Eff ects of salinity, carbonate alkalinity, and pH on physiological indicators of nutrition transporter for potential habitat restoration of amphipod Eogammarus possjeticus*
XUE Suyan , MAO Yuze , , WANG Jinye , FANG Jianguang ZHAO Fazhen
1 Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture, Shandong Provincial Key Laboratory of Fishery Resources and Eco-environment, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology, Qingdao 266071, China
3 College of Marine Science and Engineering, Qingdao Agriculture University, Qingdao 266109, China
Abstract The eff ects of three environmental factors, salinity, carbonate alkalinity, and pH, on the survival, feeding, and respiratory metabolism of Eogammarus possjeticus (Amphipoda: Gammaridae) were investigated experimentally. The results show that E. possjeticus could tolerate a broad salinity range. The 24-h lowest median lethal salinity was 2.70, and the highest was 47.33. The 24-h median lethal alkalinity and pH were 23.05 mmol/L and 9.91, respectively; both values decreased gradually with time. Diff erent values of salinity, carbonate alkalinity, and pH resulted in signifi cant diff erences in the cumulative mortality ( P <0.05). The ingestion rate and feed absorption effi ciency were signifi cantly aff ected by the coupling of the three environmental factors ( P <0.05). With increases in carbonate alkalinity, salinity, and pH, both ingestion rate and feed absorption effi ciency exhibited a downward trend, indicating a decline in feeding ability under high salinity and more alkaline water conditions. The coupling of salinity, carbonate alkalinity, and pH also had a signifi cant eff ect on respiration and excretion ( P <0.05). The oxygen consumption rate increased fi rst and then decreased with increasing carbonate alkalinity. Under the same carbonate alkalinity values, the oxygen consumption rate increased with increasing salinity. Under the same carbonate alkalinity and salinity, the oxygen consumption rate initially increased and then decreased with increasing pH. The O:N ratio fi rst increased and then decreased with increasing carbonate alkalinity. When carbonate alkalinity was less than 6 mmol/L, the O:N ratio increased with increasing salinity and decreased with increasing pH. The results demonstrate that changes in salinity, carbonate alkalinity, and pH had a measurable impact on the osmotic pressure equilibrium in E. possjeticus and aff ected the energy supply mode (i.e. ratio of metabolic substrate).
Keyword: Eogammarus possjeticus; saline and alkaline water; survival; feeding; respiratory metabolism
1 INTRODUCTION
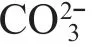
China is rich in saline and alkaline water resources, covering an area of approximately 45.87 million hectares (Lin et al., 2013). The reasons for the abundance of saline and alkaline water resources may be due to climate warming, sea-level rise, drought and precipitation reduction, and human activities (such as agricultural irrigation), etc. (Yao et al., 2010). To date, several aquatic animals, includingFenneropenaeuschinensis,Penaeusvannamei,Exopalaemoncarinicauda, andAcipenserbaeri, have been cultured successfully using saline and alkaline waters (Fang et al., 1999; Zhou et al., 2007; Huang et al., 2010; Jaff er et al., 2020). To prevent intensive aquaculture from causing adverse eff ects, such as disease and environmental pollution, we need to shift the focus toward aquaculture infrastructure that enhances the disease resistance of cultured species and improves the aquatic environment. Specifi cally, live food is added during the culture process to enhance the disease resistance of aquatic animals and improve the environment (Xue et al., 2018).
Amphipods are one of the most diverse and quantitatively dominant groups of organisms in the benthic macrofauna (Cunha et al., 1997; Dauby et al., 2001). Because amphipods are rich in nutrients, such as proteins, fats, and carbohydrates, they are a highquality natural food for aquatic animals, including fi sh and crustaceans (Xue et al., 2013; Baeza-Rojano et al., 2014). Additionally, amphipods play a key role in decomposition and remineralization processes in benthic ecosystems (Sainte-Marie, 1992), and have a potential role in remediating the benthic environment within aquaculture systems (Ananthi et al., 2011). With the gradual development of aquatic animal culture in saline and alkaline waters, the demand for live food in the culturing process has increased accordingly. Therefore, it is necessary to carry out research into the physioecological responses of food organisms to saline and alkaline waters.
In this study,Eogammaruspossjeticus, a predominant species of amphipods in marine aquaculture ponds in Shandong Peninsula (China), was used for survival, feeding, and respiratory metabolism analyses under diff erent salinity, carbonate alkalinity, and pH conditions. The aim of this study was to describe the physiological response of amphipods to saline and alkaline waters. The results provide useful data to describe the mechanisms of salinity and alkalinity tolerance in aquatic animals, and they guide the rational development and utilization of saline and alkaline water resources.
2 MATERIAL AND METHOD
2.1 Collection and cultivation of amphipods
The individuals of amphipodE.possjeticuswere collected from Laizhou Bay (37° 03′ N–37° 10′ N, 119° 29′ E–119° 30′ E) in April 2018 using a suction sampler and shifted through a 2-mm meshed sieve. The animal samples were then transported to the laboratory for three hours in three sealed plastic bags, fi lled with one-third seawater (10 L) and two-thirds of the pure oxygen. Large numbers of healthy and vigorous individuals ofE.possjeticus(the mean values and standard deviations of body size and body weight were 1.1±0.1 cm and 12.1±2.8 mg, respectively) were selected for temporary cultivation under natural seawater conditions in the laboratory. Eight corresponding incubators (60 cm long, 45 cm wide and 40 cm high) fi lled with 60 L of seawater were set up. In total, 1 500–2 000 individuals were added to each incubator. During the period of temporary cultivation, the environmental parameters of seawater were measured by the multi-parameter environmental monitoring system (YSI6600, YSI, USA) every day. The seawater temperature was 10–13°C, the salinity was 29–32 practical salinity units, the pH was 8.0–8.2, and the dissolved oxygen was 6.5–7.1 mg/L. The amphipods were cultured with the photoperiod of 12 h L:12 h D. The seawater was renewed every day and the organisms were fed with fresh macroalgae (Enteromorphasp.) once a day. The fresh macroalgae were collected from the seaside of Fushan Bay (36° 02′N–36° 03′N, 120° 21′E–120° 22′E) in April 2018. The wet weight of the macroalgae was at 150%–200% of the amphipod’s total wet body weight. The amphipods were allowed to acclimatize to the cultivation conditions for one week before the experiments.
