Cardiovascular magnetic resonance in myocardial infarction with non-obstructive coronary arteries patients: A review
Marco Gatti, Andrea Carisio, Tommaso D'Angelo, Fatemeh Darvizeh, Serena Dell'Aversana, Davide Tore,Maurizio Centonze, Riccardo Faletti
Abstract
Key words: Cardiovascular magnetic resonance; Acute coronary syndrome unobstructed coronaries; Acute myocardial infarction; Acute myocarditis; Takotsubo cardiomyopathy
INTRODUCTION
The diagnosis of myocardial infarction with non-obstructive coronary arteries(MINOCA) necessitates documentation of an acute myocardial infarction (AMI)according to the universal definition, non-obstructive coronary arteries, using invasive coronary angiography (ICA) or coronary computed tomography angiography (CCTA) and no clinically overt cause for AMI[1]. Possible causes of MINOCA include ischemic diseases such as coronary plaque - with less than 50%stenosis-rupture or erosion, coronary embolism, coronary dissection and microvascular coronary spasm or myocardial disorders such as myocarditis,Takotsubo (TS) and other cardiomyopathies, and finally non-myocardial disorders such as pulmonary embolism[2]. Historically patients with MINOCA represent a clinical dilemma with subsequent uncertain clinical management[1,3,4]. Differential diagnosis is crucial to choose the best therapeutic option for ischemic and nonischemic patients[5]. A recent study stated that cardiovascular magnetic resonance(CMR) could identify the cause of MINOCA in 74% of cases[3]. Therefore, since CMR is able to analyze cardiac structure and function simultaneously and provides tissue characterization, it “should be a mandatory test[6]” to evaluate the patients, providing valuable information for clinical decision making.
Moreover, a study[7]showed that the CMR allows stratification of patients with worse outcomes which resulted in therapeutic changes in almost half of them. In particular, a CMR confirmation or exclusion of myocardial infarction (MI) allows tailored medical therapy, including secondary prevention and avoiding the use of antiaggregant therapy and the subsequent bleeding risks[3]. Furthermore, CMR can promptly identify many underlying conditions responsible for MINOCA, such as acute or chronic myocarditis, TS and other cardiomyopathies. A summary of the causes of MINOCA that can be identified by CMR is shown in Figure 1. In this review is discussed the features of CMR in MINOCA, from exam protocols to imaging findings.
CMR STUDY PROTOCOL
In MINOCA patients the CMR study should be performed within 7 d from symptom onset in order to prevent false negative results or underestimation of the disease extent[8]. It should also be underlined that the examination should not be performed too early, but at least 24 h after disease onset, to avoid too early or overt signs of pathology. Furthermore, in case of negative CMR but with clinical evidence of myocardial involvement, it may be useful to repeat the test between 1 and 2 wk after the initial study to make the correct diagnosis[9].
A CMR study protocol to evaluate MINOCA patients should include evaluation of cardiac structure and function with cine imaging, presence and pattern of myocardial edema with T2-weighted short-tau inversion recovery (T2w-STIR) image and presence and pattern of myocardial injury with late-gadolinium enhancement (LGE)imaging. Moreover, the use of new semiquantitative tissue characterization techniques, T1 and extracellular volume (ECV) and T2 mapping are recommended,due to their excellent sensitivity, specificity and diagnostic accuracy in detection of myocardial damage[10].

Figure 1 Cardiovascular magnetic resonance findings in patients with myocardial infarction with non-obstructive coronary arteries. Adapted from Pasupathy S et al[13]. CMR: Cardiovascular magnetic resonance; MINOCA: Myocardial infarction with non-obstructive coronary arteries.
AMI
The AMI criteria for MINOCA are defined by the “Fourth Universal Definition of Myocardial Infarction”: (1) Clinical signs of ischemia; (2) Abnormal cardiac troponin value; and (3) at least one of: Symptoms of cardiac ischemia, electrocardiogram (ECG)changes (ischemic or development of Q-waves), imaging evidence of and ischemic pattern or identification of coronary thrombus by ICA or autopsy[11]. The diagnosis of MINOCA requires non obstructive coronary arteries on ICA or CCTA (no stenosis ≥50%)[12]. MINOCA patient characteristics differ from those of other AMI and coronaropathic patients because they are younger, more often female with fewer traditional cardiovascular risk factors[13].
