lntra-procedural arrhythmia during cardiac catheterization: A systematic review of literature
Fatima A Shaik, David J Slotwiner, Gregory M Gustafson, Xuming Dai
Abstract
Key words: Catheterization; Coronary angiography; Percutaneous coronary intervention;Ventricular fibrillation; Ventricular tachycardia
INTRODUCTION
Cardiac catheterization procedures performed in the cardiac catheterization laboratory (CCL) often include right heart catheterization (RHC); left heart catheterization (LHC); and coronary angiography with or without intra-coronary interventions. Cardiac catheterization is one of the most commonly performed procedures in the modern healthcare system. In 2014, there were more than 1 million inpatient diagnostic cardiac catheterizations and 480000 coronary angiography performed in the United States alone[1]. Given the nature of the intracardiac or intracoronary instrumentation as part of the cardiac catheterization procedure,cardiac arrhythmias are common and often unavoidable. We systematically reviewed the published literature to provide a comprehensive overview of the incidence rates,impact on outcomes and potential approaches to minimize the risk of cardiac arrhythmias during cardiac catheterization procedures.
Catheter-induced cardiac arrhythmias during RHC may occur as soon as the catheter tip enters the right atrium, and while advancing through the right atrium,right ventricle, right ventricular outflow tract and the pulmonary artery. Observed arrhythmias include supraventricular arrhythmias [premature atrial contraction,supraventricular tachycardias (SVTs, including atrial fibrillation (AF), atrial flutter)],ventricular arrhythmias, premature ventricular contractions (PVCs), non-sustained or sustained ventricular tachycardia (NSVT or VT) and ventricular fibrillation (VF), as well as various conduction disturbances, such as right bundle branch block (RBBB)and complete heart block (CHB), especially in the setting of pre-existing left bundle branch block (LBBB)[2,3].
LHC studies typically include measuring the left ventricular pressures and performing left ventriculography with catheters crossing the aortic valve and positioned in the left ventricle, in addition to performing coronary artery angiography. Depending on the coronary angiographic findings, a percutaneous coronary intervention (PCI) may subsequently be performed. In addition,intravascular imaging such as intravascular ultrasound (IVUS) or optimal coherence tomography (OCT) may be used to examine coronary artery anatomy. Fractional flow reserve (FFR) may be applied to assess the hemodynamic significance of a coronary artery stenosis. This review summarizes arrhythmic complications of these procedures. Recently developed structural heart interventional procedures,i.e.transcatheter valvular therapies for valvular disease, often involve rapid pacing and have a potential to cause significant injury to the conduction system. Arrhythmias associated with structural heart interventions are not included in this review.
MATERIALS AND METHODS
We screened the titles and abstracts of studies against predefined terms, using PubMed, EMBASE and Cochrane databases (Table 1). The title and available abstracts of all returned articles were reviewed to identify relevant articles for a full-length review and follow-up of their references. We synthesized the following review according to the procedure and arrhythmia types. Meta-analysis was not performed due to the tremendous heterogeneity in inclusion criteria, equipment used in cardiac catheterization, and the arrhythmia definitions among reported studies.
RESULTS
Cardiac arrhythmic during RHC
RHC may be performed in the CCL, at the bedside of intensive care unit (ICU) or in the operating room. The majority of published studies on arrhythmias during RHC were about RHC procedures performed in the ICU or operating room settings. There have been no head-to-head comparisons about the incidence rates of significant arrhythmias or conduction disturbances during RHC performed in the ICU, operating room and CCL settings. The differences of arrhythmias occurring during RHC using different types or sizes (5 Frenchvs7 French) of balloon tipped catheters was not studied either.
Catheter-induced conduction disturbance during RHC
Right sided conduction disturbances, whether transient or permanent were observed infrequently (less than 1%) during RHC, which rarely resulted in the requirement of permanent pacemakers[4-7]. Damenet al[2]reported 2 catheter-induced RBBB during 1400 RHCs (0.14%). Ranuet al[8]retrospectively reviewed charts of 349 patients who underwent RHC and discovered that only 1 patient developed CHB (0.3%) required the removal of the pulmonary artery catheter and insertion of a temporary transvenous pacing wire for 36 hours until the patient recovered normal conduction.CHB could occur during RHC in patients with pre-existing LBBB[4,9-12]. In the setting of pre-existing LBBB, Damen found 1 out of 16 patients with LBBB experienced transient CHB requiring temporary pacing (6.3%)[2]. Morriset al[10]reported 82 procedures in the ICU setting, during which 7 French balloon-tipped flow directed catheters were used in patients with LBBB, and there was no occurrence of CHB. Based on this, the investigators recommended against routine placement of temporary transvenous pacing wires during RHC in patients with LBBB. The incidence of conduction disturbances during RHC which is performed routinely in the CCL for heart failure,pulmonary hypertension or cardiogenic shock has not been well documented.
