Description of Cephalodella changdensis sp. nov. (Rotifera: Notommatidae) from Hunan Province, China*
WEI Nan , , JERSABEK Christian D. , YANG Yufeng ,
1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510530, China 2 Department of Biosciences, University of Salzburg, A-5020 Salzburg, Austria 3 Department of Ecology and Institute of Hydrobiology, Jinan University, Guangzhou 510632, China
Abstract A new freshwater rotifer Cephalodella changdensis sp. nov. discovered in two locations in Changde, Hunan, China, is described morphologically. The new species is characterized by body shape moderately elongate, double eyespots frontally, toes conical and almost straight, about one fourth of total length, trophi type B (classifi cation after Wulfert, 1937), rami asymmetrical with a pair of long alulae, fulcrum inverted T-shaped (ventral/dorsal view) with dorsally directed distal projection (lateral view), and manubria asymmetrical, left one slightly longer and stronger, with J-shaped or crutch-shaped posterior ends.
Keyword: rotifer; new species; Cephalodella changdensis sp. nov.; taxonomy; trophi asymmetrical
1 INTRODUCTION
The genusCephalodella, belonging to family Notommatidae, is one of the most species-rich genera of the Phylum Rotifera. At present, 163 valid morphospecies (including subspecies) have been documented worldwide (Nogrady and Pourriot, 1995; Segers, 2007; Jersabek and Leitner, 2013). Many species withinCephalodellashow high similarity and can be diff erentiated only by carefully examining their trophi structure, which is diffi cult by light microscopy or even by scanning electron microscopy (SEM) (De Smet and Verolet, 2016). In addition, identifi cation ofCephalodellashould be based on observation of live animals, in order to ascertain presence or absence of eyespots and to properly establish body proportions and anatomical organization (Jersabek et al., 2011). This is why, Nogrady and Pourriot (1995) considered it to be the most taxonomically diffi cult genus among all rotifers.
In China, less than 50 species ofCephalodellahave been recorded to date (Zhuge et al., 1998). Most of them are widely distributed, and onlyCephalodellaqionghaiensisKoste & Zhuge, 1998 was originally recorded in China and is still regarded as a Chinese endemic. In the present study,C.changdensissp. nov. is described from two sites in Changde City, Hunan Province, based on live animal observations and SEM examination of the trophi.
2 MATERIAL AND METHOD
Rotifers were collected on fi ve occasions during diff erent seasons in 2017 (March 20, September 16, and December 13) and 2018 (June 22 and September 19) from two sites in the Huashan area at the foot of Taiyangshan Hill, Changde City, Hunan Province, South China. One was in a fast-fl owing roadside creek, with abundant but patchily distributed submerged plantLimnophilasp. The other was in a small roadside concrete ditch, with fast-fl owing shallow water and unidentifi ed weeds on the bottom.
Plankton samples from the two sites were collected using a nylon net with 30-μm mesh size. Periphytic rotifers from the roadside creek site were sampled by rinsing submerged plants in a 30-μm mesh sieve using fi ltered water which was prepared on the spot by fi ltering the site water using a 30-μm net. From each sample two subsamples were taken, one for live observation, and the other was preserved in 4% formaldehyde. A YSI-Pro Plus®multiparameter instrument (YSI, USA) was pre-calibrated and used for measuring in situ water temperature, salinity, pH, and dissolved oxygen.
Live samples were brought to the laboratory, and observed immediately using a BX51 microscope®(Olympus, Japan) equipped with a TrueChrome Metrics®camera (Tucsen, China) at a magnifi cation of (400–1000)×. Preparation of trophi for light and scanning electron microscopy (SEM) was done following De Smet (1998), using a 10% NaOCl solution to dissolve the soft parts. For taking scanning electron micrographs, an EVO MA15®(ZEISS, Germany) microscope operated at 20 kV was used. Measurement of animals was done by using Tcapture®imaging software. Trophi elements were measured by ImageJ 1.46r (https://imagej.nih.gov/ij/) (Abràmoff et al., 2004). All measurements are presented as mean ± standard deviation (SD),n= number of individuals measured. Permanent mounts of voucher specimens were prepared in glycerine glass slides according to Jersabek et al. (2010).
