Occurrence and light response of residual plastid genes in a Euglena gracilis bleached mutant strain Ofl B2*
QIN Huan , GUO Qingqing , LIU Chenchen , LI Fenglan , ZHANG Hua , CHU Zihan , WANG Jiangxin , LEI Anping ,
1 Shenzhen Key Laboratory of Marine Bioresource and Eco-environmental Science, Shenzhen Engineering Laboratory for Marine Algal Biotechnology, Guangdong Provincial Key Laboratory for Plant Epigenetics, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518000, China
2 Basis International School SZ, Shenzhen 518000, China
Abstract Euglena gracilis is a unicellular green eukaryotic microalga that features characteristics of both plants and animals. The photosynthetic function of its chloroplast is easily lost under stress resulting in bleached mutants, while the physiological role of their residual plastid DNAs remains unclear. In this study, we obtained fi ve bleached mutants by ofl oxacin (Ofl ) treatment, identifi ed 12 residual plastid genes in fi ve bleached mutants, and determined the mRNA levels in the wild type E. gracilis (WT) and one bleached mutant (Ofl B2) under dark and light stimulation conditions by quantitative reverse transcribed PCR (qRTPCR). Results show that the expression of all selected plastid genes in both WT and Ofl B2 mutant did not change signifi cantly in darkness, while their responses to light stimulation were diff erent. Under the light stimulation conditions, half of the genes did not change signifi cantly, while most of the other genes were down-regulated in Ofl B2 mutant and up-regulated in WT. Therefore, the bleached mutant retains part of the plastid genome and the plastid relic is responsive to light. Our research will help to understand the functions of residual plastid DNA and evolution of chloroplasts.
Keyword: Euglena gracilis; bleached mutant; residual plastid genes; light response
1 INTRODUCTION
Euglenais a unicellular protist that shows characteristics of both plants and animals (Bodyl, 1996). MostEuglenaspecies contain fully developed chloroplasts when autotrophically grown through the photosynthesis under light, and they can also heterotrophically grow by absorbing organic matters in the dark (Tucci et al., 2010). The chloroplasts ofEuglenaare believed to be originated from a unicellular green alga endosymbiosis (Sulli et al., 1999; Ahmadinejad et al., 2007; Yoshida et al., 2016; Zakryś et al., 2017). Unlike chloroplasts of other algae and higher plants, the chloroplasts ofEuglenahave poor stability and are easy to lose under stresses, such as antibiotics, heat, UV, etc., but retain parts of the plastid genome (Kivic and Vesk, 1974; Heizmann et al., 1981; Wang et al., 2002, 2004).Euglenacells that lost a part of their photosynthesis-related genes become bleached mutants and can stably grow and multiply (Gockel et al., 2000). Therefore,Euglenais an ideal model species for studying the function, development, and endosymbiosis of chloroplast.Euglenagracilisis the most popular and typical species, and it is usually chosen as a model forEuglena.
WhenEuglenagrows in the dark, its chloroplasts are poorly developed. Once returned to light,Euglenaturns green, reestablishes its chloroplasts, and begins to function again as a photosynthetic organism (Kivic and Vesk, 1972). In addition, when treated with UV or ofl oxacin (Ofl ),E.graciliscells will permanently lose their chloroplasts’ photosynthetic functionality and become bleached mutants, while most mutants will still have residual plastids and partial plastid genomes (Sulli et al., 1999; Ahmadinejad et al., 2007; Yoshida et al., 2016; Zakryś et al., 2017). Residual plastids were observed in a bleached mutant ofE.gracilisby transmission electron microscopy (Kivic and Vesk, 1974; Wang et al., 2004), and a deleted chloroplast genome was further demonstrated by Heizmann's hybridization method (Heizmann et al., 1976, 1982; Hussein et al., 1982). Moreover, chloroplast rRNA was detected in 25 bleached mutants, and the majority of these rRNAs are similar in number to those found in wild typeE.gracilis(Heizmann et al., 1976, 1982; Hussein et al., 1982).