Catheter-induced ventricular arrhythmia during RHC
During RHC, both advancing and withdrawing the balloon tipped catheter through the right atrium, right ventricle or pulmonary artery (PA) may cause arrhythmias[2,13,14]. Since the initial report of the improved design of the flexible,balloon-tipped, flow-directed catheter for RHC or a PA catheter placement by Swan and Ganz, it has been universally adopted in clinical practice. In Swanet al[15,16]'s initial experience and some subsequent experiences in the CCL, the risk of ventricular arrhythmia was minimal. However, during RHC or PA catheter placement at the bedside in the ICU or OR settings, there were higher rates of various degrees ofventricular arrhythmias such as singlet PVCs, runs of couplets, consecutive PVCs, VT(non-sustained or sustained) and VF, as high as 85% in some reports (Table 2). All the published reports on ventricular arrhythmias related to RHC were single center studies with either retrospective or prospective designs. There are no uniform definitions in reporting the types of arrhythmias. Table 2 provides the most complete list of published data on the incidence of ventricular arrhythmias during RHC. Most of the observations confirmed that ventricular arrhythmias observed during RHC are generally short-lived and self-limited[17].
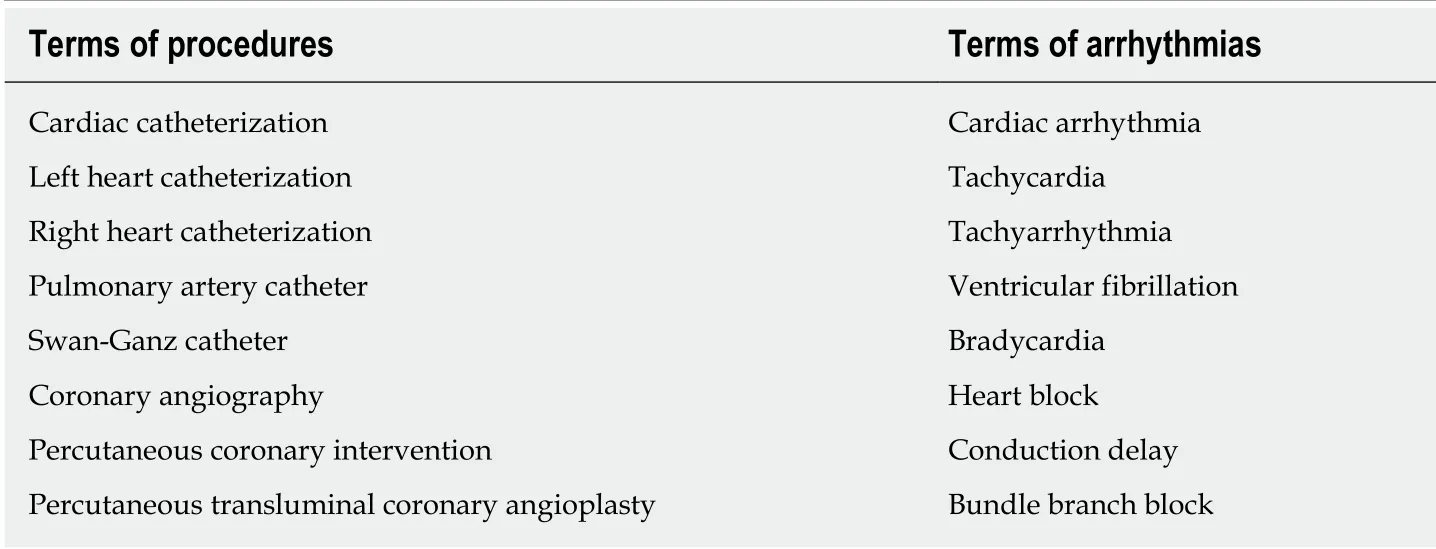
Table 1 Terms describing cardiac catheterization procedures and arrhythmias used in the combination for database search
Severe life-threatening arrhythmias, such as sustained VT and VF can occur during RHC but are very rare[19]. Wennevoldet al[3]reported that only 2 VT and 2 VF episodes occurred during more than four thousand RHC (< 0.1%) performed from 1947 to 1963(before the design of balloon tipped Swan-Ganz catheter). The incidence of sustained VT or VF, requiring anti-arrhythmia treatment either by medication or cardioversion,is relatively low (0.26%[19], 1%[20], 1.5%[21], 4.7%[17,22-28]). Bergmannet al[19]reported that no episodes of VT and 1 episode of VF requiring defibrillation occurred out of 380 RHCs (0.3%) performed for patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. However, Gwaket al[13]reported a 2%incidence rates for VT or VF episodes requiring either withdrawal of the catheter or defibrillation, in their prospective observation of 100 PA catheter placements in the OR for liver allograft transplant recipients.