In the absence of relevant coronary arteries disease, myocardial ischemia may be triggered by a disorder of epicardial arteries and/or malfunction in the microcirculation. Many atherosclerotic plaques show positive-outward remodeling with high risk features such as a lipid-core and a thin fibrous-cap. In this plaque, the intermittent and partial thrombosis produces distal embolization, with potential superimposed vasospasm, which may be responsible for MINOCA. Sometimes these alterations are not visible on ICA, in this sense, the use of techniques such as intravascular ultrasound (IVUS) that allow direct visualization of the vessel wall can play a key role in evaluation of the lesion[14]. In addition, an assessment with IVUS helps to predict future events based on the evaluation of plaque characteristics.Thrombosis may be a trigger to AMI in plaque disruption, coronary artery spasm, or in the absence of these conditions may be the cause of MI. Hereditary or acquired thrombotic disorders can give coronary thrombosis. Spasm of the coronary arteries may theoretically lead to AMI pathogenesis. It represents hyper-reactivity of the vascular smooth muscle to endogenous vasospastic agents, but can also happen in the presence of exogenous vasospastic agents (e.g., cocaine or methamphetamines). In accordance with this, a recent study reported a rate of positive provocative tests in 46% of patients with MINOCA[15]. Positive testing was associated with an increased rate of death from any cause and cardiac death during follow up[15]. Calcium channel blockers and nitrates can be used to treat patients with vasospastic angina.
Spontaneous coronary artery dissection is a rare cause of AMI that is characterized by non-traumatic and non-iatrogenic displacement of the coronary arterial wall with the development of a false lumen filled with intramural hematoma. Dissection may not always be evident on ICA, resulting in the diagnosis of MINOCA and coronary artery intramural hematomas accounting for 25% of MI in women under the age of 50[16]. It is still unclear why coronary dissection occurred. However, fibromuscular dysplasia is present in other vascular beds in most cases when screening is performed:Changes in intima-media composition due to hormones, pregnancy and delivery have also been implicated.
CMR imaging is a key diagnostic tool to be employed in MINOCA patients with suspected AMI (Figure 2). Imaging for contractile function is important to localize the area of injury and to evaluate the biventricular function and volume. Myocardial edema is evaluated on T2w-STIR images and LGE allows for myocardial damage localization and gives insight into mechanisms. An area of LGE in the subendocardium (or a transural extension) indicates an ischemic cause of injury, but it does not specify the particular mechanism of ischemia, while a non-ischemic appearance of LGE (mesocardial or subepicardial localization) speaks in favor of other myocardial disorders such as myocarditis and other cardiomyopathies. Dastidaret al[3]reported that CMR, as a noninvasive imaging technique, can diagnose 3 out of 4 patients with MINOCA presentation; the strongest predictors of mortality are CMR diagnosis of cardiomyopathy and presence of ECG changes with ST-segment elevation.
ACUTE MYOCARDITIS
The term acute myocarditis refers to an inflammatory condition. Inflammation of the myocardium may occur as a result of exposure to external antigens such as viruses,bacteria, protozoa, drugs, toxins or as an autoimmune condition. While historically enteroviruses and coxsackievirus B were the most common identified pathogens,parvovirus B19 and human herpesvirus 6 are currently the most frequent myocarditis related infections[17]. CMR is indicated for patients with new onset or persisting symptoms suggestive of myocarditis (dyspnea, orthopnea, palpitations, chest pain,effort intolerance), in case of recent or ongoing myocardial injury (ventricular disfunction, ECG abnormalities, increase in troponin) or if viral etiology is suspected with no evidence of coronary stenosis on CCTA or ICA[9].