2.2 Experimental design and sampling
2.2.1 Cumulative mortality and median lethal dose
Three tests were conducted (salinity, carbonate alkalinity, and pH). The cumulative mortalities under diff erent salinities (1, 5, 10, 15, 20, 25, 30-control, 35, 40, 45, 50, and 55), carbonate alkalinities (3-control, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, 42, and 45 mmol/L) and pH values (8.0-control, 8.5, 9.0, 9.5, 8.0, 10.0, and 10.5) were studied. The seawater pumped from the seacoast of Fushan Bay was shipped to the laboratory and adjusted to the desired salinities by adding crude sea-salt or mixing with aerated tap water. The carbonate alkalinity of seawater was adjusted by adding sodium bicarbonate (Macklin Ltd., Shanghai, China), and the pH values were maintained for the experimental requirement through the use of HCl (0.1 mol/L) and NaOH (0.1 mol/L). During the experiments, the temperature was kept at (16.5±1.5)°C, and other conditions were the same as temporary cultivation.
Experiments were conducted using 2-L plastic tanks fi lled with 1 L of continuously aerated water with the above salinity, carbonate alkalinity, and pH values. The amphipods were randomly selected from temporary incubators and transferred directly to the experimental tanks using a plastic pipette. The mortality rate and the median lethal dose at the time points of 24, 48, 72, and 96 h were calculated. Each test tank had 30 individuals. The control and experimental groups were tested in triplicate.
The calculation of median lethal dose:
Cumulative mortality data were used to estimate median lethal dose (LD50) and their respective confi dence intervals (95%) at diff erent experimental levels, using the improved Trimmed Spearman Karber method (Huang and Xu, 2017). The formula was as follows:
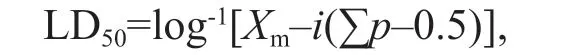
whereiis the group distance, that is, the logarithm diff erence of two adjacent groups of processing conditions;Xmis the logarithm of the maximum processing condition;pis for each treatment group mortality (mortality is expressed in decimal); Σpis for the sum of each treatment group of mortality.
Calculation of 95% confi dence interval of LD50was based on the following equation:
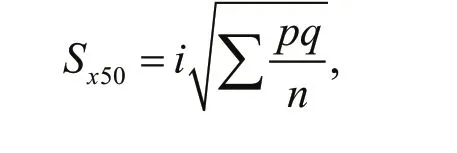
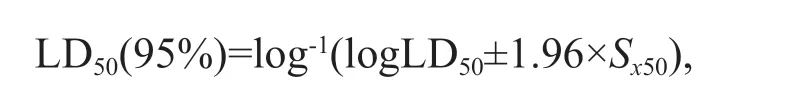
whereSx50is the standard error of logLD50;qis survival rate of all treatment groups,q=1–p;nis the number of amphipods in each group.
In this paper,LSal50,LCa50, andLpH50were respectively used to represent the LD50of salinity, carbonate alkalinity, and pH.
2.2.2 Determination of ingestion, respiration, and excretion
In order to study the coupling response ofE.possjeticuson carbonate alkalinity, pH, and salinity, 36 treatments including four carbonate alkalinities (3-control, 6, 9, and 12 mmol/L), three pH values (8.0-control, 8.5 and 9.0) and three salinities (20, 30-control and 40) were tested to determine the ingestion and respiratory excretion ofE.possjeticus.
The experiment was initiated after 3 days of an adaptation period. Before each feeding, uneaten food was removed and faeces were syphoned out from each tank. Each feeding group was fed with algae (1g wet weight). Faeces that accumulated at the bottom of the tanks after 24 h of feeding were syphoned into large beakers and uneaten algae were carefully removed with forceps. Each test tank had 30 individuals. The control and experimental groups were tested in triplicate. Calculation formula of feeding index was as follows:
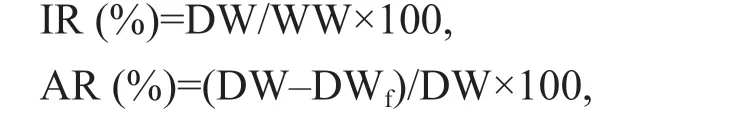
where IR is ingestion rate, DW is dry weight of feed eaten, WW is wet weight of amphipods; AR is absorption rate, DWfis dry weight of faeces.
Respiration was calculated by comparing the dissolved oxygen content of closed 1-L bottles fi lled with seawater and containing 30 individuals to that in a reference bottle with no animals, after 4-h incubation in each test. Ammonia excretion was measured at the end of the respiratory experiments. No food was provided during the respiration and excretion tests. The oxygen consumption rate [OR, mg/(g·h)] and ammonia excretion rate [NR, mg/(g·h)] were calculated based on the changes in the initial and fi nal dissolved oxygen and ammonia nitrogen concentrations. The calculation formula was as follows:
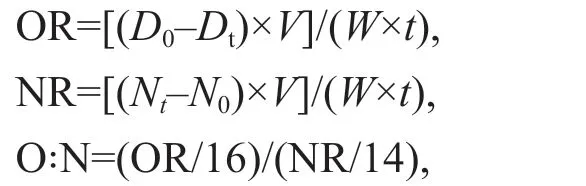
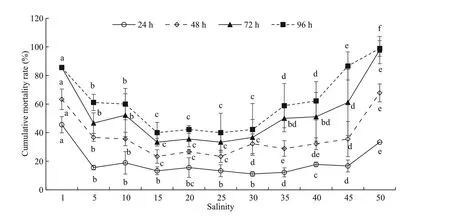
Fig.1 The cumulative mortality rates of E. possjeticus under diff erent salinities
whereD0andDtare the dissolved oxygen content of the reference bottle with no animals and the experimental group respectively,N0andNtare the ammonia nitrogen concentrations in water of the reference bottle with no animals and the experimental group (mg/L).Vis the breathing bottle volume (L),Wis the body weight of the individuals (g fresh weight),tis experiment duration (h) and O:N is the ratio of oxygen consumption rate and ammonia excretion rate.