Cardiac arrhythmias during LHC, coronary angiography and intervention
Ventricular arrhythmias during LHC and coronary interventions:Much attention was paid to the occurrence of malignant ventricular arrhythmias - VF, VT and ventricular arrest/asystole (VA) during the early decades of coronary artery angiography (CAG). There were many reports from experienced single centers as well as multicenter registries detailing ventricular arrhythmias. Table 3 provides a comprehensive list of published reports of incidence rates of malignant ventricular arrhythmias during CAG. Gauet al[29]reported an unusually high incidence rate of VF(12%) in their single center study of 75 cases of selective CAG. Excluding this outlier,the median reported incidence rates of ventricular arrhythmias during diagnostic CAG is 0.9% with a range of 0.1% to 1.7%. Taken together the published data reported total of 163090 cases with 1260 incidences of malignant ventricular arrhythmias that resulted in an accumulated incidence rate of 0.77%. In the period of 1960s, ventricular arrhythmias occurred at the rate of 1.1% in CAG in the reported series (134 incidences in 11747 cases); in the 1970s, the rate was 1.0% (738 events in 73097 cases); in the 1980s, 0.8% (216 events in 26231 cases); and in the 1990s, 0.6% (136 events in 24142 cases). More recently, there were two reports from the same institute in China that included more than 18365 and 27798 diagnostic CAG respectively, using 4 or 5 French catheters. Due to the potential overlap of cases in these two reports, only the later report which included the larger sample size was included in our cumulative calculation. The incidence rate of VF was reported to be 0.1%. The temporal trends show that the incidence rates of malignant ventricular arrhythmias during diagnostic CAG have steadily declined from 1.1% to as low as 0.1% in contemporary practice(Table 3). Figure 1 provides a graphic view of the trend of reported incidence rates of VT/VF.
Percutaneous transluminal coronary angioplasty (PTCA) or percutaneous coronary intervention (PCI) has become the most commonly used approach to revascularize obstructive coronary artery disease both in stable ischemic conditions and acute myocardial infarctions. Ventricular arrhythmias are commonly encountered during PCI. In an early study of 1500 PTCA cases, Dorroset al[30]reported an incidence rate of1.6% of VF and 0.5% of sustained VT required intervention. Subsequent reports of the rate of ventricular arrhythmias from both single center experiences and registries ranged from 0.84% to 4.3%[31-36]. Addalaet al[31]have so far reported the largest single center cohort, with more than 19000 PTCA cases and 164 events of VF (0.84%). Based on the published data (255 events in 23882 PTCA cases), the cumulative incidence rates of VF/VT during PTCA in patients with stable or unstable angina was calculated to be 1.1%. Mehtaet al[35]and Haret al[36]both reported a higher incidence of VF during primary PCI for ST-elevation myocardial infarction (STEMI), 4.3% and 4.1%respectively (Table 3). Available data in the literature suggests that the incidence rates of ventricular arrhythmias during PCI in patients with stable and acute coronary artery disease have been relatively constant in the past two decades of practice. NCDR CathPCI registry®and ACTION registry®did not collect information about intraprocedural arrhythmias during diagnostic CAG and PCI until the newest version of data collection form (version 5) for CathPCI registry®was implemented in July 2018.

Table 2 List of studies reported the incidence rate of ventricular arrhythmia during right heart catheterization

1Malignant definition: Premature ventricular contractions (PVCs) with couples or > 3 consecutive PVCs.2Malignant definition: PVC couples, or ≥ 3 consecutive PVCs with heart rate > 120 bpm).3Malignant definition: ≥ 3 consecutive PVCs with heart rate > 100 bp. RHC: Right heart catheterization; VA: Ventricular arrest (asystole); OR: Operating room; ICU: Intensive care units (including medical ICU, surgical ICU and cardiac ICU); PVC: Premature ventricular contractions; VT: Ventricular tachycardia; PAC: Pulmonary artery catheter; VF: Ventricular fibrillation; CCL: Cardiac catheterization laboratory; cPVCs: Consecutive PVCs; DCCV:Direct current cardioversion; NSVT: Non-sustained VT.
Without timely termination, malignant ventricular arrhythmias could be lifethreatening. Intrinsic build-in telemetry monitoring by trained staff in CCL has proven to be effective. In the reported series, the episodes of VT/VF during diagnostic LHC and CAG left minimal impact on long term outcomes. Gauet al[29]reported that all 9 episodes of VF in their first 75 CAG experiences (12% incidence rate) were successfully defibrillated without impacts on outcomes. Others reported the same successful immediate restoration of normal rhythm from intra-procedural VF/VT episodes without adverse sequelae during the hospitalization[30,31,37-39]. The prognosis of patients with stable coronary artery disease was more governed by the status of CAD and left ventricular dysfunction and other comorbidities, rather than the occurrence of VT/VF during the procedure[40].
