Assessing the eff ects of oyster/kelp weight ratio on water column properties: an experimental IMTA study at Sanggou Bay, China*
FANG Jinghui , FANG Jianguang CHEN Qionglin , MAO Yuze JIANG Zengjie , ,DU Meirong GAO Yaping LIN Fan
1 Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao); Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China 2 Key Laboratory of Marine Biotechnology of Zhejiang Province, School of Marine Sciences, Ningbo University, Ningbo 315211, China
Abstract Integrated Multi-Trophic Aquaculture (IMTA) is an eff ective method for sustainable aquaculture as species from diff erent trophic levels could reduce negative eff ects from fed species in the environment. A proper proportion of diff erent trophic species in an IMTA system could improve the aquaculture production and environmental sustainability. At present, research on the proper proportions for farming species is scarce. We investigated the eff ects of IMTA modes of oyster ( Crassostrea gigas) and kelp ( Saccharina japonica) in diff erent weight ratios on water quality and carbonate system in a closed enclosure experiment for three days in the Sanggou Bay in Shandong Province, China, in December 2017. Nine collocation modes in oyster:kelp weight ratio were tested showing as 24:3, 24:2, 24:1, 16:3, 16:2, 16:1, 8:3, 8:2, and 8:1. The water parameters were determined at 17:00 on Day 1 (D1), and 6:00 and 17:00 on Days 2 (D2) and 3 (D3). As two-way ANOVA showed, all increased parameters (dissolved oxygen (DO), pH, chl a, the carbonate system and p CO 2) were signifi cantly related to oyster-kelp modes, and interaction between modes and time were also signifi cant ( P <0.05). On the 3 th day, the 8:3 mode was the highest in DO, pH, chl a, ( P <0.05), and dissolved inorganic carbon (DIC), CO 2, and p CO 2 were the lowest ( P <0.05). According to previous references and the results of this study, the appropriate oyster:kelp proportion at the beginning of winter is from 8:2 to 8:3. The results of this study may help government to optimize the aquaculture structure of Sanggou Bay.
Keyword: Integrated Multi-Trophic Aquaculture (IMTA); Pacifi c oyster; Crassostrea gigas; kelp; Saccharina japonica
1 INTRODUCTION
Aquaculture is a fast-growing food-producing sector in the world, which is crucial to meet the need of growing human population on the Earth. The State of the World Fisheries and Aquaculture (SOFIA) states that “in terms of global production volume, that of farmed fi sh and aquatic plants combined surpassed that of capture fi sheries in 2013. In terms of food supply, aquaculture provided more fi sh than capture fi sheries in 2014” (FAO, 2016). Driven by the growing demand for aquaculture, many environmental problems appeared, which hampered the sustainable development of aquaculture, particularly for the species that need feed input. Only 17% of the nitrogen in fi sh feed can be retained in fi nfi sh, while the rest 83% are wasted in the environment (Wen et al., 2007). Furthermore, Wu (1995) found that 1%–15% of formulated fi sh feed and 40% of fresh feed are wasted. Half of sedimentary organic carbon is originated from aquaculture fi sh feed (Gillibrand et al., 1996). The nitrogen input from the feed in aquaculture pond or cage could amounted to 54.7%–95% of the total nitrogen input in the system (Briggs and Fvnge-Smith, 1994; Chang et al., 2006; Zhang, 2012). Therefore, designing a system to reduce the negative eff ects in aquaculture production is an urgent task.
Sanggou Bay (37°01′N–37°09′N, 122°24′E– 122°35′E), located in Shandong Province, China, is a typical IMTA area that houses more than 30 important aquaculture species, including kelp (Saccharinajaponica), oyster (Crassostreagigas), mussel (Mytilusedulis), scallop (Chlamysfarreri), abalone (Haliotisdiscushannai), and sea cucumber (Apostichopusjaponicus) (Zhang et al., 2007). Some of them have been successfully co-cultured in IMTA mode. Shellfi sh-seaweed collocation is the base of the IMTA modes (Tang et al., 2013; Fang et al., 2016). In 2015, 65 000 ton of oyster (C.gigas), one of the mostly cultured shellfi sh, was produced in Sanggou Bay (data from local government), and kelp (S.japonica) is the main cultured seaweed in Sanggou Bay, and 84 500 ton was produced in 2015 (Ning et al., 2016). In order to determine the optimal ratio of oyster to kelp co-culture in Sanggou Bay at the beginning of winter (the kelp culturing period), water quality and carbonate system were investigated within the frame of diff erent oyster-kelp modes.
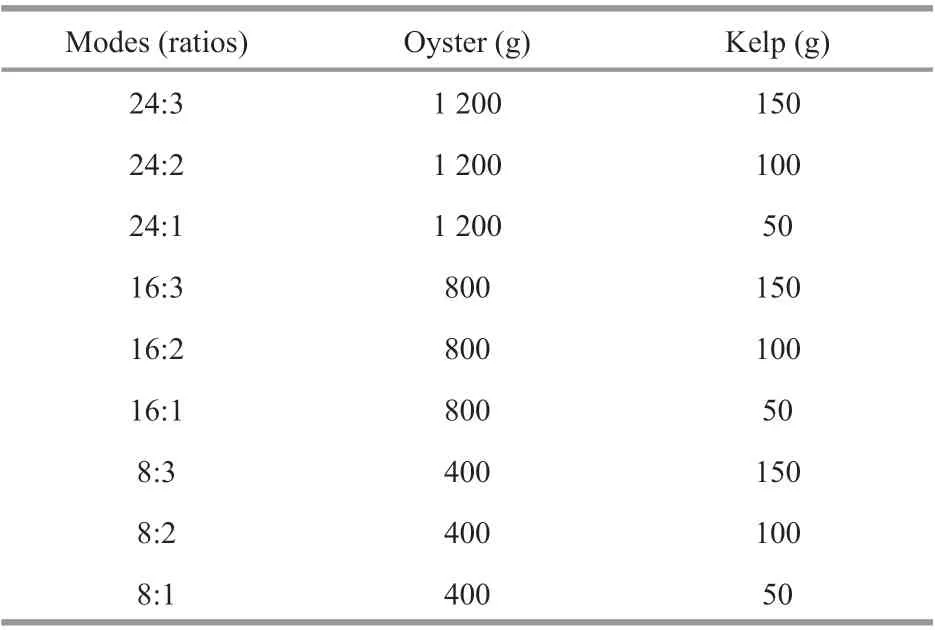
Table 1 The total weight ratios of oyster and kelp in the modes design of oyster integrated with kelp
2 MATERIAL AND METHOD
2.1 Experimental design and management
The experiment was conducted in Sanggou Bay, China, in December 2017 (Fig.1). KelpS.japonica(whole plant wet weight: 1.65±0.76 g, mean±SD,n=30) and oysterC.gigas(wet weight: 36.26±3.89 g, mean±SD,n=30) were collected locally. Kelps were tied on ropes and oysters were cleaned and put into a net bag. Both organisms were hung onto a transparent polyethylene plastic bag (1.0 m3) that was securely sealed with a cap, after expelling all air bubbles. Each bag had a special cap that can be opened and closed easily. Nine collocation modes in oyster:kelp weight ratio were tested showing as 24:3, 24:2, 24:1, 16:3, 16:2, 16:1, 8:3, 8:2, and 8:1 (Table 1). The number of oysters is shown in Table 2. Each treatment included 30, 60, and 90 kelps and 3 replications in each treatment.