Dissolved oxygen was determined by a multiparameter handheld monitor (SmarTROLL-MP, In-Situ, USA), and ammonia nitrogen was tested by sodium hypobromate oxidation (General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and Standardization Administration of the People’s Republic of China, 2008).
2.3 Data analyses
Statistical analyses were carried out using the Excel and IBM SPSS statistic software. Obtained data were analyzed by means of one-way ANOVA with repeated measures. When signifi cant main eff ects were observed, Tukey test was used for post hoc analysis. A probability ofP<0.05 was chosen as the signifi cant level in all analyses.
3 RESULT
3.1 Cumulative mortality and median lethal dose
3.1.1 Salinity
The cumulative mortality ofE.possjeticusunder diff erent salinity conditions is shown in Fig.1. Following a sudden rise or fall in salinity, cumulativemortality increased gradually. Relatively high mortality was observed when salinity was <5 or >45. With unchanged salinity, cumulative mortality increased after longer periods of time.LSal50values at various time points and corresponding 95% confi dence intervals were obtained using a modifi ed Karber’s method (Table 1). With elapsed time, there were gradual increases in theLSal50l(24 h=2.70; 48 h=5.75; 72 h=7.59; 96 h=10.67), whileLSal50hdecreased gradually (24 h=47.33; 48 h=41.78; 72 h=35.41; 96 h=23.00). One-way ANOVA revealed a signifi cant eff ect of salinity on the mortality ofE.possjeticus(P<0.05).
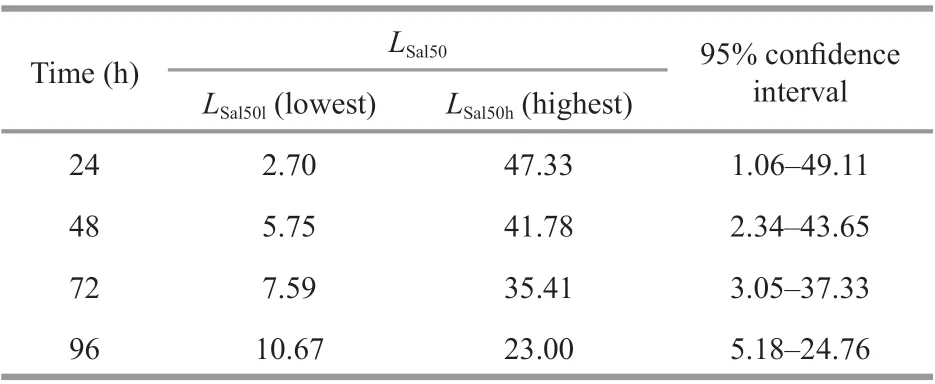
Table 1 The median lethal salinity ( L Sal50) and 95% confi dence interval of E. possjeticus
3.1.2 Carbonate alkalinity
The cumulative mortality ofE.possjeticusunder diff erent carbonate alkalinity levels is depicted in Fig.2. There were signifi cant increases in the cumulative mortality (P<0.05) with increased carbonate alkalinity over time.LCa50values at various time points and corresponding 95% confi dence intervals are provided in Table 2. With increased time,LCa50ofE.possjeticusdecreased gradually from 23.05 (24 h) to 17.14 (48 h), 10.90 (72 h), and fi nally 7.35 (96 h) in mmol/L.