Whether ventricular arrhythmias in the setting of acute myocardial infarctions have an impact on outcomes has been a controversial question. Mehtaet al[35]reported that the occurrence of VT/VF during primary PCI did not influence PCI success, inhospital or one-year outcomes, compared to patients who did not have intraprocedural ventricular arrhythmias. However, Haret al[36]recently found that,compared to patients without ventricular arrhythmias, the occurrence of intraprocedural VF/VT requiring cardioversion during primary PCI for STEMI was associated with increased early post-MI mortality (12.0%vs0.5% in-hospital mortality,and 24.1%vs3.6% of 30 days mortality); but not late mortality.
Atrial tachy-arrhythmia in LHC and coronary interventions:Bourassaet al[37]reported a 0.17% atrial tachy-arrhythmia (AF and SVT) in 5250 CAG cases in their single center study. Balloon inflation during PTCA was found to increase P wave dispersions and as well as the maximum duration of P waves[41]. which may result in increased risk of AF. There are no recent studies reporting atrial tachy-arrhythmias during LHC and coronary interventions.
FFR measurement and intravascular imaging related arrhythmias:Parket al[42]reported their first intracoronary adenosine-induced AF during FFR measurement.The patient required hospitalization, and amiodarone administration which led to sinus conversion, and a medical regimen for thromboembolic event prophylaxis.More seriously, there were a total of 7 cases of intracoronary adenosine induced VF during FFR have been reported in the literature[43-45]. Various doses (from 96 mcg to 360 mcg and 480 mcg) of intracoronary adenosine boluses were delivered right before the VF occurred. Shahet al[45]reported the incidence rate of VF during FFR was 0.9% (3 cases in 326 FFR cases). They postulated that the large volume of adenosine/saline solute injection (up to 30 cc/injection) might have contributed to the induction of VF by causing ischemia. By increasing the adenosine concentration and reducing the volume of injection with a similarly high dose of adenosine, Shah reported the avoidance of VF. The overall rate of intracoronary adenosine-induced VT/VF during FFR measurement is unknown. There is no reported case of intravenous adenosine induced VF. Intracoronary papaverine is also used to induce maximum hyperemia in FFR measurement. It was well known that use of intracoronary papaverine during FFR may prolong QT interval and induce polymorphic VT and VF[46-49]. The risk of polymorphic VT (torsade de pointes) and VF has been reported to be around 1.2%-1.3%[48,50].The potential risk of cardiac arrhythmias, especially malignant ventricular arrhythmias associated with intravascular imaging such as IVUS and OCT, has been reported to be 1.1% (5 out of 468 cases). VF rate was reported in a multicenter evaluation of the safety of OCT[51]. However, transient chest pain and electrocardiographic changes (QRS widening/ST segment depression/elevation) have been observed in 47.6% cases[52].

Figure 1 Graphic view of reported incidence rates of ventricular tachycardia / ventricular fibrillation during coronary angiography. Gau et al[29] reported in 1970, an outlier with high incidence of ventricular fibrillation (VF) in their early experience of 75 cases of coronary angiography (CAG). Excluding the outlier, other reported VF/ventricular tachycardia (VT) incidence rates were consistently low with median 0.9%, (range 0.1% to 1.7%). Total reported CAG cases excluding the 75 cases in Gau et al[29] were 163015, and total VF/VT cases 1251, with the incidence rate of 0.8%. VF: Ventricular fibrillation; VT: Ventricular tachycardia.
Brady-arrhythmias and conduction disturbances during LHC and coronary angiography:The risk of conduction disturbances is low during procedures performedviafemoral artery approach and higher when using a radial artery approach. One study reported the incidence of symptomatic sinus bradycardia in patients undergoing trans-radial coronary angiography to be as high as 4.3%[53]. In almost all cases, the heart rate returned to normal with adjustment of catheter or atropine administration without residual consequences[53,54]. The etiology of this phenomenon is unclear. Perhaps catheter stimulation or stretch of subclavian,brachiocephalic arteries or ascending aorta may induce a vasovagal reaction.
DISCUSSION
Arrhythmias during right heart catheterization
Conduction disturbances were recognized during the very early practice of intracardiac catheterization[27]. It was anticipated that advancing catheters through the ventricle, would irritate the right bundle branch and its fascicular branches and might lead to transient or even permanent injuries. Fortunately the incidence of conduction abnormalities as well as ventricular arrhythmias is low and the long term implications are relatively negligible.