Fig.1 The study site
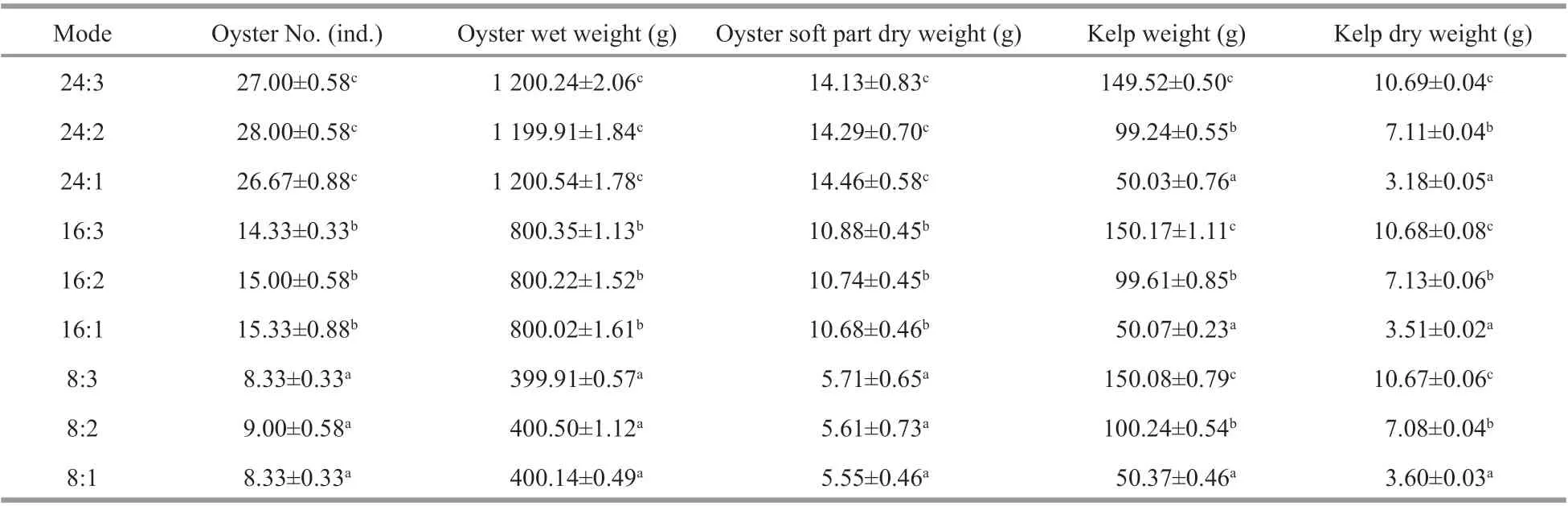
Table 2 The biomass of organisms at diff erent treatments in the experiment
The bags were randomly tied onto the fl oating frame (10 m2) in open sea and fi lled with natural seawater. The bags were placed 10–20 cm above the surface. During the experiment, samples were collected at 17:00 on Day 1 (D1), and 6:00 and 17:00 on Days 2 and 3. The time was adjusted according to local sunrise at 6:00 and sunset at 17:00. Water temperature, salinity, pH, and dissolved oxygen (DO) were measured by YSI Professional Plus (Pro Plus, USA) and pH meter (Orion 9107 BNMD, USA). Measurement work took 1.5 h each time. One liter of water sample was collected from each bag stirred in each sampling period and particles in the water samples were collected with cellulose acetate fi lters (0.45 μm, Whatman) and preserved at -20°C. An amount of 150-mL fi ltered seawater was mixed with 0.02% HgCl2saturated solution and stored and sealed in 150-mL borosilicate bottles at 4°C for total alkalinity (TA) analysis (Dickson and Goyet, 1994).
2.2 Sample analysis
2.3 Data analysis
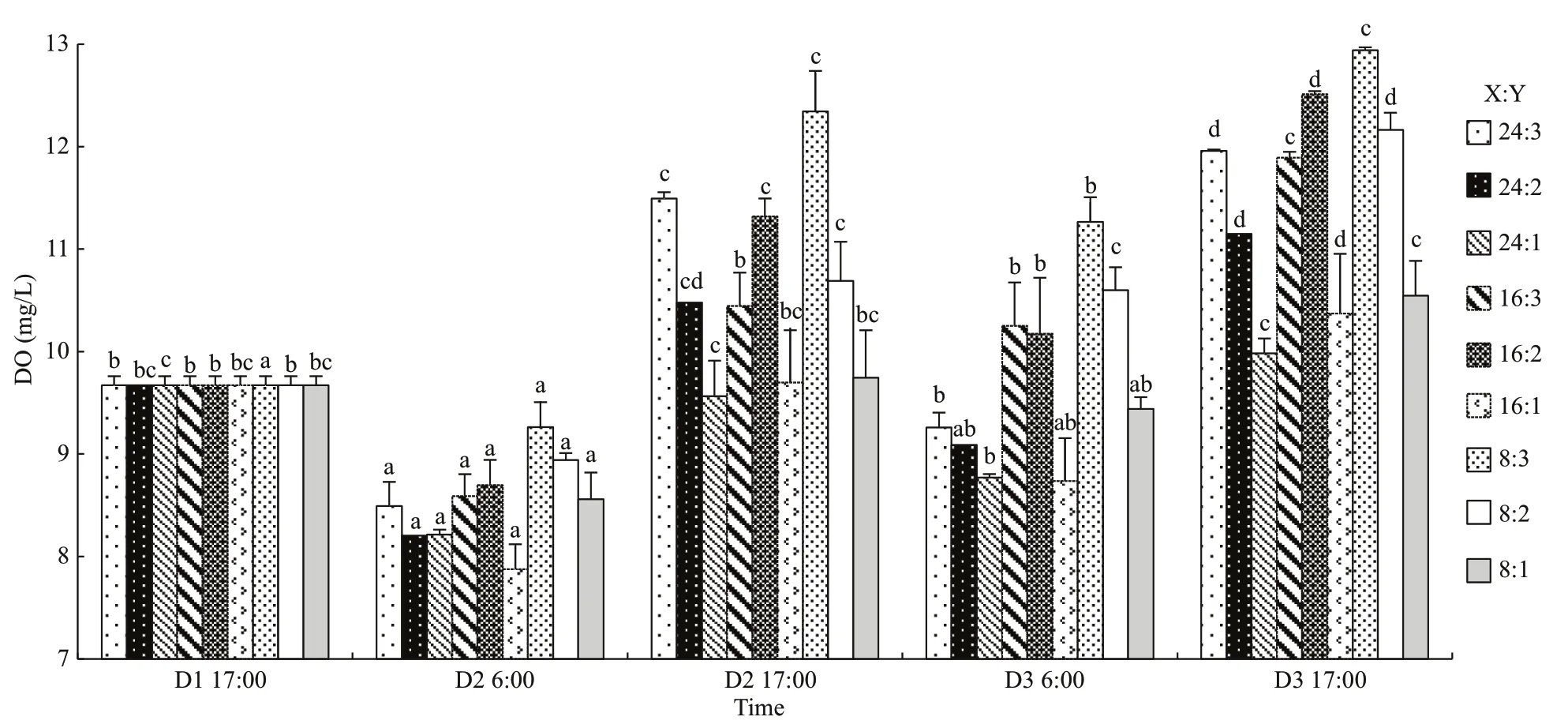
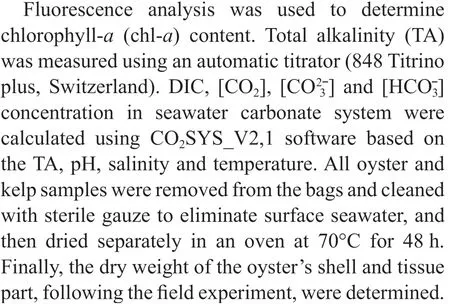
Fig.2 The dissolved oxygen of oyster-kelp modes at diff erent time
Data were analyzed using SPSS 13.0 for Windows (SPSS, USA). Prior to statistical analysis, all data were checked for homogeneity and normality of variance assumptions by (i) visually examining standardized residuals versus predicted values plots and Q-Q plots of residuals, (ii) Shapiro-Wilk tests, and (iii) Levene tests. Data were log-transformed if a normal distribution assumptions were not met. Oyster and kelp in each mode were considered together as a combined system (mode). Interaction between modes and experiment time on DO, pH, chla, and parameters of the carbonate system were tested using two-way repeated ANOVA measurements. Statistically signifi cant infl uences, proven by ANOVA, were followed by a Tukey test to determine the withintreatment eff ects. The diff erence in the organism’s initial wet weight and dry weight and biomass after experiment among treatments was tested using oneway ANOVA. All diff erences were considered statistically signifi cant atP<0.05.
3 RESULT
During the experiment, water temperature ranged 9.0–10.2°C, salinity 31.30–31.57, and no dead animals were found. The biomass of the organisms was allocated as the experiment design showed in Table 2.
3.1 Dissolved oxygen
Two-way ANOVA showed that DO was signifi cantly aff ected by oyster-kelp modes and sampling time, and that interaction between both factors was signifi cant (P<0.05, Table 3). DO was higher in the modes with higher kelp ratio (P<0.05,Fig.2). At the end of the experiment, DO was lowest in the 24:1 mode and highest in the 8:3 mode (P<0.05).
Table 3 Two-way ANOVA statistics of dissolved oxygen (DO), pH, chl a, and p CO 2 of oyster-kelp modes at diff erent sampling time
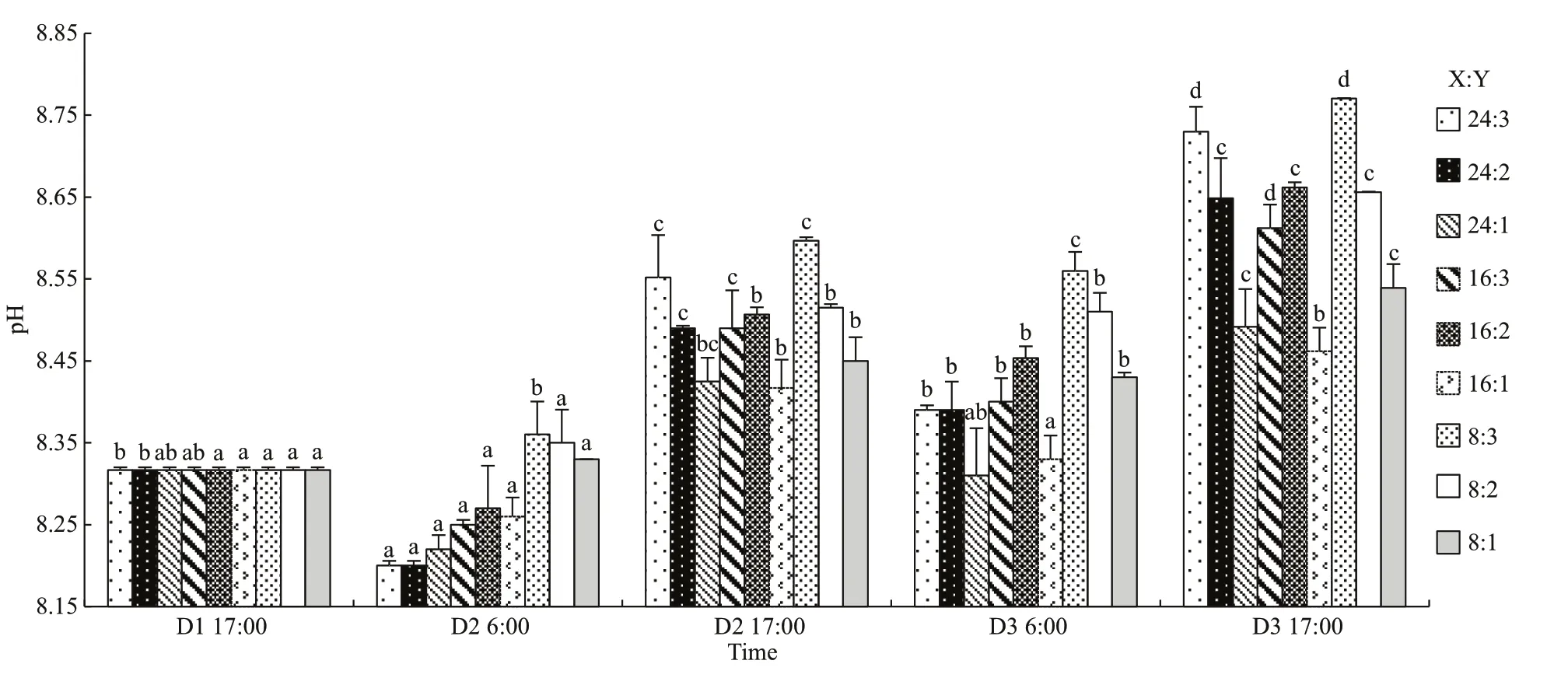
Fig.3 The pH of oyster-kelp modes at diff erent time
3.2 pH
The pH values in this experiment are often in parallel to those of the DO results (Fig.3).