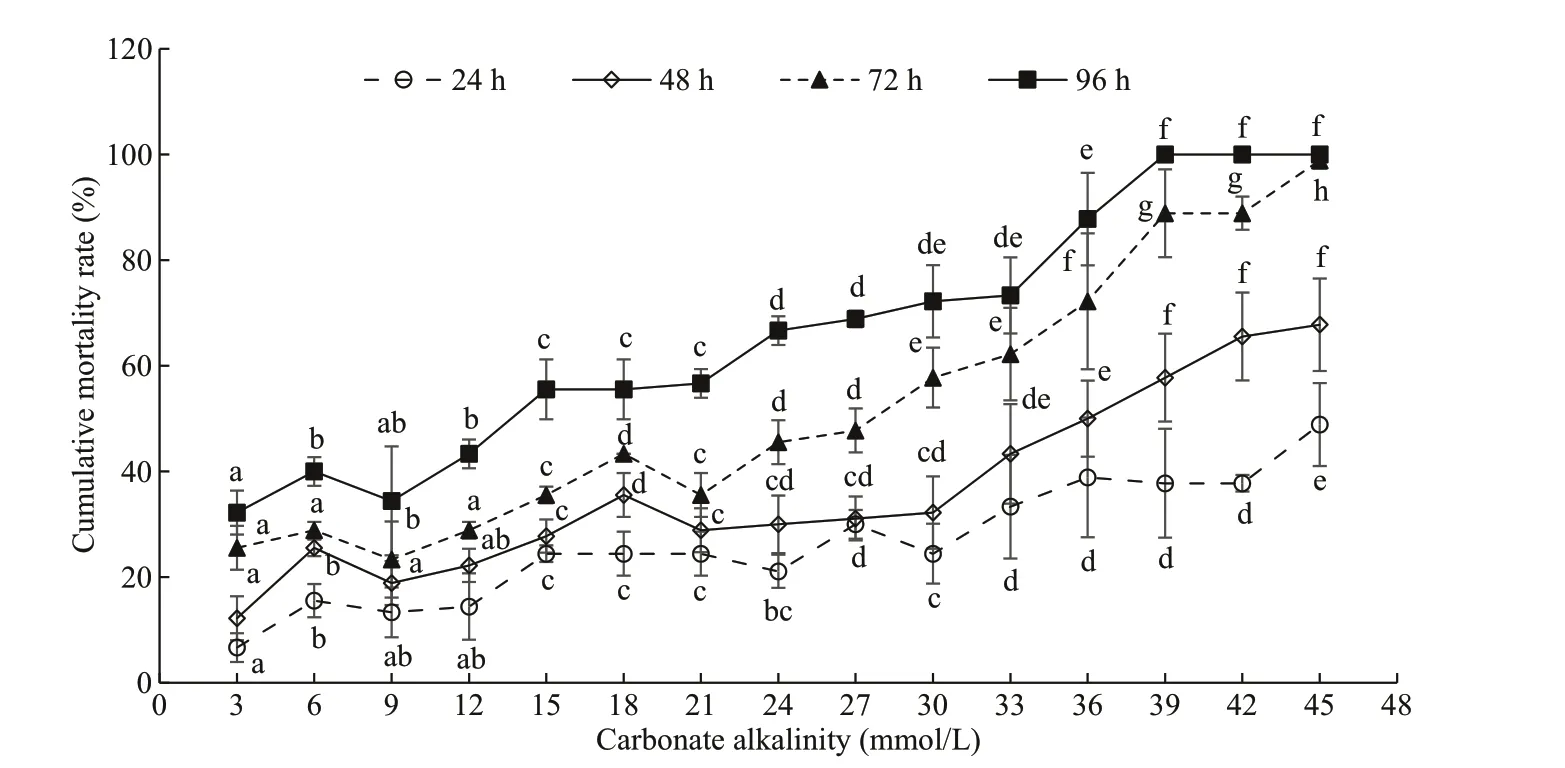
Fig.2 The cumulative mortality rates of E. possjeticus under diff erent carbonate alkalinities
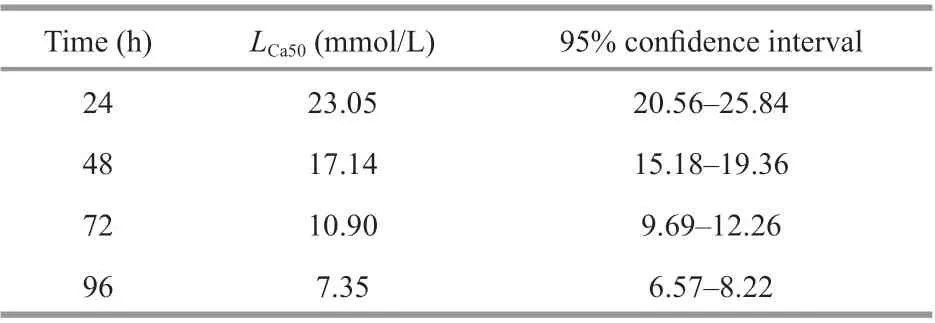
Table 2 The median lethal carbonate alkalinities ( L Ca50) and 95% confi dence interval of E. possjeticus
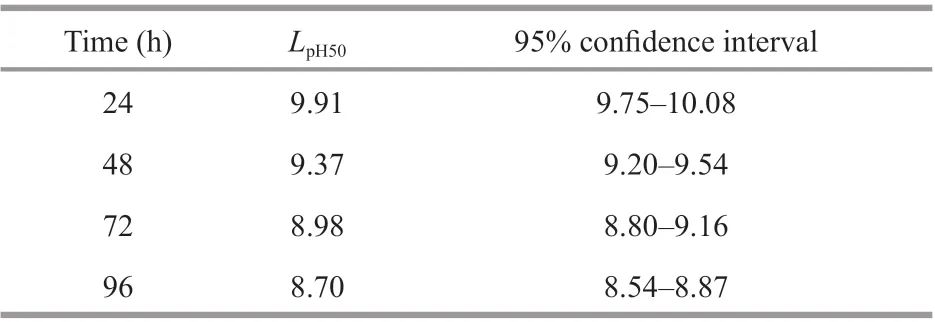
Table 3 The median lethal pH ( L pH50) and 95% confi dence interval of E. possjeticus
3.1.3 pH
The cumulative mortality ofE.possjeticusunder diff erent pH conditions is shown in Fig.3. With an increase in pH over time, the cumulative mortality rate increased signifi cantly (P<0.05).LpH50values at various time points and corresponding 95% confi dence intervals are summarized in Table 3. With time,LpH50ofE.possjeticusdecreased gradually from 9.91 at 24 h, 9.37 (48 h), 8.98 (72 h), to 8.70 at 96 h.
3.2 Ingestion rate and feed absorption effi ciency
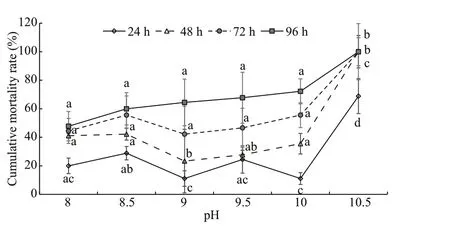
Fig.3 The cumulative mortality rates of E. possjeticus under diff erent pH
Daily ingestion rates and feed absorption effi ciencies decreased gradually with increasing carbonate alkalinity (Figs.4 & 5). Both parameters were highest at a carbonate alkalinity of 3 mmol/L and lowest at 12 mmol/L. Similarly, gradual decreases occurred in the daily ingestion rate and feed absorption effi ciency with increasing salinity and constant carbonate alkalinity. In addition, both parameters exhibited a downward trend with increasing pH under the same carbonate alkalinity and salinity. Repeated ANOVA analyses revealed signifi cant diff erences in the daily ingestion rate and feed absorption effi ciency ofE.possjeticusamong various groups under diff erent carbonate alkalinity, salinity, and pH levels (P<0.05).