Pre-disposing risk factors associated with the increased incidence of ventricular arrhythmias, in particular the risk of VT or VF requiring intervention during RHC,include myocardial infarctions, septic shock[55], pre-existing cardiac conditions[17], use of a guidewire to assist PA catheter advancement[56], prolonged procedural time and presence of valvular diseases[23,55]. Recent studies suggested that positioning the patient in the head-up and right lateral position while passing right heart catheters would allow the catheter to easily enter the right ventricular outflow tract and thereby, reduce the incidence of severe arrhythmias[28,57]. Intravenous lidocaineadministration before the catheter enters the right ventricle for prophylaxis of ventricular arrhythmias was tested, but its use remains controversial[58-60]. Due to the fact that the majority of catheter-induced ventricular arrhythmias are benign, selflimited, and rarely required medical or cardioversion, prophylactic lidocaine is not recommended during RHC.

Table 3 lncidence rate of arrhythmia in coronary angiography and percutaneous coronary interventions
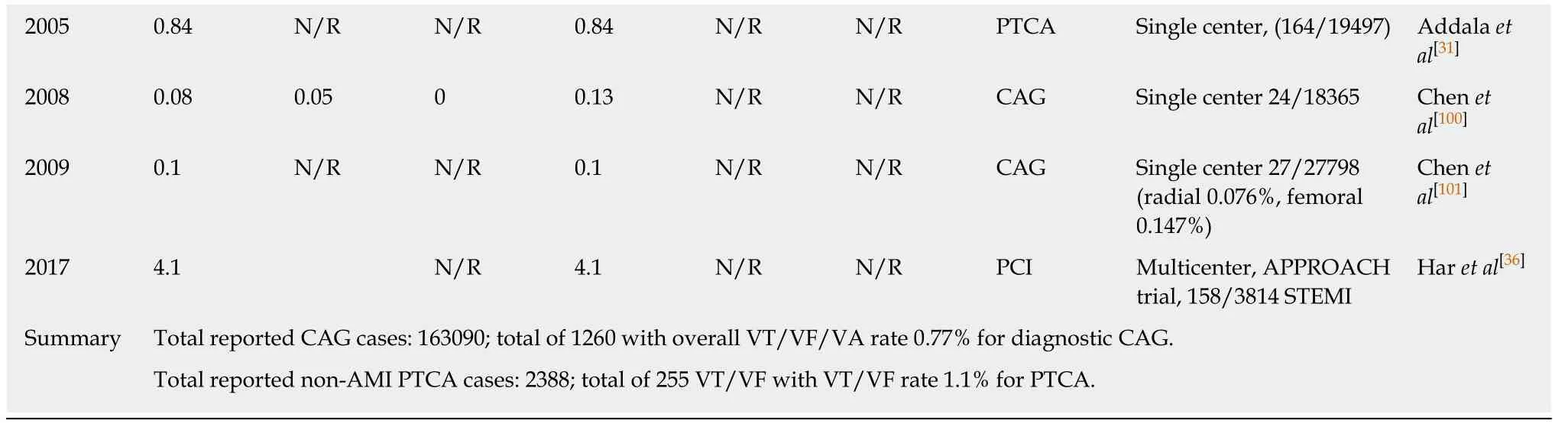
1Ventricular fibrillation and sustained ventricular arrhythmias were reported together. VF: Ventricular fibrillation; VT: Ventricular tachycardia; VA:Ventricular arrest (asystole); CAG: Coronary arteriography or angiography; PTCA: Percutaneous transluminal coronary angioplasty; PCI: Percutaneous coronary intervention; RCT: Randomized controlled trial; STEMI: ST segment elevation myocardial infarction. N/R: Not reported.
Arrhythmias during left heart catheterization
The belief that the selective injection of contrast medium into coronary arteries would result in asymmetrical hypoxia, electrical imbalance, and invariantly ventricular arrhythmias was disproved by the pioneer of coronary arteriography, Dr. Sones Jr[61,62].However, the fear of fatal ventricular arrhythmias related to coronary angiography persists. Due to the proximity of catheters, wires and other equipment to the ventricular walls during LHC, ventricular arrhythmias will unavoidably occur despite the advancement of techniques, reagents and equipment. Direct stimulation with wires and catheters of the ventricular myocardial tissues may disturb local electric activities and introduce myocardial contractions which lead to PVCs in singlets,couples or runs continuously for various lengths. Therefore, advancing equipment into the left ventricular chamber leading to frequent PVCs, non-sustained ventricular tachycardia (NSVT) with cPVCs ≥ 3 beats or ventricular tachycardia (cPVCs ≥ 30 beats) are common, up to 80% in our catheterization laboratory at New York Presbyterian Queens (Shaiket almanuscript in preparation). These ventricular arrhythmias are usually terminated by catheter manipulations (withdrawal,repositioningetc.) without significant impact on hemodynamics. Malignant ventricular arrhythmias, such as sustained VT, VF and ventricular arrest or standstill could occur but are much less common. These malignant ventricular arrhythmias usually cause hemodynamic compromise and require immediate interventions,i.e.chest wall compression, cardioversion and possibly the administration of a pharmacological agent. These malignant ventricular arrhythmias are also the topic of many case reports throughout recent decades. Understanding their potential causes,contributing factors, and approaches to minimize the risk as well as preparing to manage them when they occur are one of the core subjects of training in the interventional cardiology community.