Results of two-way ANOVA show that pH was signifi cantly diff erent among the oyster-kelp modes and sampling time, and interaction between both factors was signifi cant (P<0.05, Table 3). pH was higher in the modes of more kelp (P<0.05). At the end of the experiment, pH was the lowest in the 16:1 mode and highest in the 8:3 mode (P<0.05).
pH fl uctuated signifi cantly during day and night, but the means were lower in the morning than those in the afternoon on the same day (P<0.05, one-way ANOVA). At the end of the experiment, pH was signifi cantly higher in all mode than the initial value (P<0.05).
3.3 Chl a
As two-way ANOVA statistics show, chl-aconcentration was signifi cantly diff erent among the oyster-kelp modes and sampling time. Interaction between both factors was signifi cant (P<0.05, Table 3). Chl-aconcentration demonstrated signifi cantly higher values in the modes in higher ratio of oyster:kelp (P<0.05). The chl-aconcentration was continuously increasing over the time (P<0.05).
By the end of the experiment, the modes with “24” oysters contained highest chl-aconcentrations (P<0.05, Fig.4). There was a signifi cant increase in 24:1 and 24:2 modes on 6:00 am of D3 (P<0.05). Chl-aconcentrations increased in all modes compared with those at D1 17:00 excepting for the 8:1 mode at the end of the experiment. However, no signifi cant diff erence was found between D1 17:00 and D3 17:00 in the 8:2, 8:3 or 16:1 modes (P>0.05).
3.4 Carbonate system and p CO 2
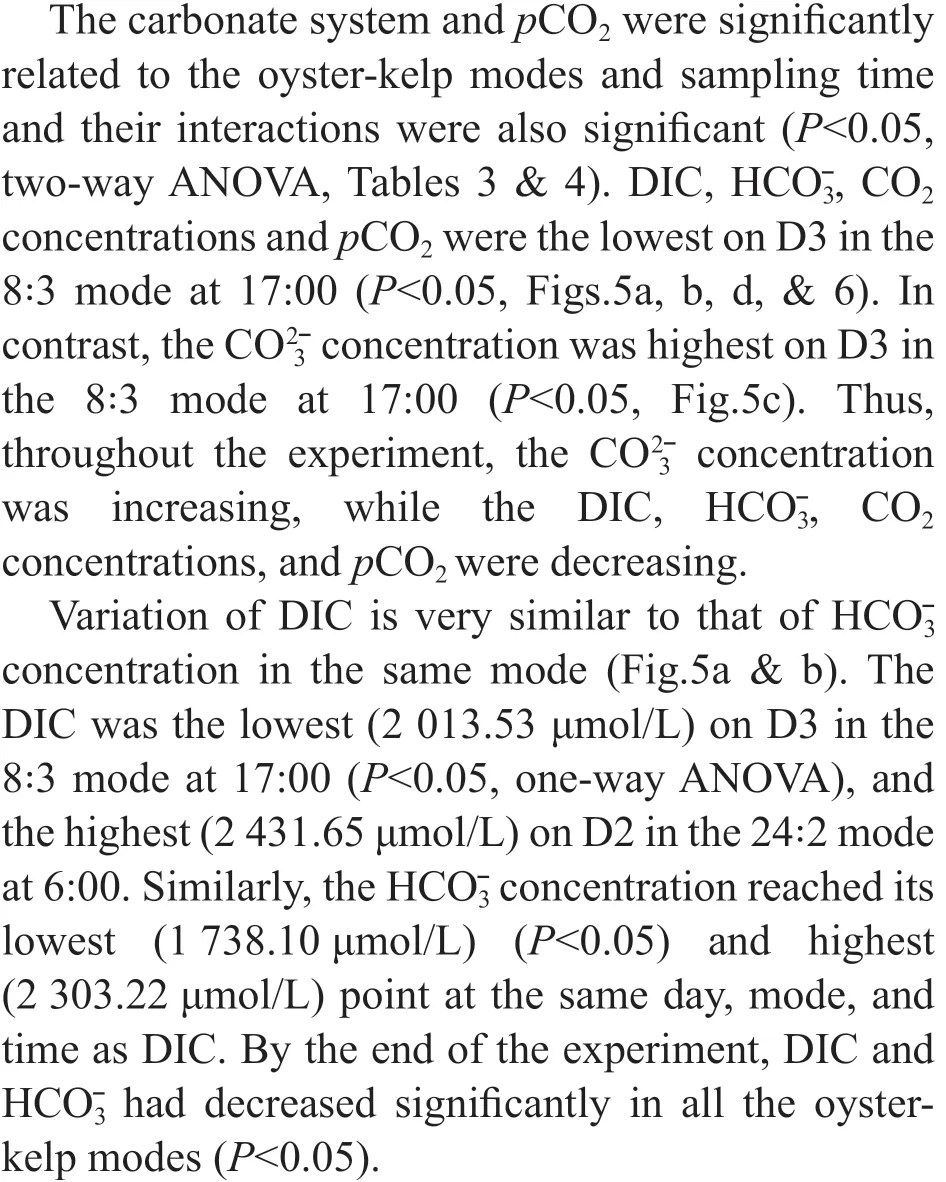
Fig.4 The chl a of oyster-kelp modes at diff erent time
The fl uctuations of CO2concentration andpCO2were very similar. Both of them fl uctuated signifi cantly during day and night, but means were lower in the afternoon than those in the morning on the same day (P<0.05, one-way ANOVA, Figs.5d & 6). The higher CO2concentration andpCO2were in the modes with higher oyster ratio. Both of them decreased signifi cantly at the end of the experiment in all oysterkelp modes (P<0.05).pCO2dropped as low as 134.31 μatm in the 8:3 mode.