Euglenagracilisplastid genome consists of 143 170 bp, a total of 97 proteins and gene loci, including 46 protein-coding genes, and 51 RNAcoding genes (Bennett and Triemer, 2015; Hadariová et al., 2017). Except for the presence of three copies of the rRNA gene, each gene is present in a single copy matter (Hallick et al., 1993; Hadariová et al., 2017).Euglenalongais a naturally-occurring, colorless, non-photosynthetic euglenoid, which looks like bleachedE.gracilisin appearance. The discovery of a circular 73-kb plastid genome inE.longa, which is about half the size of the circular 143 kb inE.gracilis, suggested that the colorlessE.longacould not simply be a naturally bleachedE.gracilis(Gockel et al., 1994; Eberhard et al., 2002). All the genes encoding photosynthetic proteins were lost from theE.longaplastid genome except for therbcLgene encoding the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (Gockel et al., 1994). Furthermore, the divergence between the plastid genomes inE.gracilis, andE.longaappears to have involved in a selective loss and rearrangement ofE.longaplastid genes rather than an expected random loss (Siemeister and Hachtel, 1989; Gockel et al., 1994; Eberhard et al., 2002). It is essential to understand if the loss of plastid genes in the bleached mutant ofE.graciliswas selective.
Most previous studies on the bleached mutants ofE.gracilisfocused on the presence or absence of plastid genomes, and few have studied the plastid genes and their functions (Buetow, 1982; Vesteg et al., 2009). To understand the function of residual plastid DNA, the present study compared the expression level of their genes in WT and bleached mutant under light stimulated conditions.
2 MATERIAL AND METHOD
2.1 Strains and culture conditions
EuglenagracilisCCAP 1224/5Z was purchased from CCAP (Culture Collection of Algae and Protozoa, UK) and maintained in our laboratory. WT and the bleached mutants were grown in EG medium (Supplementary Table S1) under the condition of 12 h/12 h light (100±10 μmol/(m2·s)/dark at 22°C. Solid plates were supplemented with 2% agar.
2.2 Experimental set-up
To obtain the bleached mutants, Ofl was added to the medium to a fi nal concentration of 100 μmol/L, and 100 cells were spread on the EG solid medium plate. After 7 d, fi ve white mutants (Ofl B1, Ofl B2, Ofl B3, Ofl B4, and Ofl B5) were picked from the plate and transferred to liquid EG medium for continuous cultivation. These bleached colonies have been cultivated for more than 2 years in our lab with no reversed phenotype (greening), indicating the phenotypic stability of these mutants, and that the bleaching was permanent.
The growth curves of the WT and fi ve bleached mutants ofE.graciliswere monitored. The fi ve mutants were further confi rmed bleached by UVvisible spectrophotometer, and their residual plastid genes were determined by PCR. Quantitative real time PCR (qRT-PCR) was used to obtain expression level of the plastid residual genes in WT and one of the bleached mutant (Ofl B2), which were grown for 5 d under dark conditions and then remained in the dark or stimulated by light conditions for 0.5 and 3 h. Thus, fi ve treatments were labeled as D0, D0.5, D3, L0.5, and L3. All the control and experimental samples were in triplicates.
2.3 Detection of plastid genes with PCR
The total DNA of WT and the fi ve mutants was extracted using the TransGen Biotech DNA isolation Kit (EE141-01). Briefl y, cells grown at the log phase were harvested by centrifugation at 8 000×gfor 2 min, and resuspended in 400 μL of Plantzol containing 10 μL of Proteinase K and 10 μL of RNase A (10 mg/mL). The suspension was then incubated at 55°C for 0.5 h to completely lyse the cells. An equal volume of phenol:chloroform:isoamyl alcohol (25:24:1) was added, vortexed and mixed, centrifuged at 12 000×gfor 5 min, and the supernatant was removed to a new Eppendorf tube. An equal volume of isopropanol was added, vortexed well and centrifuged at 12 000×gfor 15 min at 4°C. The precipitated DNA was washed with 70 % ethanol and resuspended in 50 μL deionized water for further use.