3.3 Oxygen consumption and ammonia excretion rates, and the O:N ratio
With increasing carbonate alkalinity, oxygen consumption rates fi rst increased and then decreased (Fig.6). Among various salinity and pH conditions, oxygen consumption rates were highest at a carbonate alkalinity of 6 mmol/L and lowest at 12 mmol/L. Oxygen consumption rates exhibited an upward trend with increasing salinity under the same carbonate alkalinity. In contrast, it fi rst increased and then decreased with increasing pH under the same carbonate alkalinity and salinity. Ammonia excretion rates tended to increase with increasing pH when carbonate alkalinity was <6 mmol/L. Little variation was observed in ammonia excretion rates under various salinity and pH conditions when carbonate alkalinity was greater than 9 mmol/L (Fig.7). The O:N ratios initially increased and then decreased with increasing carbonate alkalinity. Under a carbonate alkalinity of <6 mmol/L, the O:N ratios fi rst increased with increasing salinity but then decreased with further increasing pH. Under a carbonate alkalinity >6 mmol/L, various salinity and pH conditions yielded relatively low O:N ratios and showed little variation (Fig.8). Repeated ANOVA analyses revealed signifi cant diff erences in the oxygen consumption and ammonia excretion rates as well as the O:N ratios ofE.possjeticusamong various groups under diff erent carbonate alkalinity, salinity, and pH levels (P<0.05).
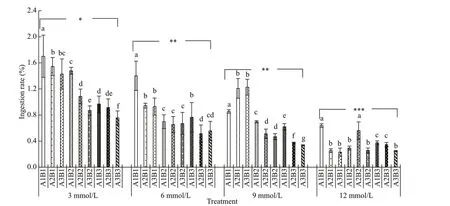
Fig.4 The ingestion rate of E. possjeticus at diff erent salinities, carbonate alkalinities, and pH
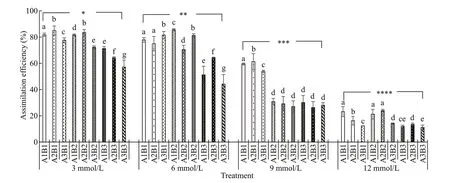
Fig.5 The assimilation effi ciency of E. possjeticus at diff erent salinities, carbonate alkalinities, and pH
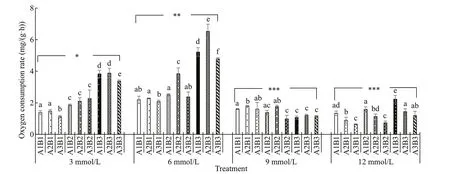
Fig.6 The oxygen consumption rate of E. possjeticus at diff erent salinities, carbonate alkalinities, and pH
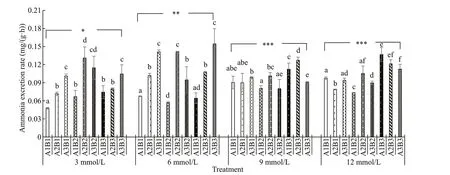
Fig.7 The ammonia excretion rate of E. possjeticus at diff erent salinities, carbonate alkalinities, and pH
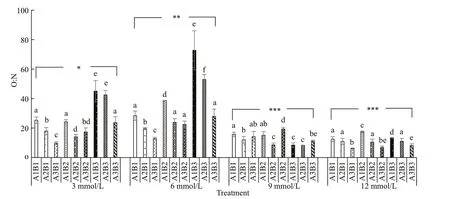
Fig.8 The O:N ratio of E. possjeticus at diff erent salinities, carbonate alkalinities, and pH
4 DISCUSSION
4.1 Salinity, carbonate alkalinity, and pH tolerance
The eff ects of salinity on amphipods are highly diverse and species specifi c (Normant and Lamprecht, 2006). For example, the biomass ofGammaruschevreuxiis closely linked to salinity (Subida et al., 2005), whereasG.locustademonstrates good salinity tolerance with its survival and growth not aff ected signifi cantly within a certain range (Neuparth et al, 2002). In the present study, theLSal50lat 24 h ofE.possjeticuswas 2.70 and the correspondingLSal50hwas 47.33, indicating a broad salinity tolerance range for this species. The survival strategy of amphipods isr-selection (natural populations commonly kept at low densities by density-independent mortality should evolve high intrinsic rate of growth, but be unable to have superior performance at high population densities) (Mueller and Ayala, 1981); strong fecundity and high biomass albeit with high mortality (Duff y and Hay, 2000). In addition to the increased cumulative mortality ofE.possjeticusin various treatment groups, the mortality of the control seawater group also increased with time. Thus, the 95% confi dence interval of theLSal50values forE.possjeticusat various time points might be an underestimation.
The carbonate alkalinity in aquaculture water is positively signifi cant within a certain range to the survival and growth of aquatic animals, but it likely has adverse eff ects beyond a certain limit (Zhao et al., 2016). Yang et al. (2004) reportedLCa50values for juvenilePenaeusvannameiof 14.29 (24 h), 12.55 (48 h), and 12.01 mmol/L (96 h) under pH values of 7.50–8.72. Similar to prior studies, carbonate alkalinity was found to be positively correlated with the mortality ofE.possjeticus. In other words, the mortality ofE.possjeticusincreased with increasing carbonate alkalinity. TheLCa50at 24 h ofE.possjeticuswas 23.05 mmol/L under pH values of 7.98–8.35. With time, the cumulative mortality ofE.possjeticusincreased such that at 48 h, 72 h, and 96 h, theLCa50values changed to 17.14, 10.90, and 7.35 mmol/L, respectively. These results demonstrate thatE.possjeticushas a higher tolerance to carbonate alkalinity thanP.vannameiover a short period. However, because of species diff erences, such as survival strategy, the mortality ofE.possjeticusincreases with time and its tolerance to carbonate alkalinity declines considerably relative toP.vannamei.