4 DISCUSSION
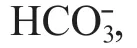
Table 4 Two-way ANOVA results of CO 2, , , and DIC in the seawater of oyster-kelp modes at diff erent sampling time
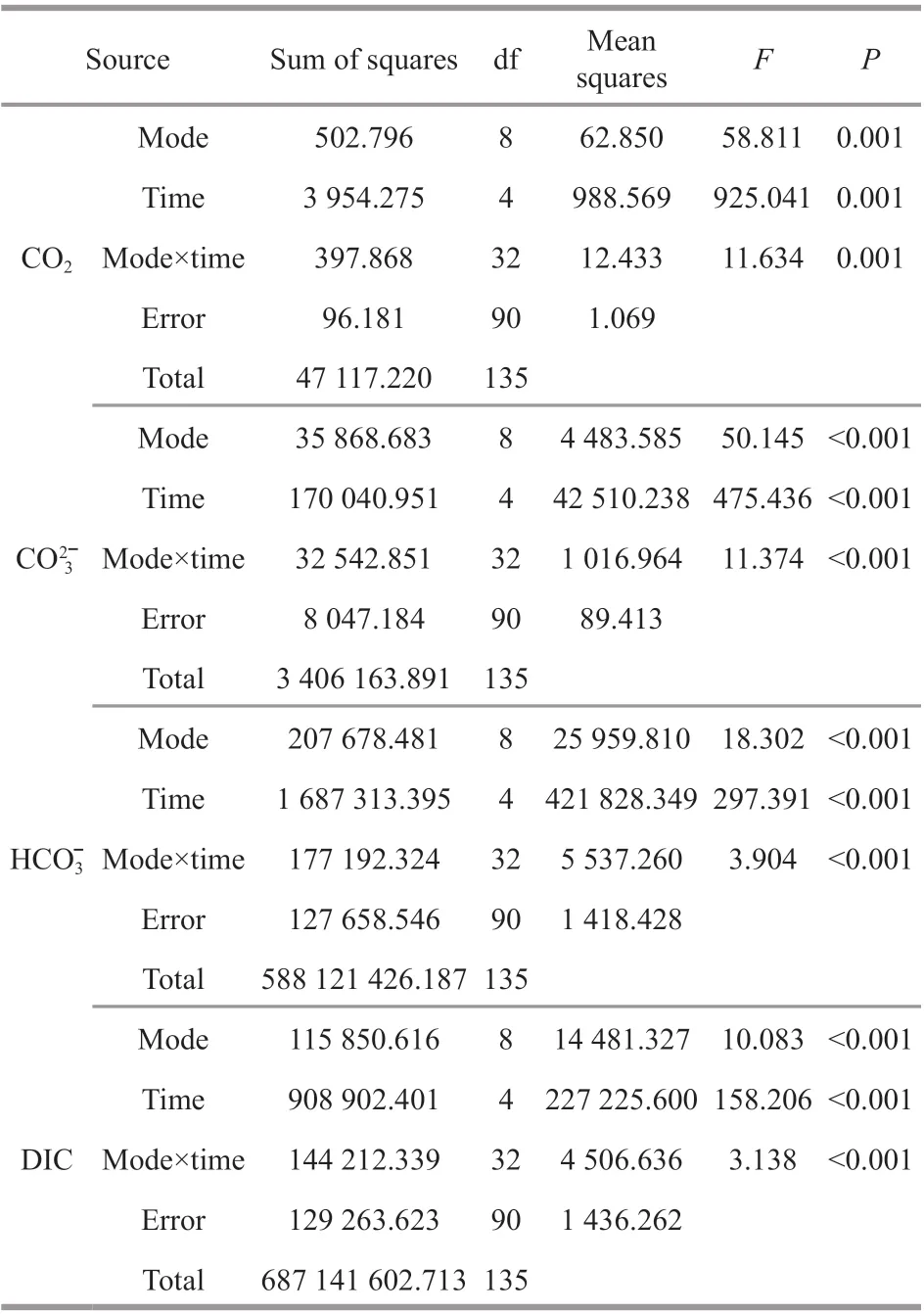
Table 4 Two-way ANOVA results of CO 2, , , and DIC in the seawater of oyster-kelp modes at diff erent sampling time
Source Sum of squares df Mean squares F P CO 2 Mode 502.796 8 62.850 58.811 0.001 Time 3 954.275 4 988.569 925.041 0.001 Mode×time 397.868 32 12.433 11.634 0.001 Error 96.181 90 1.069 Total 47 117.220 135 CO 32ˉ Mode 35 868.683 8 4 483.585 50.145 <0.001 Time 170 040.951 4 42 510.238 475.436 <0.001 Mode×time 32 542.851 32 1 016.964 11.374 <0.001 Error 8 047.184 90 89.413 Total 3 406 163.891 135 HCO 3 ˉ Mode 207 678.481 8 25 959.810 18.302 <0.001 Time 1 687 313.395 4 421 828.349 297.391 <0.001 Mode×time 177 192.324 32 5 537.260 3.904 <0.001 Error 127 658.546 90 1 418.428 Total 588 121 426.187 135 DIC Mode 115 850.616 8 14 481.327 10.083 <0.001 Time 908 902.401 4 227 225.600 158.206 <0.001 Mode×time 144 212.339 32 4 506.636 3.138 <0.001 Error 129 263.623 90 1 436.262 Total 687 141 602.713 135
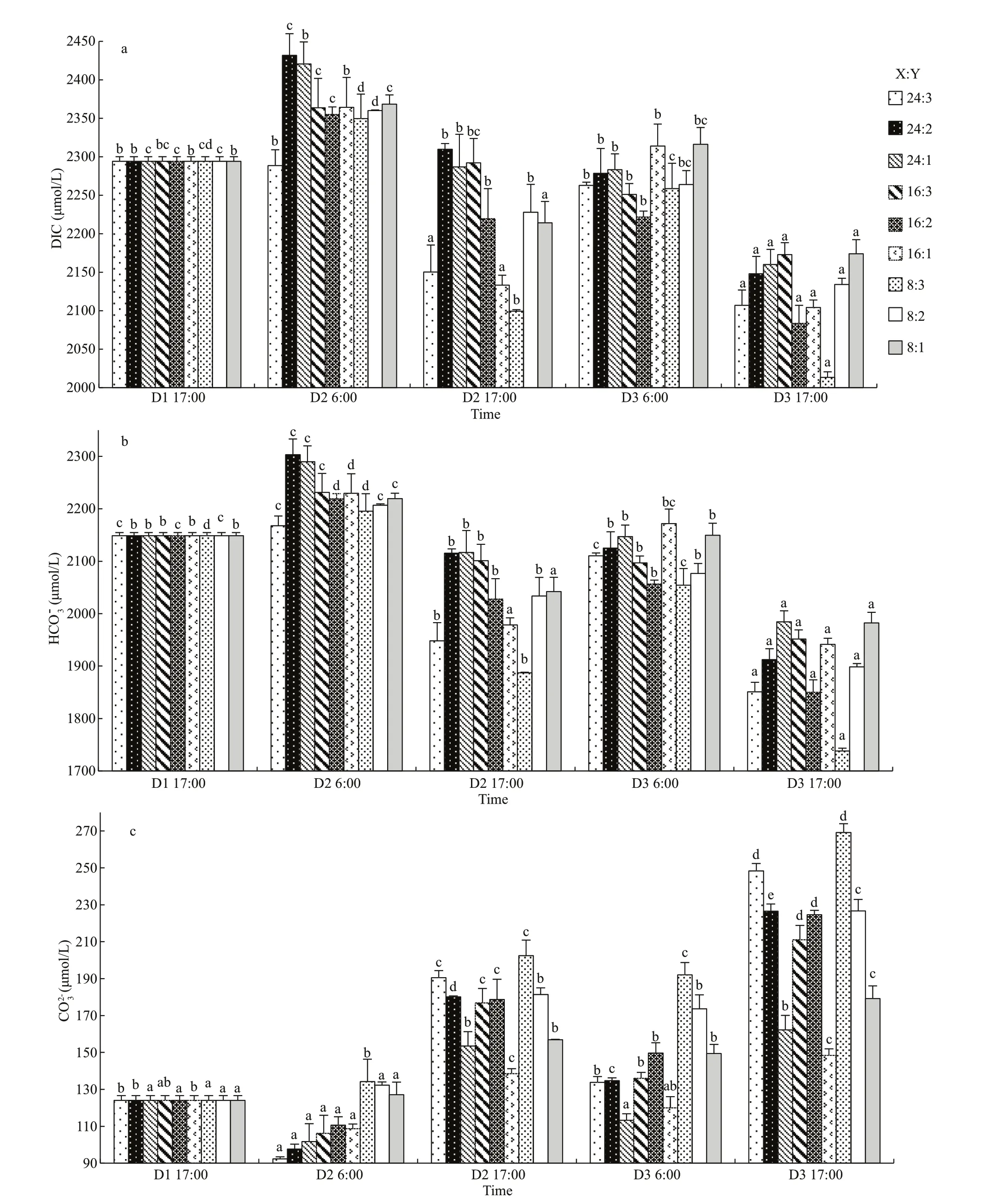
Fig.5 The variation of carbonate system in diff erent oyster-kelp modes
To be continued
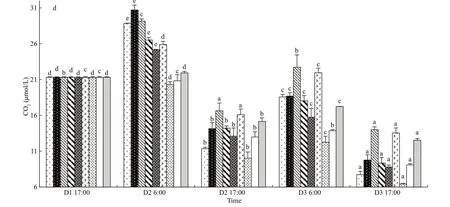
Fig.5 Continued
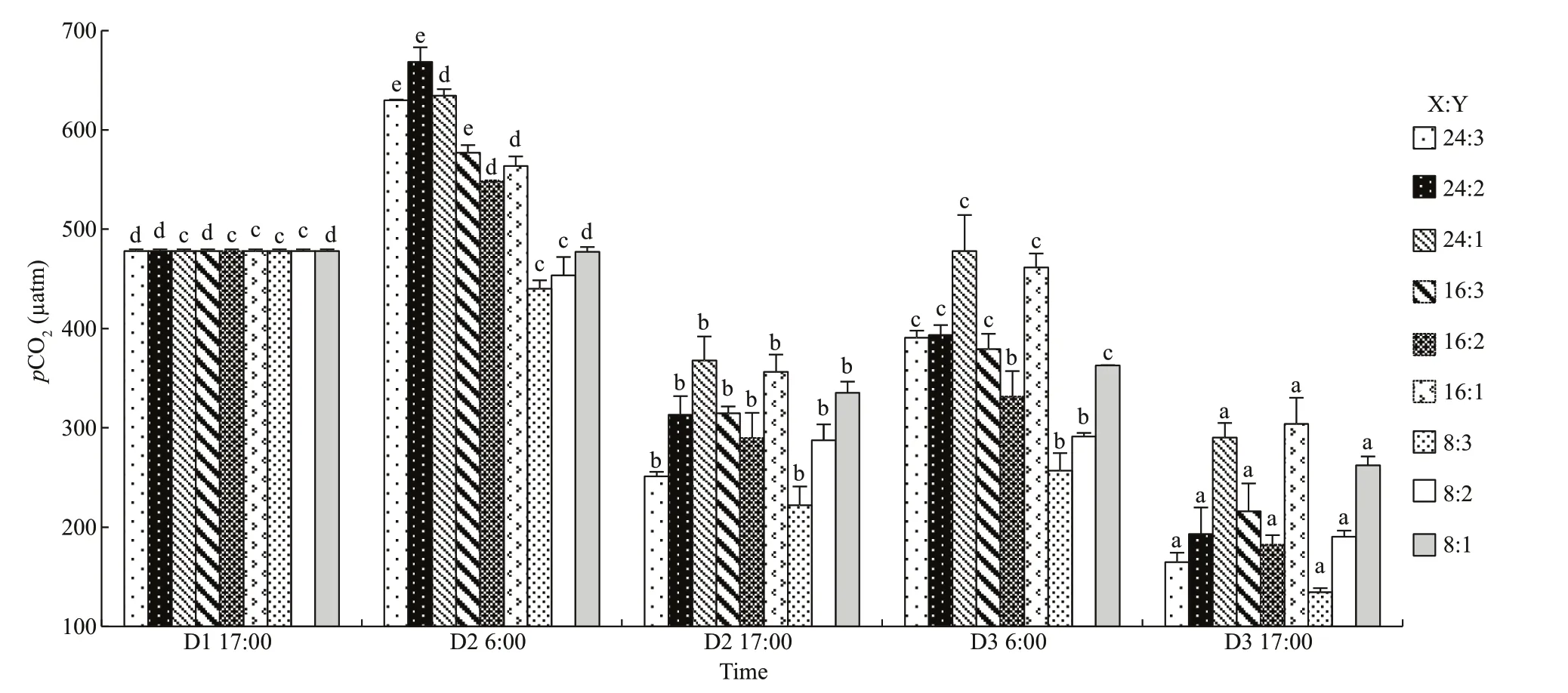
Fig.6 The fl uctuations of p CO 2 in oyster-kelp modes at diff erent time