Causes and contributing factors of ventricular arrhythmias during LHC and approaches to minimize the risk
The more than 10 folds decrease in incidence rate of malignant ventricular arrhythmias during CAG and LHC is the result of half a century's clinical and translations research. It is generally accepted that ischemic changes of myocardium,toxicities of contrast medium[63-66], and mechanical stimulations of myocardial tissue by catheter and wires, contribute to the occurrence of ventricular arrhythmias.Individual patients' vulnerability or susceptibilities to ventricular arrhythmias, often influenced by electrolyte derangement, pre-existing prolongation of QT interval, small caliber coronary arteries, or the severities and acuities of coronary artery disease, also play important roles. Furthermore, the operators' experience and approach in performing the LHC and coronary intervention also dictate outcomes (Table 4).
Atrial tachy-arrhythmia in LHC and coronary interventions
Contrary to RHC, LHC is performedviaa retrograde approach. There is no direct contact of instruments with atrial structures. Direct stimulation of the atrium causing arrhythmia is rare during LHC and coronary interventions. Thus, the findings of ourliterature review are not surprising.
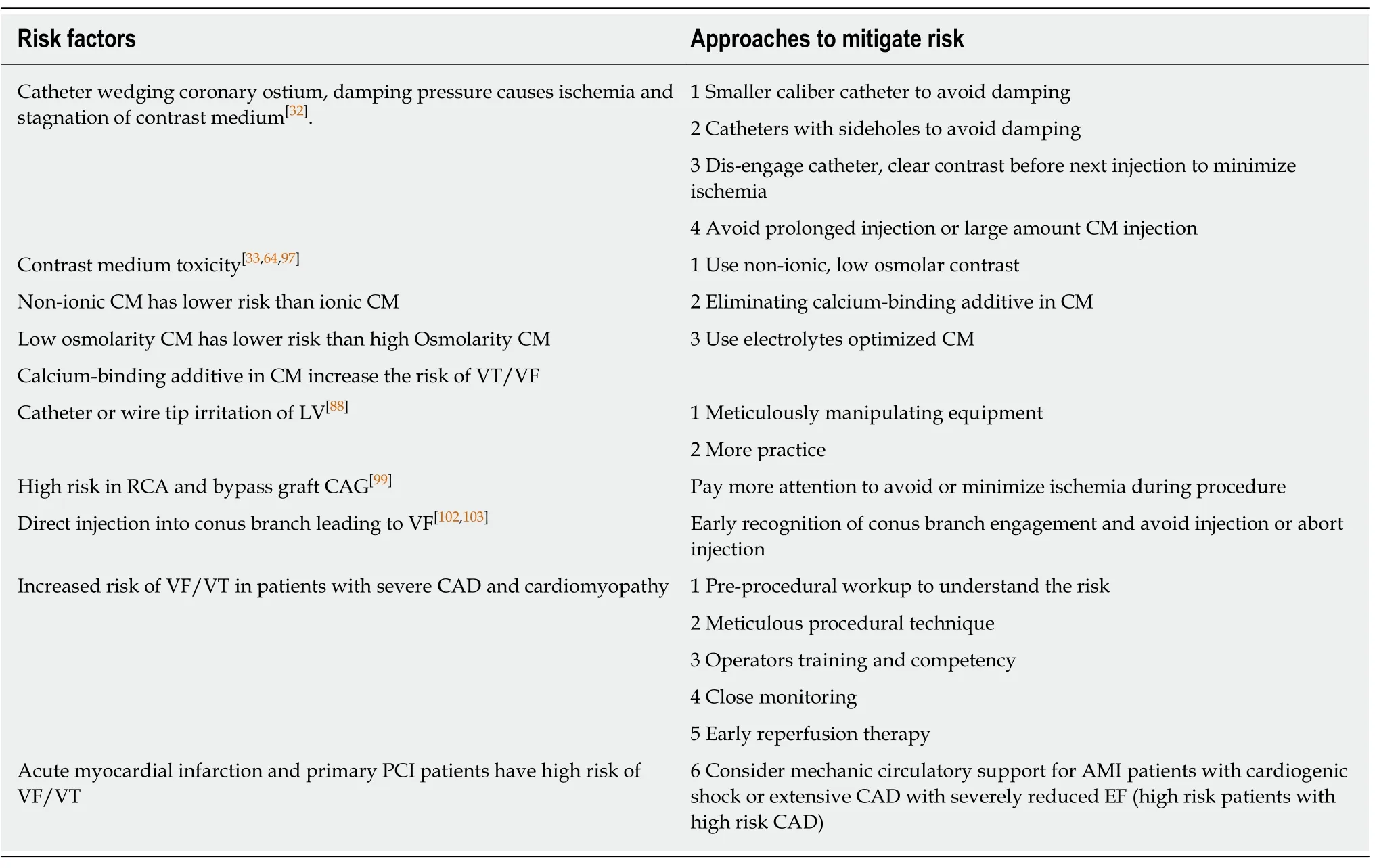
Table 4 Known risk factors for ventricular tachycardia / ventricular fibrillation during coronary angiography and percutaneous coronary intervention and approaches to mitigate the risk
FFR measurement and intravascular imaging related arrhythmias during LHC and coronary interventions
In symptomatic patients with moderate coronary artery stenosis, guidelines supported by robust clinical evidence recommend the use of FFR to guide the clinical decision making process[67]. The risk of arrhythmias during FFR measurement involves instrumentation of the coronary arteries with guidewires, catheters and contrast medium, as well as the pro-arrhythmic effects[68]of adenosine, which is the most commonly used agent to induce maximum hyperemia[69]. Intravenous infusion of adenosine at 140 mcg/kg/min or intracoronary bolus injection at the doses of 60 mcg, 120 mcg, up to 480mcg, are generally safe and well tolerated, largely owing to its very short half-life. Adenosine induced transient sinus bradycardia, AV block, and sinus tachycardia are common and expected physiologic effects on the heart rhythm.Adenosine-induced arrhythmias and conduction disturbance are short-lived and selflimited without the need of special treatment. The current gold standard for FFR studies is to use intravenous adenosine to induce hyperemia, especially when taking into consideration the reported risk of severe ventricular arrhythmias which using intracoronary adenosine and papaverine. However, head-to-head safety data is not available.
Because OCT involves high volume contrast injections to disperse blood components during image acquisition the incidence of ventricular arrhythmias is higher. This may cause chest pain, electrocardiographic changes and even ventricular arrhythmias - all three of which have been reported in the literature. There are no particular concerns regarding IVUS studies causing ventricular arrhythmias.
Brady-arrhythmias and conduction disturbances during LHC and coronary angiography
Brady-arrhythmias have been recognized since the very early experiences and are relatively common during LHC and coronary angiography[70].Direct toxicity of contrast medium and stimulation of chemoreceptors, other vasovagal reactions induced by pain and anxiety,etc. were the proposed mechanisms of these arrhythmias[70-72].Lately, with the growing popularities of trans-radial catheterization,coiling of the catheters and direct stimulation of the aortic arch and carotid sinus receptors was also noted to cause sinus bradycardia[54].
An infrequent yet significant conduction disturbance associated with LHC and CAG is LBBB and/or CHB. As opposed to the right bundle, the trunk of the left bundle is generally short and immediately divides into two fascicles. The left bundle branch is also broadly distributed over the left septal surface in a diffuse fanlike structure. To some extent, these anatomic features of the left bundle protect it from mechanical damage during catheter instrumentation of the left ventricle. Some patients, however, may have anatomic variations, which include a left bundle that extends undivided for 20 mm or more, making the left bundle vulnerable. Shimamotoet al[73]reported 3 patients, without any known conduction abnormalities or evidence of infarction prior to LHC, who developed LBBB, without a change in heart rate during coronary angiography. Of these patients, only one eventually developed a permanent LBBB and none had significant complications. The recognition of the possibility of developing LBBB is particularly important when patients have preexisting right bundle branch and/or fascicular blocks, which could potentially require permanent pacemaker implantation if persistent CHB occurs[74-76]. Furthermore, the His bundle travels through the membranous septum in immediate proximity to the posterior sinus of Valsalva and runs just under the left ventricular endocardium. It is thus, anatomically vulnerable to mechanical trauma during LHC and CAG. A single touch of these structures by the catheter tip may cause intra-His bundle injury resulting in CHB[75,77-80].
Understanding the risk factors for development of brady-arrhythmias and conduction disturbances during LHC and CAG helps the operator to be prepared should these arrhythmias occur and compromise hemodynamics, which will require either administration of atropine and other drugs, and/or emergent transvenous pacing. However, given the low incidence as well as relatively rapid recovery in most of the cases, prophylactic temporary transvenous pacing as performed earlier in practice[79]is no longer recommended. In recent years, there has been a growing interest in using coronary catheters and guidewires for left ventricular pacing in order to reduce resource utilization and avoid the risks of transvenous wire placement[81-84].