The classifi cation ofCephalodellatrophi follows Wulfert (1937, 1938) and Fischer and Ahlrichs (2011), while terminology of trophi structures follows De Smet and Verolet (2016).
3 TAXONOMY
Phylum ROTIFERA Cuvier, 1812
Class EUROTATORIA De Ridder, 1957
Subclass MONOGONONTA Plate, 1889
Superorder PSEUDOTROCHA Kutikova, 1970
Order PLOIMA Hudson & Gosse, 1886
Family NOTOMMATIDAE Hudson & Gosse, 1886
GenusCephalodellaBory de St. Vincent, 1826
Cephalodellachangdensissp. nov.
3.1 Type locality
A fast- fl owing roadside creek (29°7′57.81″N, 111°40′30.58″E) and a small roadside concrete ditch (29°7′20.46″N, 111°39′49.19″E), near Maojiashan Village in Dingcheng District, Changde City, Hunan Province, China.
3.2 Type material
Holotype: a female in a permanent glycerine glass slide mount deposited in the Museum of Biology, Sun Yat-sen University, Guangzhou, China (SYS ROT00019).
Paratypes: four females from type locality, in a permanent glycerine slide mount each. Two females in the Museum of Biology, Sun Yat-sen University, Guangzhou, China (SYS ROT00020, SYS ROT00021); one female in the Museum of Hydrobiological Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China (MHBS R GD 2019010003); one female in the Academy of Natural Sciences of Drexel University, Philadelphia, USA (ANSP 2139).
Additional material: numerous specimens from the roadside creek site preserved in 4% formaldehyde solution with added glycerine in Eppendorf vials, all kept in the collection of the fi rst author at South China Sea Environmental Research Center in South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou, China.
3.3 Diagnosis
Female about 150–160-μm long. Body moderately elongate, weakly arched dorsally, almost fl at ventrally, slightly compressed laterally. Head large, in average 26% of body length. Foot short, in average 12% of body length. Tail relatively large, almost as long as foot. Toes medium-sized, in average 24% of total length, near conical, evenly tapering into acutely pointed tips, straight or very weakly recurved dorsally. Two frontal red eyespots. Trophi type B, moderately asymmetrical, with more strongly developed left side. Rami with two acutely pointed, long alulae and 9–10/7 (left/right) teeth on inner margins. Fulcrum relatively long, inverted T-shaped (ventral/dorsal view), with dorsally recurved posterior half and large distal projection (lateral view). Manubria asymmetrical, right side shorter and weaker, lacking typical lamellae on shaft, J-shaped or crutch-shaped in posterior half.
3.4 Etymology
This species is named after the city of Changde, Hunan Province, China, where the new species was found.
3.5 Description of female
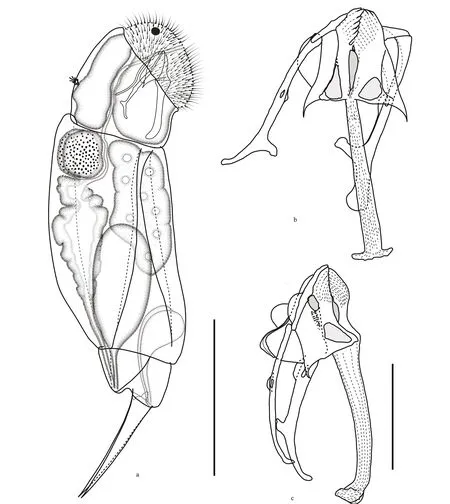
Fig.1 Cephalodella changdensis sp. nov.