Furthermore, IMTA system may aff ects the seawater’s carbonate system, which plays an important role in the coastal carbon cycle (Tang et al., 2011). It has been reported that the shellfi sh culture, without macroalgae, could be an appreciable net carbon source (Han et al., 2013). At the end of a 36 h-experiment, pH, and DO signifi cantly reduced and CO2concentration signifi cantly increased in shellfi sh in treatment with no seaweed. However, shellfi sh integrated with seaweed could generate a carbon sink system, which would aid in off setting the negative environmental eff ects facilitated by shellfi sh culture (Han et al., 2013). Yet, fi nding the optimal proportions of diff erent trophic species for particular IMTA systems is diffi cult. Jiang et al. (2010) published good suggestions for optimal co-cultivation proportion of caged fi sh andLaminariaorGracilariabased on the nitrogen balance in Nansha Bay, China. However, the IMTA system is a complex ecological system containing many environmental parameters. The optimal proportion of diff erent trophic species in the IMTA system depends on numerous variables that show fl uctuations during the culturing period in every mode in this study. Based on our results with respect to the variation of DO, carbonate system andpCO2, the optimal proportion of oyster and kelp was below 24:1. pH continued to increase over the time, indicating that the appropriate oyster-kelp combination could reduce CO2concentration and create a carbon sink to resist ocean acidifi cation. However, as the chl-aconcentration changes, keeping an oyster:kelp ratio of 24:1–24:3 would induce a phytoplankton bloom in the system.