Primers were designed for PCR and qRT-PCR according to the chloroplast genome ofE.gracilis(Hallick et al., 1993) and were synthesized by Guangzhou Ige Biotechnology Company (Supplementary Table S2). A total of 50 μL of PCR reaction system was set up: 25 μL of 2×Es Taq MasterMixa (Dye), 1 μL of upstream primer (10 μmol/L), 1 μL of downstream primer (10 μmol/L), 2 μL of 300 ng/μL DNA template, and 21 μL of sterilized ddH2O. The PCR amplifi cation was started with denaturation at 95°C for 3 min, followed by 35 cycles of 10 s at 95°C, 20 s at 56°C, and 10 s at 72°C, and a fi nal extension of 5 min at 72°C. The PCR products ofrpl16-rpl5,rpl5-rps14andrps8-rps2from WT and mutants were purifi ed and sequenced by Guangzhou Ige Biotechnology company.
2.4 qRT-PCR
qRT-PCR was conducted to obtain expression level of the plastid residual genes of WT and Ofl B2 bleached mutant:rps2,rps3,rps8,rps14,rpl5,rpl14,psbA,psaC,petB,rbcL, and5s rRNA. NuclearActingene was used as controls.
Total RNAs of WT and Ofl B2 mutant were extracted using the TaKaRa RNAiso Plus Kit (D9108A). Briefl y, cells were harvested by centrifugation at 8 000×gfor 2 min, and resuspended in 1 mL of RNAiso Reagent. 200 μL of chloroform (1/5 volume of RNAiso Reagent) was added, vortexed for 15 s and stood at room temperature for 5 min, centrifuged at 12 000 r/min for 15 min at 4°C, and the supernatant was transferred to another new Eppendorf tube. An equal volume of isopropanol was added, mixed gently and stood at room temperature for 10 min, centrifuged at 12 000×gfor 10 min at 4°C, and the supernatant was removed. The precipitated RNA was washed with 75% ethanol, resuspended in 20-μL RNase-free water, and stored at -80°C if not used immediately. Three microgram RNA of each sample was quantitatively reverse transcribed into cDNA using TaKaRa’s PrimeScriptTMRT reagent Kit with gDNA eraser (RR047A). The cDNA of each sample was used for Quantitative PCR (qPCR).Actingene fromE.graciliswas used as an internal control for the data normalization. qPCR reaction system was set up: 5-μL Fast SYBRTM Green Master Mix (2x), 0.8-μL upstream primer (10 μmol/L), 0.8-μL downstream primer (10 μmol/L), 3-μL template, and 0.4 μL of sterilized ddH2O. The profi le of qPCR included 40 cycles of 95°C for 15 s and 60°C for 30 s. Each qRT-PCR measurement was performed in triplicates. Expression analysis was performed by the 2-ΔΔCtmethod using the analysis software provided by the Bio-Rad qPCR instrument.
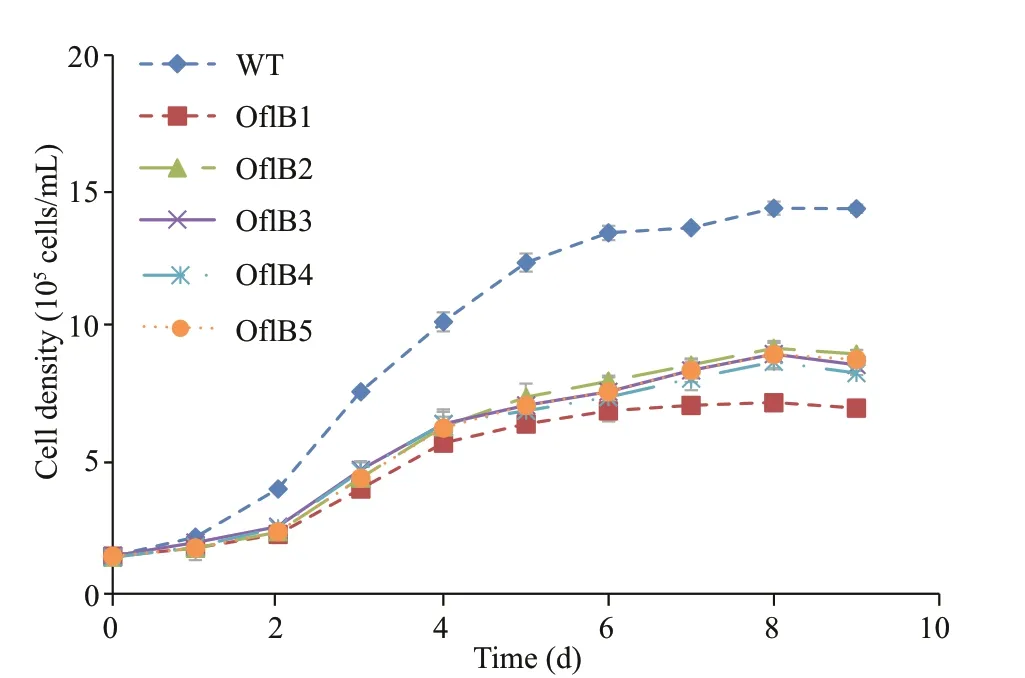
Fig.1 Growth curves of WT and the fi ve mutants (Ofl B1-5)
2.5 Statistical analysis
The data of light/dark and diff erent time treatments of WT and Ofl B2 mutant were analyzed using a parametric two-way analysis of variance (ANOVA) (light/dark treatmentn=2, time treatmentn=3). To compare all diff erences among three times during light and dark treatment, one-way analysis of variance (ANOVA) was used. The statistical analysis was carried out by SPSS 17.0 for windows.