Another important ecological factor aff ecting the survival of cultured organisms is pH. Aquatic organisms all have a pH tolerance range and an optimal range. When pH in the external environment deviates outside the optimal range, it will aff ect the normal physiological activities of aquatic organisms and can even cause death (Wu et al., 2012). Our results found that greater deviations of water pH from the control group led to higher mortality rates forE.possjeticus; as time elapsed, the cumulative mortality ofE.possjeticusalso increased gradually. These observations are consistent with previous fi ndings from crustaceans, such asPalaemonetessinensis(Wang et al., 2002; Jiang et al., 2017). This study obtained 24 h, 48 h, 72 h, and 96 hLpH50values forE.possjeticusof 9.91, 9.37, 8.98, and 8.70, respectively.E.possjeticusexhibits a relatively low tolerance to pH compared with shrimp, such asP.sinensis(96 hLpH50=10.52) (Jiang et al., 2017). This low pH tolerance is closely associated with its species characteristics, suggesting thatE.possjeticusis more sensitive to environmental change.
4.2 Eff ects of coupled salinity, carbonate alkalinity, and pH on feeding and respiratory metabolism
Aquatic animal feeding behavior directly refl ects bodily response to the external environment. For environmental changes within a certain range, such as salinity, carbonate alkalinity, and pH fl uctuations, aquatic animals can alter their metabolic status via ion regulation and the activation or shutdown of the intracellular free amino acid pool, leading to a continuous increase in metabolism and ingestion rate. However, once changes in environmental conditions exceed a suitable range, the result is an inhibitory eff ect on the metabolism and ingestion rate (Zhang et al., 2012; Cai et al., 2017). It has been shown that feeding, growth, and feed utilization effi ciency of fi sh are closely related to salinity and alkalinity in the aquatic environment. The optimal range of environmental salinity and alkalinity levels is benefi cial for feeding, growth, and feed utilization in fi sh; however, when these exceed the suitable range, feeding, growth, and feed utilization effi ciency all decrease. This phenomenon may be because salinity and alkalinity aff ect the digestive enzyme activity and energy allocation in fi sh (Lv et al., 2007). Feng et al. (2009) found that when salinity and pH exceeded the optimal range forBrachionusurceus, it signifi cantly reduced the ingestion rate. Similarly, we observed a downward trend in the ingestion rate and feed absorption effi ciency ofE.possjeticuswith increasing carbonate alkalinity, salinity, and pH. This result indicates that the feeding ability ofE.possjeticusdeclined under high salinity and alkalinity conditions, which may then aff ect the normal growth and reproduction of this species.
Diff erent environmental conditions can aff ect respiratory metabolism and the energy supply mode in aquatic animals. To adapt to aquatic environments with diff erent salinities, diverse ion compositions, and varied pH, aquatic animals must have effi cient ionic and osmotic pressure regulation mechanisms (Hwang and Lee, 2007). For example, when some marine fi sh species, such asAnguillajaponicaandIctalurusnebulosus, are exposed to isotonic salinity, their oxygen consumption drops to their lowest rates; deviation from isotonic salinity leads to an increase in oxygen consumption rates (He et al., 2016). Similarly, changes in the aquatic carbonate alkalinity aff ect osmotic pressure equilibrium in bulimia barbelBarbuscapito, and its oxygen consumption rate increases with increasing alkalinity (Geng et al., 2017). When pH deviates from a certain range, it aff ects the amount and speed of dissolved oxygen that aquatic animals can acquire from the external environment (Jiang et al., 2017). When pH deviates from the optimal level, aquatic animals change their metabolic conditions to adapt to their external environment, leading to either reduction (Savant and Amte, 1995; Cao et al., 2015) or elevation (Zhang et al., 2009) in respiratory metabolism. In this study, the oxygen consumption rate ofE.possjeticusincreased with increasing salinity, whereas it initially increased and then decreased with increasing carbonate alkalinity and pH. These observations indicate that changes in salinity, carbonate alkalinity, and pH have an impact on the osmotic pressure equilibrium inE.possjeticus. For example, when carbonate alkalinity ≤6 mmol/L,E.possjeticusincreased its metabolism to meet the energy demand; however, when carbonate alkalinity ≥9 mmol/L, respiratory metabolism was inhibited substantially. Moreover, under low carbonate alkalinity conditions, respiratory metabolism fi rst increased and then decreased with increasing pH, whereas under high carbonate alkalinity conditions, respiratory metabolism gradually decreased with increasing pH. These results demonstrate that the same species may adopt diff erent strategies to cope with various environmental stresses (Jiang et al., 2017).
O:N ratio can refl ect the ratio of protein, carbohydrate and fat metabolism of animals in a specifi c state, and it is an important index to refl ect the energy and material utilization of animals (Ruyet et al., 2004). If energy is mainly provided by protein, the value of O:N ratio is about 7 or less (Mayzaud, 1976). If the energy is provided mainly by protein and fat, the O:N ratio is about 24 (Ikeda, 1974). If the energy is mainly provided by fat or carbohydrates, the O:N ratio will be greater than 24 or even infi nite (Conover and Corner, 1968). In this study, the analysis of O:N ratios showed that coupled changes in salinity, carbonate alkalinity, and pH could aff ect the energy supply mode (i.e. metabolic substrate ratio) inE.possjeticus. The O:N ratio was >24 under low carbonate alkalinity (≤6 mmol/L), high salinity (40), and low pH (8.0), indicating that the energy consumed byE.possjeticuswas mainly supplied by fats. In contrast, the O:N ratio was <24 under high carbonate alkalinity (≥9 mmol/L) as well as low carbonate alkalinity (≤6 mmol/L), low salinity (20), and high pH (9.0). This result indicates that the energy consumed byE.possjeticusunder these conditions was supplied by proteins mainly. The evidence indicates that under diff erent environmental conditions, crustaceans can adjust their energy supply mode to cope with environmental changes (Jiang et al., 2017). In this study, we found that the energy supply mode shifted from fats to proteins under high carbonate alkalinity and pH conditions.