Body (Figs.1a & 2) moderately elongate and slightly compressed laterally, ventral margin almost straight or weakly curved, dorsal margin slightly gibbous when not well-extended. Lorica soft and smooth, trunk with three ill-defi ned plates, two dorsolaterally, one ventrally. Ventral and dorsal plates separated by wide, posteriorly fl aring lateral sulci. Connecting folds of plates conspicuous in wellextended animals. Dorsal plates separated by distinct V-shaped dorsal sulcus. Head relatively large, with average length 26% (22%–28%) of body length, widest posteriorly in dorsal/ventral view, weakly defl exed, off set by distinct neckfold, occasionally with inconspicuous lips. Corona strongly oblique, markedly convex, often with protruding epipharyngeal elements. Dorsal antenna in posterior third of head. Foot short, with average length 12% (12%–13%) of body length, very slightly defl exed, trapezoidal in lateral view. Tail relatively large, almost as long as foot segment, with well-developed fold in dorsal. Toes medium-sized, with average length 24% (23%–25%) of total length, almost conical, broadest at their base in lateral view, evenly tapering into sharply pointed tips, with almost straight ventral margin, and straight or very weakly recurved dorsal margin. Brain long, saccate, extending till neckfold, connecting to a pair of red-pigmented frontal eyespots. No retrocerebral organ. Mastax large, slightly shorter than head. Oesophagus longer than mastax. Gastric glands irregularly spherical in dorsal/lateral view, full of rounded glandular granula. Stomach yellowish, wall thick, indistinctly separated from moderately long intestine. Anus underneath tip of tail. Protonephridial bladder very large when completely fi lled. Vitellarium with 8 nuclei. Pedal glands large, near comma-shaped in lateral view, extending into trunk.

Fig.2 Light microscope photographs of Cephalodella changdensis sp. nov.
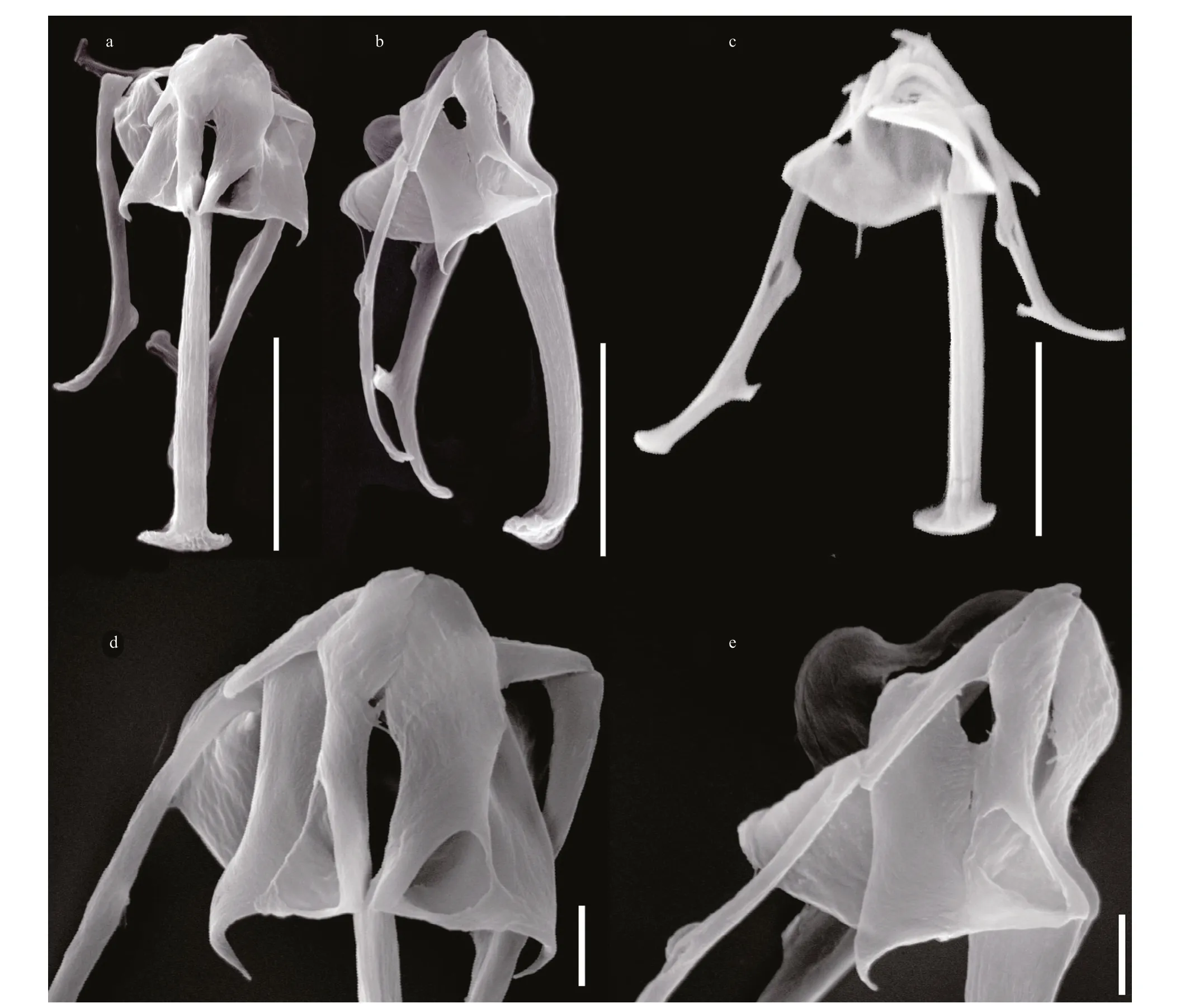
Fig.3 Cephalodella changdensis sp. nov., scanning electron microscope photographs of trophi