3 RESULT
3.1 Characteristics of the bleached mutants of E. gracilis
All the fi ve mutants (Ofl B1, Ofl B2, Ofl B3, Ofl B4 and Ofl B5) presented similar growth curves but their growths were obviously lower than that of WT (Fig.1). All samples were inoculated at the same initial cell density of 1.4×105cells/mL, but since the mutants seemed to experience a more obvious lagged growth before entering into the logarithmic growth phase, the cell density of WT was much higher than that of the mutants from day 2 to 8 (Fig.1). The highest cell density of WT (1.36×106cells/mL) was achieved at the end of the logarithmic phase at day 8 and was much higher than that of the mutants, which was only 0.9×106cells/mL for the Ofl B2 mutant (Fig.1).
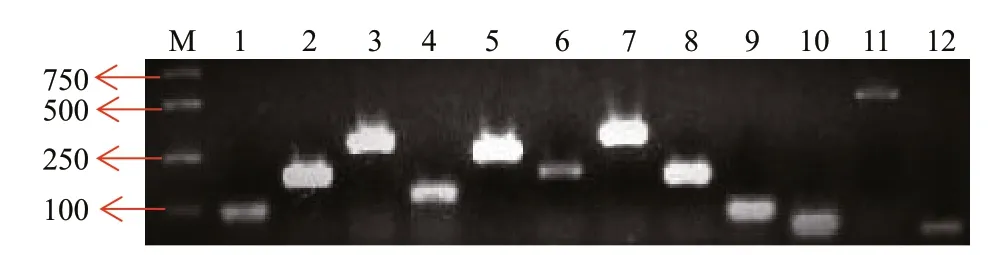
Fig.2 Detection of residual plastid genes in Ofl B2 mutant
UV-visible spectrophotometer was used to scan the pigment absorption spectrum of WT and the fi ve mutants. Results showed that WT had high absorption in the blue and red regions (sharp absorption peaks at 433 nm and 665 nm) (Supplementary Fig.S1), which coincided with the absorption spectrum of intact chloroplast that contains predominantly chlorophyll and some carotenoids. However, no absorption peak spectrum was observed in all fi ve mutants.
3.2 Detection of plastid genes with PCR
Considering the bleached phenotype of these mutants, it would be interesting to examine whether and how many genes were preserved in the fi ve mutants. We fi rst examined 50 chloroplast-relevant genes (Supplementary Table S2) in a randomlyselected mutant Ofl B2 using PCR, and 13 genes (rps2,rpl5,rps14,petB,psbA,5s rRNA,rpl14,rps3,psaC,rpl16,rps8,actin, andrbcL) (Supplementary Table S2) were detected (Fig.2). Among these genes,rps2,rps3,rps8,rps14,rpl5,rpl14andrpl16encode for ribosomal proteins,psaCandpsbAfor photosynthetic proteins,petBfor b6 protein and the core component of the cytochrome b6f protein complex, andrbcLfor the large subunit of RuBisCO. We did not test the other 37 genes in all the other 4 mutants, while we focused on the 13 genes existed in Ofl B2. So we then examined these 13 genes in the other four mutants (Ofl B1, Ofl B3, Ofl B4, and Ofl B5), and the results indicated the existence of these genes in all mutants (Fig.3).
Among the 12 genes detected, 6 genes (rpl16,rpl14,rpl5,rps8,rps14, andrps2) were closely distributed to each other, which made it possible to explore therpl16-rps2inter-regions between these 6 genes with appropriate primers (Supplementary Fig.S2). These regions of WT and Ofl B2 were therefore amplifi ed with the primer pairs ofrpl16-rpl5,rpl5-rps14, andrps8-rps2, and the PCR products were sequenced and assembled, indicating that the lengths ofrpl16-rps2region in WT and Ofl B2 mutant were 4 122 bp and 4 114 bp, respectively (Supplementary Sequence S1). Alignment analysis with BLAST showed that the similarity between the two sequences were 0.99, and 51 mutations with 10 deletions/insertions and 41 point mutations existed in Ofl B2 mutant compared with WT. Interestingly, most of these mutations existed in therps8-rps2region (Supplementary Sequence S1).
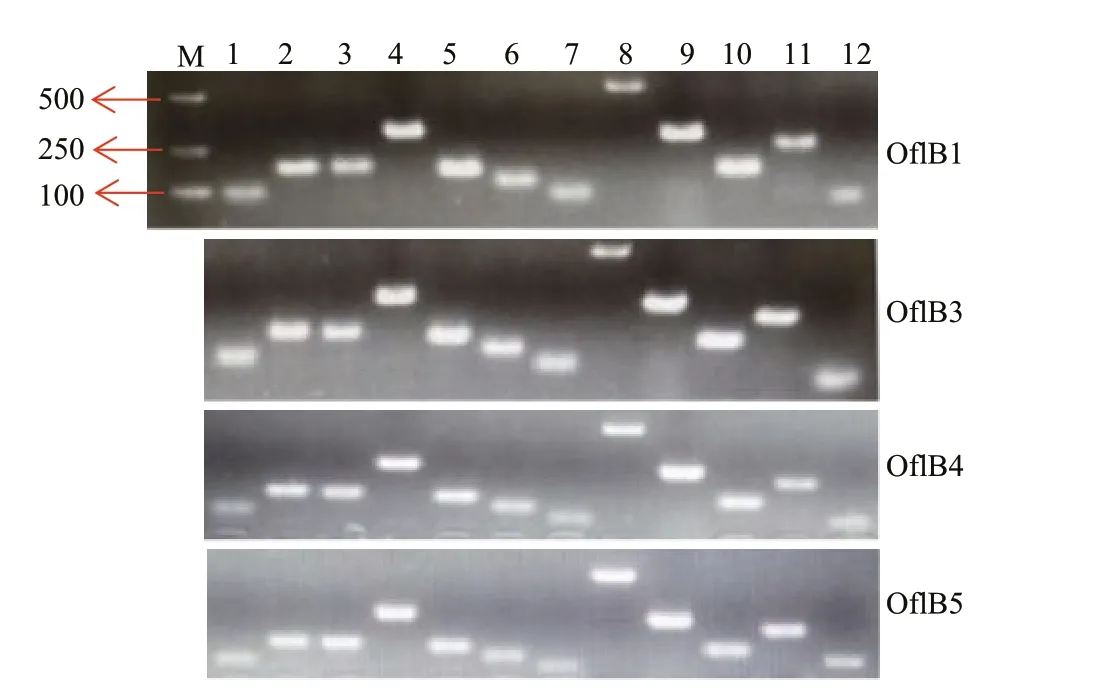
Fig.3 Detection of residual plastid genes in Ofl B1, Ofl B3, Ofl B4, and Ofl B5 mutants
3.3 Expression of residual plastid genes
The transcription levels of 11 plastid genes (rps2,rps3,rps8,rps14,rpl5,rpl14,psbA,psaC,petB,rbcL, and5s rRNA) (expectrpl16, due to its low expression level) in WT and Ofl B2 mutant under dark or lightstimulation conditions were analyzed by qRT-PCR. According to the two-way ANOVA test, eff ects of light-dark and diff erent treatment time on and their interactions with the expression of residual plastid genes were gene specifi c (Table 1). The light-dark eff ect in WT was only signifi cant on fi ve genes, which wererpl14,rpl5,rps14,5s rRNA, andpsbA; and the time eff ects in WT were signifi cant onrpl14,rpl5,rps14, andpetB. The light-dark eff ect in Ofl B2 was signifi cant on seven genes, includingrps3,rps8,rpl5,rps14,5s rRNA,psbA, andpetB, and the time eff ects in Ofl B2 were signifi cant onrps2,rps8,rpl5,5s rRNA, andpetB. The light-dark or time showed insignifi cant eff ects on the other genes.