5 CONCLUSION
In this study, the three environmental factors, salinity, carbonate alkalinity, and pH, showed signifi cant eff ects on the survival, feeding, and respiratory metabolism ofE.possjeticus. The results suggest that the changes in salinity, carbonate alkalinity, and pH have a measurable impact on the osmotic pressure equilibrium inE.possjeticusand aff ect the energy supply mode. This fi nding leads us to speculate that the population development ofE.possjeticuswould be limited in aquatic environments with high salinity, alkalinity, and pH values.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are available on reasonable request from the fi rst author.
7 ACKNOWLEDGMENT
We are grateful to the anonymous reviewers for their constructive comments and suggestions that help us to improve the manuscript. We also thank Kara Bogus, PhD, from Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
Ananthi P, Santhanam P, Nandakumar R, Ananth S, Jothiraj K, Dinesh K S, Balaji P B, Jayalakshmi T. 2011. Production and utilization of marine copepods as live feed for larval rearing of tiger shrimpPenaeusmonodonwith special emphasis on astaxanthin enhancement.IndianJournalofNaturalSciences, 2: 494-503.
Baeza-Rojano E, Hachero-Cruzado I, Guerra-García J M. 2014. Nutritional analysis of freshwater and marine amphipods from the Strait of Gibraltar and potential aquaculture applications.JournalofSeaResearch, 85: 29-36.
Cai Q J, He W H, Peng Z R, Li X X, Cui L X, Zhang A. 2017. Eff ects of pH and salinity on heart rate and feeding behavior ofDaphniamagna.JournalofShanghaiOceanUniversity, 26(3): 415-421. (in Chinese with English abstract)
Cao S M, Song B, Wang L M, Sun K, Wang H Y. 2015. Eff ect of temperature, salinity and pH on respiration and excretion in gammaridEogammarussinensis.JournalofDalianOceanUniversity, 30(5): 519-523. (in Chinese with English abstract)
Conover R J, Corner E D S. 1968. Respiration and nitrogen excretion by some marine zooplankton in relation to their life cycles.JournaloftheMarineBiologicalAssociationoftheUnitedKingdom, 48(1): 49-75.
Cunha M R, Sorbe J C, Bernades C. 1997. On the structure of the neritic suprabenthic communities from the Portuguese continental margin.MarineEcologyProgressSeries, 157: 119-137.
Dauby P, Scailteur Y, De Broyer C. 2001. Trophic diversity within the eastern Weddell Sea amphipod community.Hydrobiologia, 443(1-3): 69-86.
Duff y J E, Hay M E. 2000. Strong impacts of grazing amphipods on the organization of a benthic community.EcologicalMonographs, 70(2): 237-263.
Fang W H, Wang H, Lai Q F, Huang C N. 1999. Eff ect of K+,and HC(C) concentrations on the survival ofProtosalanxhyalocraniuslarvae.MarineFisheries, (2): 66-68. (in Chinese with English abstract)
Feng L, Wang J H, Tang X X. 2009. Study on feeding ecology of rotiferBrachionusurceus.MarineEnvironmentalScience, 28(4): 349-354. (in Chinese with English abstract)
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People's Republic of China. 2008. GB 17378.4-2007 The specifi cation for marine monitoring, Part 4: seawater analysis. China Standard Press, Beijing. p.111-113. (in Chinese with English abstract)
Geng L W, Jiang H F, Xu W. 2017. Eff ects of carbonate alkalinity on oxygen consumption and ammonia excretion in bulimia barbelBarbuscapito.ChineseJournalofFisheries, 30(6): 30-33. (in Chinese with English abstract)
He Q, Chang Y M, Su B F, Sun B, Sun X W, Liang L Q. 2016. Eff ects of carbonate alkalinities on oxygen consumption, ammonia excretion and ammonia excretion gene expression inLeuciscuswaleckiiDybowski.JournalofShanghaiOceanUniversity, 25(4): 551-558. (in Chinese with English abstract)
Huang N Y, Fan Q G, Gu X L, Xia L J. 2010. Diet composition of juvenile Siberian sturgeons (Acipenserbaeri) cultured in inland reservoir in western China and its’ relations with benthic organism resources.MarineFisheries, 32(3): 313-319. (in Chinese with English abstract)
Huang R, Xu F K. 2017. Study on the acute toxicity of rotenone toLateolabraxjaponicasandChaeturichthysstigmatiasRichardson.TransactionsofOceanologyandLimnology, (1): 96-101. (in Chinese with English abstract)
Hwang P P, Lee T H. 2007. New insights into fi sh ion regulation and mitochondrion-rich cells.ComparativeBiochemistryandPhysiologyPartA:Molecular&IntegrativePhysiology, 148(3): 479-497.
Ikeda T. 1974. Nutritional ecology of marine zooplankton.MemoirsoftheFacultyofFisheries,HokkaidoUniversity, 22: 1-97.
Jaff er Y D, Saraswathy R, Ishfaq M, Antony J, Bundela D S, Sharma P C. 2020. Eff ect of low salinity on the growth and survival of juvenile pacifi c white shrimp,Penaeusvannamei: A revival.Aquaculture, 515: 734561.
Jiang H B, Bao J, Jiang C J, Fu P P, Yu Y H, Li X D. 2017. Eff ects of pH on survival and respiratory metabolism of Chinese Grass Shrimp (Palaemonetessinensis).ChineseJournalofZoology, 52(2): 322-330. (in Chinese with English abstract)
Lin T T, Lai Q F, Yao Z L, Lu J X, Zhou K, Wang H. 2013. Combined eff ects of carbonate alkalinity and pH on survival, growth and haemocyte parameters of the Venus clamCyclinasinensis.FishandShellfi shImmunology, 35(2): 525-531.
