers or from non-tertiary centers, and tended to report on patients who were younger at presentation[36].
Grading of evidence
Rating the quality of evidence by using GRADE is now becoming a recommended step in evidence-based synthesis and it is the most widely adopted tool for grading the quality of evidence and for making recommendations[52]. Previous meta-analyses that addressed the efficacy of tacrolimus and MMF did not include effect estimates for biochemical remission[15]or only provided inconclusive analysis of the effect of MMF as a second-line treatment[16]. Thus, they could not make comprehensive or reliable recommendations. Here we have assessed the evidence using GRADE, which proved to be of very low quality for our primary and secondary outcomes due to the risk of bias and inconsistency. Our analysis therefore offers a starting point for understanding the comprehensive evidence for using tacrolimus and MMF in refractory AIH patients, and our results can provide information for decision-makers, with the ultimate goal of improving clinical outcomes and enhancing patient care.
The natural course of AIH has been clearly outlined and the efficacy of first-line treatment has been well established in naive AIH, although little was known about second-line treatments in refractory AIH. Collectively, our results, along with earlier results, suggest that tacrolimus may be superior to MMF as a therapy in patients not responding to first-line therapy, while MMF is considered a suitable second-line therapy for patients who are intolerant to the standard treatment. However, these results have been based on observational studies. So far, there have been no clinical trials comparing these treatments directly. In the absence of randomized controlled trials, the existing data on the efficacy of tacrolimus and MMF as second-line treatment modalities for AIH patients remain inconclusive; the current evidence has been mainly derived from several retrospective, mostly single-center, case series or reports[17,18,29-47], or based on expert opinion. This means the evidence is of poor quality due to significant levels of bias. Thus, the reproducibility of results may vary considerably across studies. As a consequence, the recently published guidelines[8,49], have led to great differences in the management of refractory AIH patients. The AASLD guideline states that patients with treatment failure should be managed with higher dose firstline therapy before considering second-line treatments[8]. Whereas the European Association for the Study of the Liver guideline suggests using high dose first-line treatment or alternative medications such as MMF, 6-mercaptopurine or 6-tioguanine, despite reconfirmation of diagnosis and adherence[49]. These differences do not provide any insight into which second-line therapy to consider first for these patients, and emphasize the need to conduct standardized, prospective, and preferably randomized studies, with standardized definitions of therapeutic endpoints[53].
In summary, we found most of the evidence was of low-quality. Given the reported side-effects and mortality rates, we do not feel able to offer recommendations for future research on second-line treatment modalities for refractory AIH. More larger, controlled and prospective studies are required to compare alternative drugs with first-line treatments in patients with AIH.
Study limitations
Our study was limited by the low methodological quality and small numbers of patients in the studies covered by our meta-analysis.
CONCLUSION
Tacrolimus might be a promising alternative drug in the efficient treatment of AIH patients who did not respond to first-line treatments (it was effective in 59.1% of those non-responders treated as second-line), while MMF is considered effective in 73.5% of patients who proved to be intolerant to first-line therapy. However, we found that the quality of evidence is not high, and it is thus questionable whether these results should be worked into a clinical guideline. Well-planned, prospective, multicenter studies of second-line treatments for patients with AIH would help to define the optimal dose, treatment schedule, required duration, and treatment endpoints. In addition, such studies should perform close monitoring of the side-effects. Future prospects in AIH treatments, especially in refractory patients, will be establishing individualized approaches to develop more effective and better tolerated novel therapies.
ARTICLE HIGHLIGHTS

Research methods
A systematic review and meta-analysis of the available data were performed. We reviewed the literature, focusing on our aim to identify, appraise, select and synthesize all the high-quality evidence available. We calculated pooled event rates for three outcome measures, defined as biochemical remission, adverse events, and mortality, with their corresponding 95% confidence intervals. Random effects model was applied whenever there was significant heterogeneity between studies. The GRADE approach was used to assess the quality of evidence for primary and secondary outcomes.
Research results
Overall, 21 observational studies, comprising 584 patients with AIH who were unable to tolerate or respond to first-line treatment, met our eligibility criteria. Tacrolimus is efficient in treating patients who did not respond to first-line treatments, yielding a biochemical remission rate of 59.1%, while MMF is considered effective for patients who are intolerant to the first-line therapy, yielding a biochemical remission rate of 73.5%. Moreover, the overall quality assessments using GRADE proved to be very low for all our outcomes in both treatment groups.
Research conclusions
The available evidence shows tacrolimus and MMF are in practice considered effective for AIH patients who are non-responder or intolerant to first-line treatment, but we found no high-quality evidence to support this statement and the translation of these findings to AIH clinical guidelines is questionable.
Research perspectives
Well-planned, prospective, multicenter studies of second-line treatments for patients with AIH would help to define the optimal dose, treatment schedule, required duration, and treatment endpoints. In addition, such studies should perform close monitoring of the side-effects.
ACKNOWLEDGEMENTS
We specially thank Sijtsma K, medical information specialist at the University Medical Center Groningen, for help in designing the literature search strategy. We thank Senior J for editing the text.
 World Journal of Gastroenterology2020年38期
World Journal of Gastroenterology2020年38期
- World Journal of Gastroenterology的其它文章
- Role of artificial intelligence in the diagnosis of oesophageal neoplasia: 2020 an endoscopic odyssey
- Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma
- Comparative study between bowel ultrasound and magnetic resonance enterography among Egyptian inflammatory bowel disease patients
- Monitoring hepatitis C virus treatment rates in an Opioid Treatment Program: A longitudinal study
- Endoscopic ultrasound-measured muscular thickness of the lower esophageal sphincter and long-term prognosis after peroral endoscopic myotomy for achalasia
- Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis