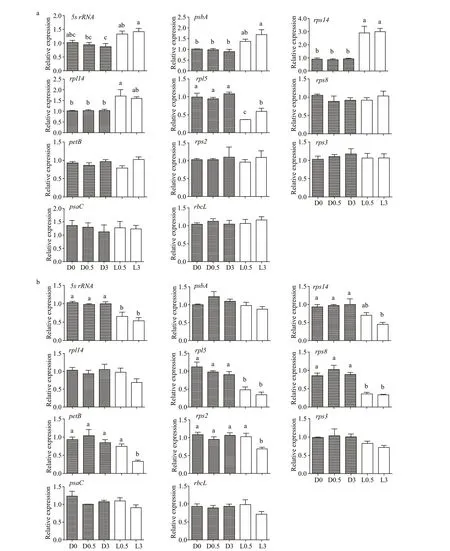
Fig.4 Expression of residual plastid genes in WT (a) and Ofl B2 (b) under dark (D) or light (L) conditions for 0.5 and 3 h (mean and standard deviation, n=3)
Results of multiple comparisons showed that the expression of all the 11 plastid genes in WT and Ofl B2 mutant did not change signifi cantly in the dark, and then continued in the dark for 0.5 h and 3 h (Fig.4a & b) (P>0.05), indicating that the expression of these genes were stable in dark conditions, whereas response to light stimulation varied among the genes and was diff erent between WT and Ofl B2 mutant. For WT, only generpl5was signifi cantly down-regulated, whereas four genes (5s rRNA,rps14,rpl14i, andpsbA) were signifi cantly up-regulated under light stimulation conditions (Fig.4a, Table 1) (P≤0.05). For Ofl B2 mutant, the expression levels of genes5s rRNA,petB,rpl5,rps8,rps14, andrps2were signifi cantly down-regulated under light stimulation conditions, and the decrease was more remarkable in longer light stimulation: the expression of genespetBandrps2remained more or less the same as that in dark conditions after 0.5-h light stimulation but dropped sharply after 3-h light stimulation (Fig.4b, Table 1) (P≤0.05). No signifi cant diff erence was observed in the expressions of the other six (rps2,rps3,rps8,psaC,petB, andrbcL) and fi ve (rps3,rpl14,psbA,psaC, andrbcL) genes in WT and Ofl B2 from dark to light stimulations, respectively (P>0.05) (Fig.4a & b).
4 DISCUSSION
Ofl is considered as a fl uoroquinolone antibacterial drug that can cause loss of chloroplast photosynthetic function inEuglena. It is an inhibitor of DNA gyrase (bacterial topoisomerase type II) and directly inhibits plastid DNA replication. As the cells divide, the newEuglenacells lose their chloroplasts and cannot eff ectively perform photosynthesis, forming a white bleached mutant (Krajčovič et al., 1989; Križková et al., 1998; Schwartzbach and Schiff , 1974). However, Ofl did not bleach two green algal species,Scenedesmusobliquus(Qin et al., 2012) andChlamydomonas(lab data, unpublished). In addition, the presence of Ofl has little eff ect on the growth ofE.gracilis(Schwartzbach and Schiff , 1974; Krajčovič et al., 1989; Križková et al., 1998), while it has a toxic eff ect on the growth and physiological status ofS.obliquus(Qin et al., 2012). The mechanism by which Ofl can bleachEuglenabut other species of algae remains unclear.