Trophi (Figs.1b, 1c & 3) virgate, type B according to Wulfert (1937, 1938) and Fischer and Ahlrichs (2011), moderately asymmetrical, with more strongly reinforced left side. Rami composed of subbasal, basal and dorsal chambers. Subbasal rami chambers strong asymmetrical. Right side fairly narrow, with larger, postero-anteriorly elongated triangular fenestra. Left side relatively large and broad, with smaller rounded-triangular fenestra. Left/right rami tip with 9–10/7 well-fused teeth on inner margin. Basal rami chambers shallow with large fenestrae opening ventro-laterally, dorsal margin of chambers extending into acute and long, posteriorly pointed alulae. Dorsal chambers very shallow with very large lateral fenestrae, dorsal margin of chambers with inconspicuous fl abella, ribbon-like shaft, composed of fused sclerite elements. Fulcrum very long, narrow proximally in ventral view, gradually thickening towards distal part and widely extended bilaterally at distal end, giving it an inverted T-shaped appearance; in lateral view widest proximally, slightly narrower and with curved ventral margin towards distal part, strongly recurved dorsally at distal end. No basal fulcrum apophysis. Unci single-toothed, acute rodshaped with broadly expanded dorsal lamina. Left uncus stronger than right one. Tooth slightly longer than shaft. Manubria asymmetrical. Left manubrium slightly longer and stronger, with straight anterior half, a longitudinal fenestra in median chamber, small semicircular lamellae in dorsal manubrial chamber, and crutch-shaped posterior half. Right manubrium shorter and more slender, with anterior half and manubrial foramen similar as in left manubrium, but posterior J-shaped or crutch-shaped. Pleural rod long, S-shaped in lateral view.
Male and eggs remain unknown.
3.6 Measurement
Body (n= 8 swimming females): total length 150.1–158.4 μm (mean= 153.3±3.7 μm,n= 8), body length (excl. toes) 111.5–120.1 μm (mean= 116.8±4.1 μm,n= 8), head length 24.5–33.2 μm (mean= 29.9±3.5 μm,n= 8), head height 31.5–34.0 μm (mean= 32.7±1.0 μm,n= 8), trunk length 68.8–73.2 μm (mean= 71.2±1.9 μm,n= 8), trunk width 38.2–41.8 μm (mean= 40.0±1.8 μm,n= 3), foot length 13.9–14.7 μm (mean= 14.5±0.4 μm,n= 8); toe length 35.5–37.6 μm (mean= 36.5±0.9 μm,n= 8), ratio of total length/toe length 4.0–4.3 (mean= 4.2±0.1,n= 8).