Chl-acontent represents phytoplankton biomass, and phytoplankton is the main food for suspension feeders like shellfi sh (Sarà and Mazzola, 2004; Rueda et al., 2005; Liu et al., 2011). In our study, chl-aconcentration signifi cantly increased in the 24:1, 24:2, and 24:3 modes. It also increased in other modes by the end of the experiment, excepting for the 8:1 mode. Furthermore, the chl-aconcentration increased with increasing oyster biomass. The confl icts with the concept that oysters feed on the phytoplankton and occurs because the growth condition for the smaller phytoplankton improved when the oysters (C.gigas) feed on larger phytoplankton (>5 μm) as opposed to smaller ones (<5 μm). Dupuy et al. (2000) found that the percentage of picoeukaryotes retained by fi ltering oysters (C.gigas) was less than 2%, which means that oyster fi ltered the larger phytoplankton leaving the smaller cells in the column. Correspondingly, nutrients produced from the oysters could increase the phytoplankton growth. Lu et al. (2015) found that the biomass ofSynechococcus(<2 μm) increased signifi cantly after 36 h of culturing with scallop (Chlamysfarreri) in transparent polyethylene bags. P-enrichment released by scallops signifi cantly stimulated the picoplankton growth (<2 μm) by providing a food source for nanofl agellates as the <5 μm treatment also excludes the predators of 2–5 μm nanofl agellates. Thus, it is indicated that the increase in chl-acontent was due to picophytoplankton growth in the system. Therefore, in our study, nutrients released by oysters were enough for kelp absorption in diff erent modes except for the 8:1 mode. In order to simultaneously avoid eutrophication and food shortage for shellfi sh, the chl-aconcentration should be kept at a stable level. Thus, the optimal proportion of oyster and kelp for IMTA is 8:2–8:3 at the beginning of winter in Sanggou Bay.
5 CONCLUSION
In conclusion, the IMTA system is an important framework for aquaculture sustainability, as it mitigates the negative eff ects from fed aquaculture species and organic extractive species. Seaweeds, as an inorganic extractive species, play an important role in the IMTA system. More seaweeds in the IMTA system result in more nutrients and carbon source being removed from water column. The proportion of diff erent trophic species in the IMTA system is diffi cult to determine due to the fl uctuations of diff erent environmental parameters. According to the previous references and results of this study, the optimal proportion of oyster and kelp is 8:2–8:3 at the beginning of winter in Sanggou Bay, which will aid government in optimally adjusting the Sanggou Bay aquaculture structure.
6 DATA AVAILABILITY STATEMENT
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Abreu M H, Varela D A, Henríquez L, Villarroel A, Yarish C, Sousa-Pinto I, Buschmann A H. 2009. Traditional vs. integrated multi-trophic aquaculture ofGracilariachilensisC. J. Bird, J. McLachlan & E. C. Oliveira: productivity and physiological performance.Aquaculture, 293(3-4): 211-220, https://doi.org/10.1016/j.aquaculture.2009.03.043.
Ahn O, Petrell R J, Harrison P J. 1998. Ammonium and nitrate uptake byLaminariasaccharinaandNereocystisluetkeanaoriginating from a salmon sea cage farm.J.Appl.Phycol., 10(4): 333-340.
Briggs M R P, Fvnge-Smith S. 1994. A nutrient budget of some intensive marine shrimp ponds in Thailand.Aquac.Res., 25(8): 789-811.
Chang J, Tian X L, Dong S L, Wang D P, Bao J, Ma S, Sun Y C, Sun J. 2006. An experimental study on nitrogen and phosphorus budgets in polyculture of shrimp, bivalve and seaweed.Period.OceanUniv.China, 36(S1): 33-39, https://10.3969/j.issn.1672-5174.2006.z1.006. (in Chinese with English abstract)
Chopin T, Robinson S M C, Troell M, Neori A, Buschmann A H, Fang J. 2008. Multitrophic integration for sustainable marine aquaculture.In: Jørgensen S E, Fath B D eds. Encyclopedia of Ecology. Elsevier, Amsterdam. p.2 463-2 475, https://doi.org/10.1016/B978-008045405-4.00065-3.
Chopin T, Troell M, Reid G K, Knowler D, Robinson S M C, Neori A, Buschmann A H, Pang S J. 2010. Integrated multi-trophic aquaculture. Part I. Responsible practice provides diversifi ed products, biomitigation.GlobalAquac.Advocate, 5: 38-39.
Dickson A G, Goyet C. 1994. Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Sea Water, version 2. ORNL/CDIAC-74 US Department of Energy, Washington, DC. p.4-26.
Dupuy C, Vaquer A, Lam-Höai T, Rougier C, Mazouni N, Lautier J, Collos Y, Le Gall S. 2000. Feeding rate of the oysterCrassostreagigasin a natural planktonic community of the Mediterranean Thau Lagoon.Mar.Ecol.Prog.Ser., 205: 171-184, https://doi.org/10.3354/meps205171.
Fang J G, Zhang J, Xiao T, Huang D J, Liu S M. 2016. Integrated multi-trophic aquaculture (IMTA) in Sanggou Bay, China.Aquac.Environ.Interact., 8: 201-205, https://doi.org/10.3354/aei00179.
Fang J H, Zhang J H, Wu W G, Mao Y Z, Jiang Z J, Fang J G. 2014. Carbon and nitrogen budget and environmental optimization in an integrated cage culture model of Japanese fl ounder withPerinereisaibuhitensis.J.Fish.Sci.China, 21(2): 390-397. (in Chinese with English abstract)
FAO. 2016. The State of World Fisheries and Aquaculture 2016 (SOFIA): Contributing to Food Security and Nutrition for All. The State of World Fisheries and Aquaculture, Rome.
Gillibrand P A, Turrell W R, Moore D C, Adams R D. 1996. Bottom water stagnation and oxygen depletion in a Scottish sea loch.Estuar.,Coast.Shelf.Sci., 43(2): 217-235, https://doi.org/10.1006/ecss.1996.0066.
Han T, Jiang Z, Fang J, Zhang J, Mao Y, Zou J, Huang Y, Wang D. 2013. Carbon dioxide fi xation by the seaweedGracilarialemaneiformisin integrated multi-trophic aquaculture with the scallopChlamysfarreriin Sanggou Bay, China.AquacultInt., 21(5): 1 035-1 043, https://doi.org/10.1007/s10499-012-9610-9.
Inui M, Itsubo M, Iso S. 1991. Creation of a new nonfeeding aquaculture system in enclosed coastal seas.Mar.Pollut.Bull., 23: 321-325, https://doi.org/10.1016/0025-326X(91)90694-N.