The clinical spectrum of myocarditis is wide and can be divided in three distinctive patterns[18]: (1) Infarct-like pattern, characterized by chest pain, fever, ST segment elevation on ECG and troponin rise; (2) Cardiomyopathic pattern with symptoms of left ventricle (LV) disfunction and heart failure (New York Heart Association class III or IV) without ECG, serologic or other systemic abnormalities; and (3) Arrhythmic pattern presenting with sudden ventricular arrhythmia without evidence of systemic infection/inflammation.
According to European Society of Cardiology Working group statement, the gold standard for myocarditis diagnosis is endomyocardial biopsy (EMB) after the exclusion of coronary heart disease[19]. However, latest guidelines on diagnosis and treatment of acute and chronic heart failure recommend CMR as a Class I procedure for the identification of myocarditis in patients with heart failure[20]and EMB remains recommended in patients with elevated troponin values and deterioration of cardiac impairment after maximal medical therapy. The use of CMR (Figure 3) prior to EMB could reduce false negatives due to incorrect sampling; furthermore CMR can provide information related to prognosis in patients affected by acute myocarditis and is an essential tool to rule out many possible conditions in case of MINOCA[1,2,16,21].Moreover, it must be underlined, as recently reported[8]that the typical feature of a patients with a positive CMR for infarct-like myocarditis is a young male with chest pain, ECG alteration and remarkable myocardial necrosis enzyme surge, and in such cases the biopsy may not even be performed.
A recent metanalysis reported a prevalence of myocarditis up to 33% in MINOCA patients[13]. In case of MINOCA it is therefore mandatory to perform CMR and it has been reported that its systematic use in those patients led to a significant increase in detection rate of myocarditis[22].
In 2009 an expert consensus formulated the Lake Louise Criteria (LLC) for diagnosis of myocarditis with CMR[9]As reported above, the CMR protocol used in these patients consisted in evaluation of: (1) Presence and pattern of myocardial edema with T2w-STIR images: (a) Patchy areas of high signal intensity; (b)Subepicardial or septal layer of high signal intensity; (c) Transmural high signal intensity (consistent with but not specific for myocardial inflammation); and (d)Global high signal intensity evaluated through the T2-ratio technique; (2) Evaluation of hyperemia and capillary leakage through the evaluation of myocardial early gadolinium enhancement ratio that explore the regional vasodilation as an integral feature of tissue inflammation; and (3) Presence and pattern of necrosis and fibrosis with LGE imaging: (a) Patchy areas of enhancement; (b) Subepicardial or septal layer of enhancement; and (c) Transmural enhancement (consistent with but not specific for myocardial inflammation).
The above-mentioned tissue characterization criteria are evaluated in parallel to the cardiac structure and function with cine imaging for the presence of LV dysfunction with regional or global systolic disfunction and pericardial effusion which were considered supportive criteria, neither necessary nor sufficient for diagnosis. In patients with infarct-like myocarditis the systolic function is often preserved and the presence of segmental kinetics alterations are rare[23]. The use of advanced techniques for the analysis of myocardial deformation such as CMR feature tracking technique has been reporting promising results and could identify even subclinical myocardial dysfunction[24], but requires further confirmation in larger and prospective studies[25]in order to establish its diagnostic and prognostic role.
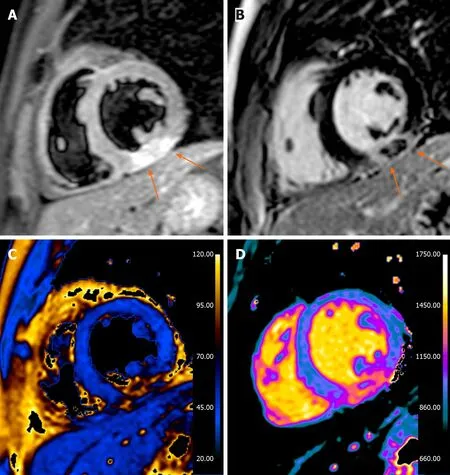
Figure 2 Thirty-three years old male presented with acute chest pain, nonspecific short-tau wave abnormalities, increased troponin values and negative invasive coronary angiography. A: Short axis T2-weighted short-tau inversion recovery image, a transmural hyperintense areas with central hypointensity is seen in the inferior wall (arrows); B: Late gadolinium enhancement image: the hypointense areas correspond with areas of microvascular obstruction and is thought to represent myocardial hemorrhage (arrows); C: T2 map; D: T1 map. The cardiovascular magnetic resonance study is in accordance with acute myocardial infarction with sign of microvascular obstruction and hemorrhage.
According to LLC, CMR findings are consistent with myocarditis if at least 2 out of 3 of the previous criteria are present, with a diagnostic accuracy of 78%[9]. A 2018 meta-analysis evaluating 22 acute myocarditis studies pointed out that using the full LLC criteria resulted in an Area under the curve (AUC) of 0.81 and individual parameter analysis resulted in 0.80 for increased T2 ratio/signal, 0.78 for early gadolinium enhancement and 0.87 for LGE[26].
However, the performance of LLC has also been discovered to be heavily dependent on clinical presentation: CMR sensitivity with classic LLC has been demonstrated to be higher in patients with infarct-like pattern (80%) compared with cardiomyopathic pattern (57%) and arrhythmic pattern (40%)[27].
Recently new semiquantitative tissue characterization technique have been developed. The mapping techniques can derive T1 and T2 relaxation time and allow ECV calculation. These techniques provide a good accuracy equals or better than traditional sequences in diagnosis of acute myocarditis[28], in particular the reported AUC for T1 mapping was 0.95, for T2 mapping was 0.88 and for ECV 0.81.
Based on these consideration, at the end of 2018, Ferreiraet al[29](Table 1) released updated LLC according to which the diagnosis of myocarditis can be made using a“two out of two” approach in presence of myocardial edema evaluation on T2w-STIR images /T2-mapping and non-ischemic myocardial injury assessment on T1-mapping, ECV or LGE. The presence of pericarditis and LV wall motion abnormalities are still present as supportive criteria.

Figure 3 Thirty-seven years old male presented with acute chest pain, increased troponin values and negative invasive coronary angiography. A: A short axis T2-weighted short-tau inversion recovery image, a subepicardial and hyperintense areas is seen in the infero-lateral wall (arrows); B: Late gadolinium enhancement image with evidence of the same alteration (arrows); C: T2 map; D: T1 map. The cardiovascular magnetic resonance study is in keeping with acute myocarditis.
The prognosis of patients with “infarct-like” myocarditis is dubious: Some authors[30]reported an association between the infarct-like pattern and major cardiovascular events, while others[23]stated a positive evolution with a good prognosis. However, the presence of LV dysfunction at baseline and of LGE predicts patients at high risk of adverse events[31]. There are not yet consistent data on the prognostic role of mapping technique, mainly due to recent introduction in clinical practice.
TAKOTSUBO SYNDROME
Takotsubo syndrome affects about 2.5% of patients presenting with troponin-positive acute coronary syndrome (ACS)[32-35]. The term was firstly introduced in 1990 by Satoet al[36]and it derives from the Japanese word for octopus trap, which resembles the shape that LV assumes at the end of systole. During the last years a number of different names have been used in literature including “apical ballooning syndrome”,“broken heart syndrome”, “stress cardiomyopathy”, and “ampulla cardiomyopathy”.
Initially regarded as a benign condition, this syndrome is still a poorly recognized heart disease, with severe complications such as death, and a prognosis that does not differ from ACS[33]. There is a gender-difference in its incidence, and post-menopausal women are the most affected (80%-90%)[33]. Symptoms are similar to acute myocardial infarction (i.e. acute chest pain, dyspnea or syncope), and can be caused by a variety of physical or emotional triggers. Several pathophysiological mechanisms have been hypothesized, including plaque disruption, multivessel spasm, baroreflex abnormalities and catecholamine surge, with proof of evidence confirming this latter to play a key role in the myocardial injury[37].
Different diagnostic criteria have been proposed for TS (Table 2); LV dysfunction associated with wall motion abnormalities not limited to a specific coronary arteryterritory is the most common[38]. As well as echocardiography and left ventriculography, CMR is able to identify LV wall motion abnormalities, and provides a more comprehensive assessment of right ventricle (RV) motility[39,40]. The most common presentation of TS is LV apical akinesia with preserved function of the remaining segments, which causes the typical “apical ballooning” appearance. Midventricular type, basal or inverted type, focal variants, and isolated or concomitant RV akinesia have also been described but with lower frequency[6]. Wall motion abnormalities are reversible and complete recovery of systolic function has been demonstrated at 3 months follow-up[41].
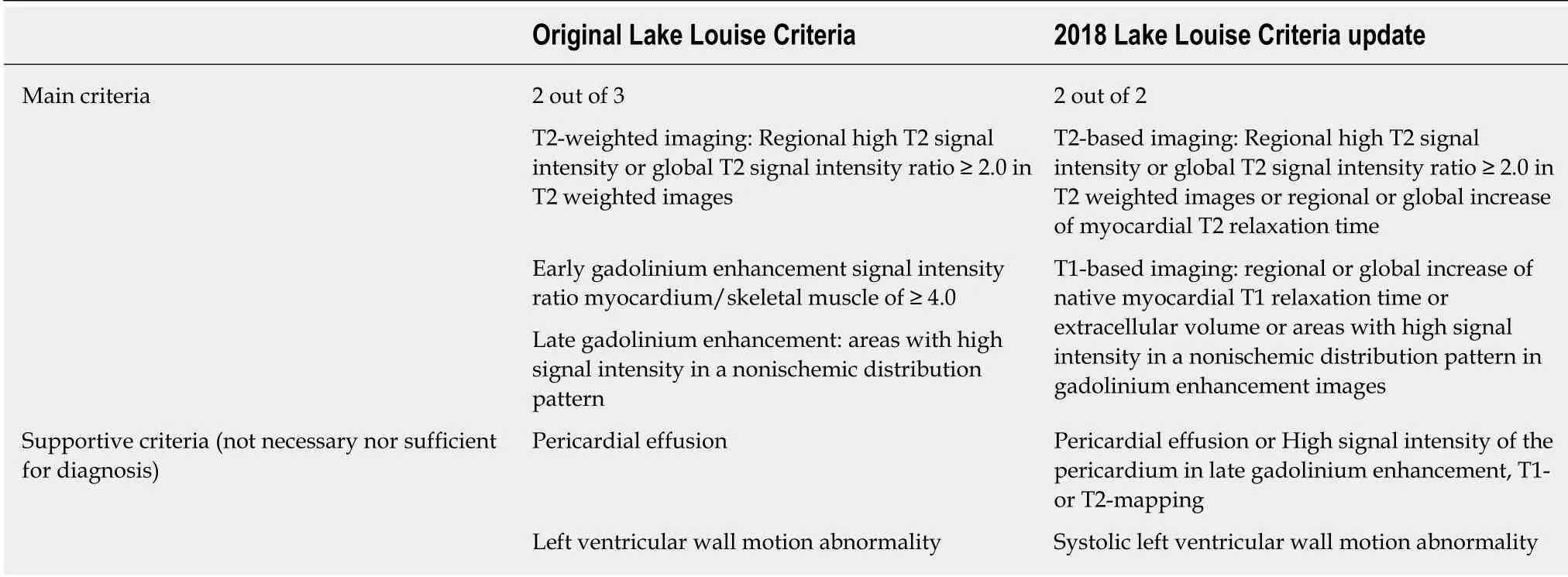
Table 1 Cardiovascular magnetic resonance diagnostic criteria for acute myocarditis
Thanks to its high spatial resolution and three-dimensional image acquisition, CMR(Figure 4) represents a widely established method to non-invasively assess myocardial tissue, within a scanning time of 30 min[42]. Histologically, patients with TS typically present contraction bands, interstitial edema and mononuclear inflammatory response, which differs from the polymorphonuclear and lymphocytic inflammation seen respectively after myocardial infarction and myocarditis[43].
On CMR myocardial edema can be visualized by means of T2w-STIR images or assessed either on T1- or T2-mapping sequences, with increased values in both ballooning and non-ballooning segments[38]. Moreover, T2*-weighted imaging after injection of ultrasmall superparamagnetic particles of iron oxide has shown a diffusely increased myocardial uptake during the acute phase of TS, confirming the pathophysiologic role of tissue-resident myocardial macrophages and keeping with the theory of a catecholamine-mediated myocardial injury in TS[44,45].
Myocardial edema and inflammatory changes usually resolve without any myocardial scarring and with complete functional recovery at 3 months follow-up[41].Usually no LGE is seen in TS patients, despite a few studies have shown subtle enhancement in akinetic segments, likely due to transient myocardial edema and delayed gadolinium washout[46,47]. However, these subtle changes should not be considered as a sign of myocardial necrosis.
In conclusion, wall motion abnormalities with matching myocardial edema distribution, and absent/subtle LGE represent the findings that allows CMR for providing a confident diagnosis of TS in patients with high serum troponin levels,and for differentiating this entity from myocardial infarction and myocarditis[48].
NEGATIVE CMR
Accordingly to a recent systematic review[49], the fourth finding at CMR per frequency(26% of cases) in case of MINOCA is the absence of wall motion abnormalities, edema or LGE. It has been known that the LGE technique has a necrosis detection threshold of about 1 g[50]. Therefore, patients with normal CMR may have either a limited necrosis or a necrosis spread over a wide area of myocardium, such that it is not highlighted. The doubt that in this subtype of patients the necrosis should be actually less extensive also comes from the fact that such patients often have lower peak troponin values at the onset of symptoms[51].
Vasospastic angina, coronary artery disease or coronary embolism may havenormal CMR findings; in these cases, IVUS may help to determine the underlying ischemic cause[52]. Moreover, another cause that must be considered when the CMR is negative is myocarditis; although diagnostic accuracy in patients with infarct-like presentation is the highest among the subtypes of myocarditis presentation, it does not reach 100%[27]. Finally, the possibility of pulmonary thromboembolism should not be ignored.

Table 2 lnternational Takotsubo Diagnostic Criteria (lnterTAK Diagnostic Criteria)[8]
All in all, the number of negative CMR in MINOCA patients could be reduced in the near future with the introduction of new mapping techniques that seem promising and able to increase the sensitivity and specificity[10]and this will be of vital importance to the management of these patients, which is still a dilemma for clinicians.
OTHER CAUSES OF MINOCA
In a small percentage of MINOCA patients the results of CMR is hypertrophic cardiomyopathy (HCM) dilated cardiomyopathy (DCM) and other causes such as pericarditis and amyloidosis.
Hypertrophic cardiomiopathy
Coronary microvascular dysfunction is a common characteristic of HCM, even in the absence of symptoms and several studies indicated that patients with HCM had an impaired coronary flow reserve that may lead to cardiac ischemia. Microvascular disfunction in patients with HCM may be caused by several mechanisms such as structural abnormalities of small vessels, inadequate capillary density, fibrosis,myocyte disarray, and increased LV end-diastolic pressure[53,54].
In an acute setting, HCM patients present with increased ventricular myocardial thickness and could have hyperintense myocardial areas at T2w-STIR images, altered first pass perfusion and/or presence of LGE. The T2 alteration are likely to be associated with edema and may represent acute ischemic damage due to microvascular dysfunction. In addition, the presence of edema has been related to ventricular arrhythmias[55]. Moreover, first pass perfusion defects are related to microvascular disfunction and in association to the presence of LGE (i.e., scar) that seems to be a marker of increased risk of non-sustained ventricular tachycardia episodes as a prognostic factor[56]. Concerning other mechanism that may cause ischemic insults, intramural coronary course has to be mentioned, however the impact of an intramural course of the coronary arteries on the clinical outcome of patients with HCM is unclear[57].