Trophi: ramus length 8.3–8.9 μm (mean = 8.6±0.2 μm,n= 5), ramus width (incl. alulae) 10.9–11.1 μm (mean= 11.0±0.1 μm,n= 2), fulcrum length 15.6–17.7 μm (mean= 16.7±0.8 μm,n= 5), fulcrum width 3.9–4.6 μm (mean= 4.3±0.4 μm,n= 3), right uncus length 6.9–7.7 μm (mean= 7.1±0.4 μm,n= 4), right manubrium length 14.5–16.3 μm (mean= 15.2±0.7 μm,n= 5), left manubrium length 16.0–18.5 μm (mean= 17.7±1.1 μm,n= 4), right manubrium crutch length 5.4–6.0 μm (mean= 5.6±0.3 μm,n= 4), left manubrium crutch length 7.1–7.5 μm (mean = 7.2±0.1 μm,n= 5).
3.7 Distribution and ecology
To date,Cephalodellachangdensissp. nov. is only known from the two sites in Changde City, collected on fi ve occasions during diff erent seasons in 2017 and 2018. It was present four times (except June 22, 2018) in plankton samples and two times (September 16, 2017 and September 19, 2018) in periphyton samples (from the submerged macrophyteLimnophilasp.) from the creek, while a perennial occurrence of the species was observed in plankton samples from the ditch. Physico-chemical characteristics of the waters in both sites were: water temperature 9.4–27.5°C, salinity 0.09–0.28, pH 5.34–7.87, dissolved oxygen 7.50–12.00 mg/L.
3.8 Comparison with related species
The peculiar shape of the fulcrum inCephalodellachangdensissp. nov., being relatively long, inverted T-shaped (ventral/dorsal view) with large dorsally extended distal projection (lateral view), can hardly be confused with other congeners.
Based on the revision of the genusCephalodellaby Nogrady and Pourriot (1995),C.changdensissp. nov. keys out toC.pachydactylaWulfert, 1937. It however diff ers fromC.pachydactylain the following aspects: (1) body moderately elongate, without retrocerebral organ and salivary glands—compared to a more sturdy body, with retrocerebral organs and several small plus one large salivary glands inC.pachydactyla; (2) toes conical, evenly tapering to acute tips, straight or weakly recurved dorsally, whereas they are stout, with tips abruptly tapering and then curved dorsally inC.pachydactyla; (3) fulcrum inverted T-shaped (ventral view), with dorsally recurved distal part terminating in a large bilaterally extended projection (lateral view), while the fulcrum distal end is spatulate (both in ventral and lateral views) inC.pachydactyla; (4) manubria clearly asymmetrical, with the left side more strongly reinforced, while they are symmetrical, carrying diff erently shaped and relatively larger crutches inC.pachydactyla; and (5) inner rami margins with 9–10/7 (left/right) teeth—but no teeth recognizable inC.pachydactyla.
Owing to the presence of double frontal eyespots and type B trophi with crutched manubria,Cephalodellachangdensissp. nov. also bears superfi cial similarity with its congenersC.cyclopsWulfert, 1937 andC.sterea(Gosse, 1887). However,C.changdensissp. nov. can be easily distinguished fromC.cyclopsby the two separated eyespots, the relatively longer toes (1/3>toe length/total length >1/5), the inverted T-shaped fulcrum (ventral view) and the asymmetrical manubria, whereas both eyespots within a common capsule, shorter toes (toe length/total length <1/5), a spatulate distal fulcrum end (ventral view) and almost symmetrical manubria can be recognized inC.cyclops. It is distinct from taxa currently attributed to the highly variableC.sterea, a complex mainly in morphology of the trophi: rami with a pair of long pointed alulae and manubria only with small semicircular lamellae are characteristics of the new species, whereas only the left ramus carrying a small alula, and manubria forming long lamellae that cover at least half of the shaft are typical features inC.sterea, in addition to the diff erently formed fulcrum, inverted T-shaped with dorsally recurved distal part inC.changdensissp. nov. of the usual form (straight in lateral view, spatulate distal end) inC.sterea. Moreover, eyespots generally lie in a single capsule inC.sterea, but they are separate inC.changdensissp. nov. Another species that shows superfi cial resemblance to the new species, in terms of trophi morphology, isC.dentataWulfert, 1937. Both species have long alulae on both rami and asymmetrical manubria without distinct lamellae, but they can safely be diff erentiated by the absence of eyespots and the more slender toes inC.dentata.