Lv F, Huang J T, Wang A M. 2007. Eff ects of alkalinity on the food consumption, growth and survival ofAllogynogemeticcruciancarp.JournalofAnhuiAgriculturalSciences, 35(22): 6 789-6 790. (in Chinese with English abstract)
Mayzaud P. 1976. Respiration and nitrogen excretion of zooplankton. IV. The infl uence of starvation on the metabolism and the biochemical composition of some species.MarineBiology, 37(1): 47-58.
Mueller L D, Ayala F J. 1981. Trade-off betweenr-selection andK-selection in Drosophila populations.ProceedingsoftheNationalAcademyofSciences, 78(2): 1 303-1 305.
Neuparth T, Costa F O, Costa M H. 2002. Eff ects of temperature and salinity on life history of the marine amphipodGammaruslocusta. Implications for ecotoxicological testing.Ecotoxicology, 11(1): 61-73.
Normant M, Lamprecht I. 2006. Does scope for growth change as a result of salinity stress in the amphipodGammarusoceanicus?JournalofExperimentalMarineBiologyandEcology, 334(1): 158-163.
Ruyet J P L, Mahé K, Le Bayon N, Le Delliou H. 2004. Eff ects of temperature on growth and metabolism in a Mediterranean population of European sea bass,Dicentrarchuslabrax.Aquaculture, 237(1-4): 269-280.
Sainte-Marie B. 1992. Foraging of scavenging deep-sea lysianassoid amphipods.In: Rowe G T, Pariente V eds. Deep-Sea Food Chains and the Global Carbon Cycle. Springer, Dordrecht. p.105-124.
Savant K B, Amte G K. 1995. Infl uence of some environmental factors on respiratory responses in the tropical estuarine crabIlyoplaxgangetica.JournalofEnvironmentalBiology, 16(4): 311-317.
Subida M D, Cunha M R, Moreira M H. 2005. Life history, reproduction, and production ofGammaruschevreuxi(Amphipoda:Gammaridae) in the Ria de Aveiro, northwestern Portugal.JournaloftheNorthAmericanBenthologicalSociety, 24(1): 82-100.
Wang W N, Wang A L, Chen L, Liu Y, Sun R Y. 2002. Eff ects of pH on survival, phosphorus concentration, adenylate energy charge and Na+-K+ATPase activities ofPenaeuschinensisOsbeck juveniles.AquaticToxicology, 60(1-2): 75-83.
Wu Y T, Lv G T, Ren S Y, Wang Z Z, Cao B. 2012. The eff ect of consumption rate and CAT, SOD enzyme activity from water temperature onMacrobrachiummipponensis.JournalofZhejiangOceanUniversity(NaturalScience), 3 1(3): 197-201. (in Chinese with English abstract)
Xue S Y, Fang J G, Zhang J H, Jiang Z J, Mao Y Z, Zhao F Z. 2013. Eff ects of temperature and salinity on the development of the amphipod crustaceanEogammarussinensis.ChineseJournalofOceanologyandLimnology, 31(5): 1 010-1 017.
Xue S Y, Mao Y Z, Li J Q, Zhu L X, Fang J G, Zhao F Z. 2018. Life history responses to variations in temperature by the marine amphipodEogammaruspossjeticus(Gammaridae) and their implications for productivity in aquaculture.Hydrobiologia, 814(1): 133-145.
Yang F Y, Sun L M, Yang X Q. 2004. Toxicity of carbonate alkalinity toPenaeusvannameijuveniles.FisheriesScience, 23(9): 3-6. (in Chinese with English abstract)
Yao Z L, Guo W F, Lai Q F, Shi J Q, Zhou K, Qi H F, Lin T T, Li Z N, Wang H. 2016.Gymnocyprisprzewalskiidecreases cytosolic carbonic anhydrase expression to compensate for respiratory alkalosis and osmoregulation in the saline-alkaline Lake Qinghai.JournalofComparativePhysiologyB, 186(1): 83-95.
Yao Z L, Lai Q F, Zhou K, Rizalita R E, Wang H. 2010. Developmental biology of medaka fi sh (Oryziaslatipes) exposed to alkalinity stress.JournalofAppliedIchthyology, 26(3): 397-402.
Zhang L Z, Yang J H, Liu J Y, Zhuang P, Zhao F, Qu L. 2009. Eff ects of water temperature, salinity, pH, and anaesthetics on oxygen consumption rate of juvenileSiganuscanaliculatus.ChineseJournalofEcology, 28(8): 1 494-1 498. (in Chinese with English abstract)
Zhang T, Sun C B, Guan R L. 2012. Eff ect of multi-factors on energy metabolism of juvenileLitopenaeusvannamei.JournalofTropicalOrganisms, 3(1): 11-15. (in Chinese with English abstract)
Zhao Y, Wu J W, Meng S, Wang Y, Wu H, Zhao J L. 2016. Eff ects of carbonate alkalinity on serum pH, ammonia concentration and related gene expression inOreochromisniloticus.JournalofSouthernAgriculture, 47(6): 1 032-1 038. (in Chinese with English abstract)
Zhou K, Lai Q F, Wang H, Huang N Y, Yao Z L. 2007. Acute toxicity eff ects of calcium and magnesium on larva ofLitopenaeusvannamei.MarineSciences, 31(7): 4-7. (in Chinese with English abstract)
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Exploring sensitive area in the tropical Indian Ocean for El Niño prediction: implication for targeted observation*
- Analysis of the typhoon wave distribution simulated in WAVEWATCH-III model in the context of Kuroshio and wind-induced current*
- Characterizing the capability of mesoscale eddies to carry drifters in the northwest Pacifi c*
- Observation system simulation experiments using an ensemble-based method in the northeastern South China Sea*
- Statistical analysis of intensity variations in tropical cyclones in the East China Sea passing over the Kuroshio*