In conclusion, diagnostic RHC, LHC, CAG, and coronary interventions are the most commonly performed invasive cardiac procedures. This systematic literature review demonstrated a 0.14%-0.3% incidence of transient RBBB during RHC in normal individuals, with a significantly higher risk of CHB (up to 6.3%) requiring temporary or permanent pacing for individuals with pre-existing LBBB. Isolated PVCs or nonsustained VT which do not require specific treatment are common (approximately 20% incidence rate in most of the reports) during RHC. Potentially life-threatening ventricular arrhythmias (sustained VT and/or VF) requiring either withdrawal of catheter or cardioversion also occur but at much lower rates (1%-1.3%). The incidence rate of diagnostic LHC and CAG causing arrhythmias has reduced 10 fold in the last half century from 1.1% to 0.1% (in modern era) due to an improved procedural techniques, better training, improved contrast medium, and equipment. Coronary interventions as well as hemodynamic assessment with FFR and intracoronary imaging (especially OCT) continue to carry an increased risk of introducing malignant arrhythmias with up to 1% incidence rate of VF requiring shocks. Rigorous and constant monitoring, and readiness to intervene are essential for the modern cardiac catheterization facility.
ARTICLE HIGHLIGHTS
Research background
Cardiac Catheterization is one of the most commonly performed procedures in the modern health care system. Given the nature of intracardiac and intracoronary manipulation of catheters during the procedure, arrhythmias are common, and potentially consequential. Understanding the incidence, risk factors and strategies to mitigate the risk bears clinical significance.
Research motivation
There are sporadic reports on the topics of intra-procedural arrhythmias during cardiac catheterization. We systematically reviewed published literature, analyzed the incidence rate,temporary trends, and predictors of atrial and ventricular arrhythmias during left and right heart cardiac catheterization. We also discussed factors and approaches to reduce arrhythmias and improve the safety of the procedures.
Research objectives
The goal of this study is to provide a comprehensive overview of the incidence rates and impact on short- and long-term outcomes of arrhythmias during cardiac catheterization, as well as understand approaches to minimize the risk of malignant arrhythmias during cardiac catheterization.
Research methods
We systematically searched PubMed, EMBASE and Cochrane databases with a combination of comprehensive terms related to cardiac catheterization procedures and various cardiac arrhythmias, then carefully reviewed and synthesized the data by types of procedure and arrhythmias.
Research results
We found a 0.14-0.3% incidence of transient right bundle branch block during right heart catheterization (RHC) in normal individuals, and a significantly higher risk of complete heart block (up to 6.3%) requiring temporary or permanent pacing for individuals with pre-existing left bundle branch block (LBBB). Isolated premature ventricular contraction or non-sustained ventricular tachycardia (VT) which do not require specific treatment are common(approximately 20% incidence rate) during RHC. Potentially life-threatening ventricular arrhythmias (sustained VT and/or ventricular fibrillation) requiring either withdrawal of catheter or cardioversion also occur but at lower rates (1.0%-1.3%). The incidence rate of diagnostic left heart catheterization and coronary angiography causing arrhythmias has significantly reduced from 1.1% to 0.1% in the last half century. However, invasive coronary intervention and hemodynamic assessment including optical computed tomography and fractional flow reserve continue to possess a significantly higher risk.
Research conclusions
Cardiac arrhythmias are common during cardiac catheterization. While the majority of arrhythmias are benign and self-limited, complete heart block in the presence of pre-existing LBBB and ventricular tachycardia during RHC could be consequential requiring interventions.As the improvement of reagents, equipment and techniques, the incidence rate of serious arrhythmias such as ventricular tachycardia/fibrillation during LHC has significantly decreased,but it continues to require constant intra-procedural monitoring and readiness to intervene.
Research perspectives
As cardiac catheterization procedure continues to serve as essential diagnostic and therapeutic tool for patients, intra-procedural cardiac arrhythmias occur at relatively low incidence rates.Understanding the types of arrhythmias, associated risk factors and the strategies to monitor and mitigate the risk continue to be essential for patient safety and procedure success. It continues to require close surveillance and exploration of best practice to minimize the risk.
ACKNOWLEDGEMENTS
The authors thank the staff in the cardiac catheterization laboratory of New York Presbyterian Queens hospital for their supports for the relevant research on cardiac arrhythmia during cardiac catheterization. We also wanted to thank the reviewers and editors for the constructive comments which helped improve the manuscript to current form.
 World Journal of Cardiology2020年6期
World Journal of Cardiology2020年6期
- World Journal of Cardiology的其它文章
- Autonomic neurocardiogenic syndrome is stonewalled by the universal definition of myocardial infarction
- Cardiovascular magnetic resonance in myocardial infarction with non-obstructive coronary arteries patients: A review
- Diagnostic and treatment utility of echocardiography in the management of the cardiac patient
- Tale of fat and fib — cardiac lipoma managed with radiofrequency ablation: A case report
- Exercise-induced torsades de pointes as an unusual presentation of cardiac sarcoidosis: A case report and review of literature