4 DATA AVAILABILITY STATEMENT
The authors declare that the data supporting the fi ndings of this study are available within the article. The original data of measurements will be available on request from the fi rst author.
5 ACKNOWLEDGMENT
The authors would like to thank LONG Aihong, YU Wenbo and LIANG Diwen for their kind help in sample collection. Comments and suggestions of the reviewers are greatly appreciated.
References
Abràmoff M D, Magalhães P J, Ram S J. 2004. Image processing with ImageJ.BiophotonicsInternational, 11(7): 36-42.
De Smet W H, Verolet M. 2016. Epibiotic rotifers ofGammaruspulex(L.) (Crustacea, Amphipoda), with descriptions of two new species and notes on the terminology of the trophi.Zootaxa, 4107(3): 301-320, https://doi.org/10. 11646/zootaxa.4107.3.1.
De Smet W H. 1998. Preparation of rotifer trophi for light and scanning electron microscopy.Hydrobiologia, 387-388: 117-121, https://doi.org/10.1023/A:1017053518665.
Fischer C, Ahlrichs W H. 2011. Revisiting theCephalodellatrophi types.Hydrobiologia, 662(1): 205-209, https://doi.org/10.1007/s10750-010-0497-z.
Gosse P H. 1887. Twenty-four more New Species of Rotifera.JournaloftheRoyalMicroscopicalSociety, 7(6): 861-871, https://doi.org/10.1111/j.1365-2818.1887.tb01590.x.
Jersabek C D, Bolortsetseg E, Taylor H L. 2010. Mongolian rotifers on microscope slides: instructions to permanent specimen mounts from expedition material.MongolianJournalofBiologicalSciences, 8(1): 51-57, https://doi.org/10.22353/mjbs.2010.08.06
Jersabek C D, Leitner M F. 2013. The Rotifer World Catalog. World Wide Web electronic publication. http://www.rotifera.hausdernatur.at/. Accessed on 2019-03-20.
Jersabek C D, Weithoff G, Weisse T. 2011.Cephalodellaacidophilan. sp. (Monogononta: Notommatidae), a new rotifer species from highly acidic mining lakes.Zootaxa, 2939(1): 50-58, https://doi.org/10.11646/zootaxa.2939.1.2.
Nogrady T, Pourriot R. 1995. Family Notommatidae.In: Rotifera. Volume 3: The Notommatidae and the Scaridiidae.In: Nogrady T ed. Guides to the Identifi cation of the Microinvertebrates of the Continental Waters of the World. SPB Academic Publishing, Amsterdam, The Hague. p.1-229, 239-248.
Segers H. 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution.Zootaxa, 1564(1): 1-104, https://doi.org/10. 11646/zootaxa.1564.1.1.
Wulfert K. 1937. Beiträge zur Kenntnis der Rädertierfauna Deutschlands. Teil III.ArchivfürHydrobiologie, 31: 592-635.
Wulfert K. 1938. Die RädertiergattungCephalodellaBory de St. Vincent. Bestimmungsschlüssel.ArchivfürNaturgeschichte, 7: 137-152.
Zhuge Y, Huang X F, Koste W. 1998. Rotifera recorded from China, 1893-1997, with remarks on their composition and distribution.InternationalReviewofHydrobiology, 83(3): 217-232, https://doi.org/10.1002/iroh.19980830305.
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Exploring sensitive area in the tropical Indian Ocean for El Niño prediction: implication for targeted observation*
- Analysis of the typhoon wave distribution simulated in WAVEWATCH-III model in the context of Kuroshio and wind-induced current*
- Characterizing the capability of mesoscale eddies to carry drifters in the northwest Pacifi c*
- Observation system simulation experiments using an ensemble-based method in the northeastern South China Sea*
- Statistical analysis of intensity variations in tropical cyclones in the East China Sea passing over the Kuroshio*