Dilated cardiomiopathy


Figure 4 Thirty eight years old female presented with acute chest pain, increased troponin values and negative coronary angiogram. A and B: Diastolic and systolic 4-chamber cine-SSFP images: there is a minimum “apical ballooning” appearance with a relative left ventricle apical akinesia with preserved function of the remaining segments, which causes the typical “apical ballooning” appearance; C and D: T2-weighted short-tau inversion recovery images with evidence of myocardial edema in apical segments (arrows); E and F: Ate gadolinium enhancement images without presence of areas of increased signal intensity; G-I: T1-map; J-L: T2-map.M-O: T1 native, extracellular volume and T2 mapping bull's eye, there is a marked increase in values in the apical segments (T1 mapping: 1350 ms; extracellular volume: 38%, T2 mapping: 70 ms). The cardiovascular magnetic resonance images are in keeping with Takotsubo syndrome.
Even if traditionally considered a “non ischemic” pathology, many studies have reported abnormalities of myocardial perfusion with moderate or severe adverse remodeling in DCM. A patient with DCM presents a dysfunction, stretched and enlarged ventricle with or without areas of LGE (i.e., fibrosis). The typical pattern of DCM fibrosis is midwall and some research groups highlighted its prognostic value[58,59]. However, the pathophysiologic basis of midwall fibrosis are not completely understood, perfusion and microvascular abnormalities are thought to be implicated[60].
A previous study[61]found that stress and rest myocardial blood flow (MBF) in patients with DCM was decreased in LGE segments relative to others, indicating an association between abnormal perfusion and myocardial fibrosis. Such results indicate that fibrosis segments may exhibit microvascular anomalies, exemplified by the failure to increase MBF under stress, while impaired perfusion may merely represent decreased demand secondary to a reduced number of myocytes. However,if these pathological findings may drive to the development of an acute ischemic pathology, it is still unknown.
Amyloidosis
Amyloidosis is a restrictive cardiomyopathy resulting from deposition of abnormal protein in the cardiac tissue[62]; the typical outcome of these patients is a diastolic dysfunction with heart failure symptoms and arrhythmias. However, some authors[63,64]highlighted a different aspect of cardiac amyloidosis: The presence of small vessel disease (intramural coronary artery), which led to fatal myocardial infarction. From a pathological point of view, a study on transplanted hearts demonstrated that the deposition of amyloid at the coronary artery level is frequent(over 90%), although it usually involves adventitia and vasa vasorum[65]. Therefore,most patients with primary systemic amyloidosis and cardiac involvement have obstructive intramural coronary amyloidosis; this finding is associated with microscopic ischemic changes demonstrating that myocardial ischemia may occur in these patients.
Pericarditis
Pericarditis may mimic myocardial infarction because of clinical presentation (chest pain may be sometimes difficult to be distinguished from ischemic pain, also because of myocardial involvement), cardiac enzyme elevations and ECG alterations. Based on its definition, in pericarditis coronary arteries must be unobstructed but they can be interested by contiguity from the inflammatory process[66]. A patient with acute pericarditis on CMR had thickening of the pericardium associated with effusion. The pericardium is hyperintense on T2w-STIR images and this is associated with LGE[67].
CONCLUSION
This review describes a summary of the main pathologies in which CMR makes it possible to do a differential diagnosis by providing therapeutic and prognostic information, underlining the importance of this imaging technique in MINOCA patients.
 World Journal of Cardiology2020年6期
World Journal of Cardiology2020年6期
- World Journal of Cardiology的其它文章
- Autonomic neurocardiogenic syndrome is stonewalled by the universal definition of myocardial infarction
- Diagnostic and treatment utility of echocardiography in the management of the cardiac patient
- lntra-procedural arrhythmia during cardiac catheterization: A systematic review of literature
- Tale of fat and fib — cardiac lipoma managed with radiofrequency ablation: A case report
- Exercise-induced torsades de pointes as an unusual presentation of cardiac sarcoidosis: A case report and review of literature