The decrease of chlorophyll is an important indicator of the bleaching ofEuglena(Thomas and Ortiz, 1995). The absorption peak of WT indicates that the pigment of WT contains chlorophyll and carotene, which is consistent with the study of the pigment composition of the predecessors (Krinsky and Goldsmith, 1960). However, the mutants did not contain any chlorophyll and carotene, which is consistent with our previous observations (Wang et al., 2002, 2004). The growth of the bleached mutants was much slower than that of WT in the light (Fig.1), asE.graciliscan be both autotrophic and heterotrophic, while bleached mutants have lost their chloroplasts’ function and could not perform photosynthesis but only grow heterotrophically. In contrast to our results, the previous research reported that the presence of Ofldid not aff ect the growth ofE.gracilis(Križková et al., 1998; Hadariová et al., 2017) perhaps caused by the heterogeneity of residual plastid genes in diff erent Ofl mutants. We used diff erent growth medium and at diff erent growth conditions from the other researches (Križková et al., 1998; Hadariová et al., 2017). As stated in Section 2.1, our strains were grown in EG medium under the condition of 12 h/12 h light (100±10 μmol/(m2·s))/dark at 22°C. Križková et al. (1998) used modifi ed CM supplemented with 0.5% sodium acetate at 27°C under permanent illumination (2 000 lx). Hadariová et al. (2017) used modifi ed CM medium supplemented with 0.8% ethanol at 27°C, with continuous illumination (30 μmol/(m2·s)) or in the dark. And these may be the reasons attribute to the diff erence. The eff ects and mechanism of Ofl on algal growth need to be further elucidated.
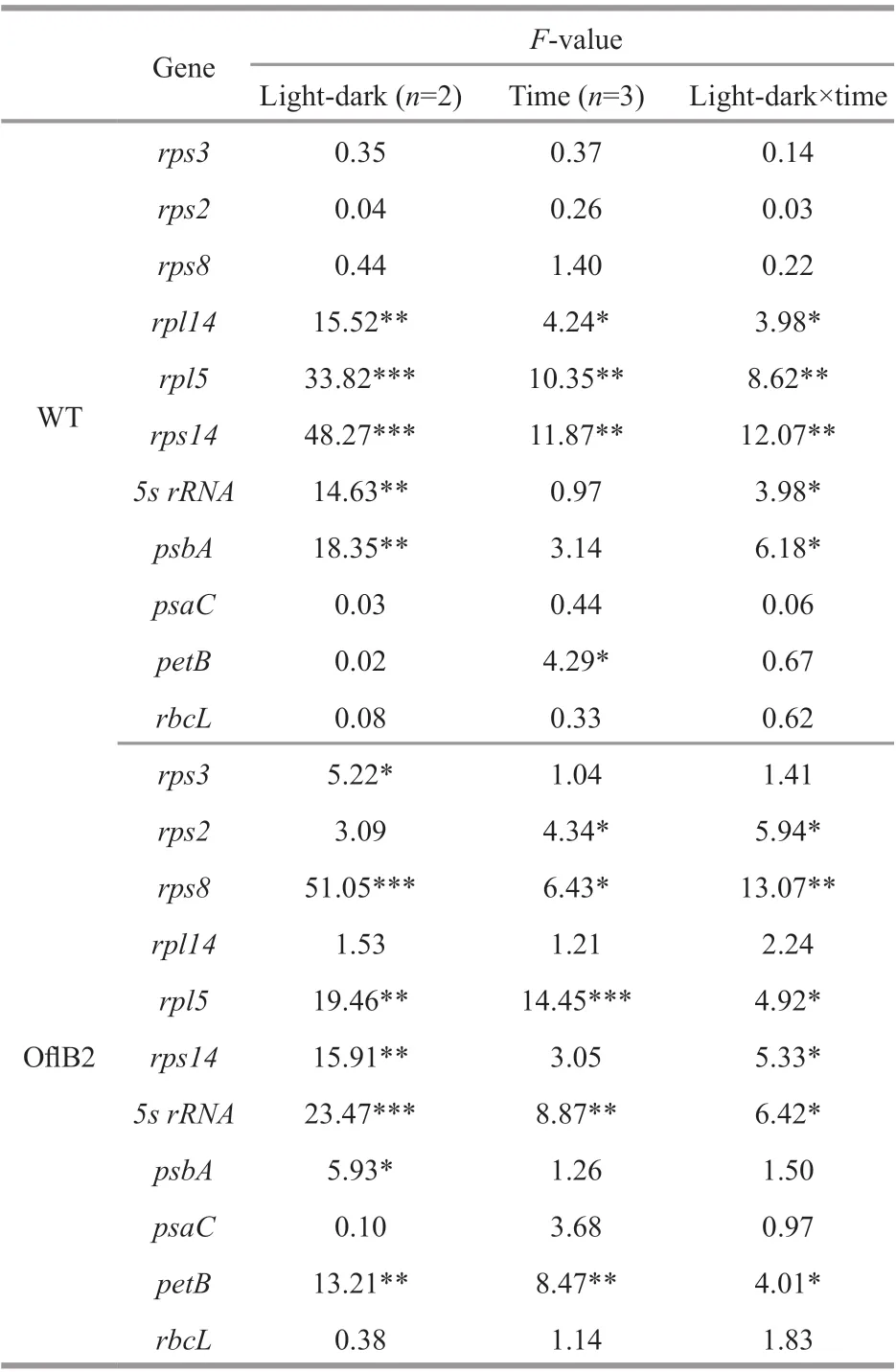
Table 1 Two-way ANOVA results showing eff ects of light/dark
Using the knownE.gracilischloroplast genome (Hallick et al., 1993), 50 genes were selected, and PCR was carried out using the DNA of WT and Ofl B2 mutant as templates. Only 12 genes were detected, and the remaining 38 genes were all detected in WT but not in the Ofl B2 mutant (data not shown). However, failing to detect these genes in the Ofl B2 mutant does not indicate that the Ofl B2 mutant has lost the undetected genes. It is possible that these genes may have been mutated or rearranged in the regions of selected primers. Similarly, the detection of genes in the Ofl B2 mutant does not mean that the Ofl B2 mutant contains the entire genetic sequence.