Jiang Z J, Fang J G, Mao Y Z, Wang W. 2010. Eutrophication assessment and bioremediation strategy in a marine fi sh cage culture area in Nansha Bay, China.J.Appl.Phycol., 22(4): 421-426, https://doi.org/10.1007/s10811-009-9474-1.
Jiang Z J, Wang G H, Fang J G, Mao Y Z. 2013. Growth and food sources of Pacifi c oysterCrassostreagigasintegrated culture with Sea bassLateolabraxjaponicusin Ailian Bay, China.Aquac.Int., 21(1): 45-52, https://doi.org/10.1007/s10499-012-9531-7.
Langan R. 2004. Balancing marine aquaculture inputs and extraction: combined culture of fi nfi sh and bivalve molluscs in the open ocean.Bull.Fish.Res.Agen., (S1): 51-58.
Lartigue J, Sherman T D. 2005. Response ofEnteromorphasp. (Chlorophyceae) to a nitrate pulse: nitrate uptake, inorganic nitrogen storage and nitrate reductase activity.Mar.Ecol.Prog.Ser., 292: 147-157.
Liu Z L, Chen J F, Liu Y L, Gao S Q, Li H L, Zhang H S. 2011. The size-fractionated chlorophyll a concentration and primary productivity in the Bering Sea in the summer of 2008.ActaOceanol.Sin., 33(3): 148-157. (in Chinese with English abstract)
Lu J C, Huang L F, Xiao T, Jiang Z J, Zhang W C. 2015. The eff ects of Zhikong scallop (Chlamysfarreri) on the microbial food web in a phosphorus-defi cient mariculture system in Sanggou Bay, China.Aquaculture, 448: 341-349, https://doi.org/10.1016/j.aquaculture.2015.06.021.
Mao Y Z, Yang H S, Zhou Y, Hu Z F, Yuan X T, You K, Wang R C. 2006. Studies on growth and photosynthesis characteristics ofGracilarialemaneiformisand its capacity to uptake ammonium and phosphorus from scallop excretion.ActaEcol.Sin., 26(10): 3 225-3 231. (in Chinese with English abstract)
Mao Y Z, Yang H S, Zhou Y, Ye N H, Fang J G. 2009. Potential of the seaweedGracilarialemaneiformisfor integrated multi-trophic aquaculture with scallopChlamysfarreriin North China.J.Appl.Phycol., 21(6): 649-656, https://doi.org/10.1007/s10811-008-9398-1.
Mazzola A, Sarà G. 2001. The eff ect of fi sh farming organic waste on food availability for bivalve molluscs (Gaeta Gulf, central Tyrrhenian, MED): stable carbon isotopic analysis.Aquaculture, 192(2-4): 361-379, https://doi.org/10.1016/S0044-8486(00)00463-4.
Ning Z M, Liu S M, Zhang G L, Ning X Y, Li R H, Jiang Z J, Fang J G, Zhang J. 2016. Impacts of an integrated multitrophic aquaculture system on benthic nutrient fl uxes: a case study in Sanggou Bay, China.Aquac.Environ.Interact., 8: 221-232, https://doi.org/10.3354/aei00144.
Pedersen A, Kraemer G, Yarish C. 2004. The eff ects of temperature and nutrient concentrations on nitrate and phosphate uptake in diff erent species ofPorphyrafrom Long Island Sound (USA).J.Exp.Mar.Biol.Ecol., 312(2): 235-252, https://doi.org/10.1016/j.jembe.2004.05.021.
Phillips J C, Hurd C L. 2003. Nitrogen ecophysiology of intertidal seaweeds from New Zealand: N uptake, storage and utilisation in relation to shore position and season.Mar.Ecol.Prog.Ser., 264: 31-48, https://doi.org/10.3354/meps264031.
Rueda J L, Smaal A C, Scholten H. 2005. A growth model of the cockle (CerastodermaeduleL.) tested in the Oosterschelde estuary (The Netherlands).J.SeaRes., 54(4): 276-298, https://doi.org/10.1016/j.seares.2005.06.001.
Sarà G, Mazzola A. 2004. The carrying capacity for Mediterranean bivalve suspension feeders: evidence from analysis of food availability and hydrodynamics and their integration into a local model.Ecol.Modell., 179(3): 281-296, https://doi.org/10.1016/j.ecolmodel.2004.03.005.
Stenton-Dozey J. 2007. Finding hidden treasure in aquaculture waste.WaterAtmos, 15(4): 10-11.
Tang Q S, Fang J G, Zhang J H, Jiang Z J, Liu H M. 2013. Impacts of multiple stressors on coastal ocean ecosystems and integrated multi-trophic aquaculture.Prog.Fish.Sci., 34(1): 1-11, https://doi.org/10.3969/j.issn.1000-7075. 2013.01.001. (in Chinese with English abstract)
Tang Q S, Zhang J H, Fang J G. 2011. Shellfi sh and seaweed mariculture increase atmospheric CO2absorption by coastal ecosystems.Mar.Ecol.Prog.Ser., 424: 97-104, https://doi.org/10.3354/meps08979.
Troell M, Joyce A, Chopin T, Neori A, Buschmann A H, Fang J G. 2009. Ecological engineering in aquaculture—potential for integrated multi-trophic aquaculture (IMTA) in marine off shore systems.Aquaculture, 297(1-4): 1-9, https://doi.org/10.1016/j.aquaculture.2009.09.010.
Wen Y M, Wei X G, Shu T F, Zhou J F, Yu G H, Li F, Huang Y Y. 2007. Forms and balance of nitrogen and phosphorus in cage culture waters in Guangdong Province, China.Chin.Geogr.Sci., 17(4): 370-375, https://doi.org/10.1007/s11769-007-0370-9.
Wu R S S. 1995. The environmental impact of marine fi sh culture: towards a sustainable future.Mar.Pollut.Bull., 31(4-12): 159-166, https://doi.org/10.1016/0025-326X(95)00100-2.
Zhang Y. 2012. Comparison of culture eff ect, discharge of nitrogen and phosphorus and environmental infl uence for three kinds of cages. Huazhong Agriculture University, Wuhan. (in Chinese with English abstract)
Zhang Z H, Lü J B, Ye S F, Zhu M Y. 2007. Values of marine ecosystem services in Sanggou Bay.Chin.J.Appl.Ecol., 18(11): 2 540-2 547. (in Chinese with English abstract)
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Exploring sensitive area in the tropical Indian Ocean for El Niño prediction: implication for targeted observation*
- Analysis of the typhoon wave distribution simulated in WAVEWATCH-III model in the context of Kuroshio and wind-induced current*
- Characterizing the capability of mesoscale eddies to carry drifters in the northwest Pacifi c*
- Observation system simulation experiments using an ensemble-based method in the northeastern South China Sea*
- Statistical analysis of intensity variations in tropical cyclones in the East China Sea passing over the Kuroshio*