It is well known that someEuglenableached mutants contain residual plastids and partial plastid genomes (Heizmann et al., 1981; Gockel et al., 2000; Wang et al., 2002, 2004). We found that theE.gracilismutants contain 12 residual plastid genes, namelyrps2,rps3,rps8,rps14,rpl5,rpl14,psbA,psaC,rpl16,petB,rbcL, and5s rRNA(Figs.2&3). In previous reports, one mutant sm5 retained16srRNA,psbD,psaA,rpl16,rps9i, andrpoBgenes, while the other mutants only retained16srRNAgene andrpoBregion but lost genesrpl3,rbcL, andatpE.16srRNAgene andrpoBare next to the replication origin, and therefore they were retained in most mutants (Wang et al., 2004). However, in this study,rps3,rpl16andrbcLgenes were retained in all mutants, and16srRNA,psbD,psaA,rps9,rpoB,atpEgenes were not detected. Moreover, in our experiment, therps3,rpl16, andrbcLgenes were retained in all mutants. The copy number ofrrn16,rrn23,rpl2,tufA,psbC,rbcL, andrpoC2genes was reduced in the presence of Ofl in theE.gracilis, and the copy number ofrpl16gene remained unchanged (Gockel et al., 2000). In addition, WgmZOfl L mutant has also retainedrpl14andrpl5genes which are present downstream fromrpl16gene, while it does not possess16srRNAgene (Krnáčová et al., 2015; Oldenburg and Bendich, 2016). This is consistent with our experiments, and our fi ve mutants also retained therpl16,rpl5, andrpl14genes. Our residual plastid genes were diff erent from that of previous research, indicating that the residual plastids genes of bleached mutants varied among diff erent treatments, perhaps even in the same treatment diff erent cells may lose diff erent part of the plastid genome. More detailed molecular analyses of stable bleached mutants and after the bleaching of variousE.gracilisstrains would be needed to explain the phenomenon of diff erential retention of plastid genes in diff erent mutants.
We planned to amplify the plastid genome of the Ofl B2 mutant by using long distance PCR with primers derived from the 13 genes detected. However, it was unsuccessful due to the unknown structure of the Ofl B2 plastid genome and distribution of these genes. Finally, 6 genes (rpl16,rpl14,rpl5,rps8,rps14, andrps2) were found to be closely distributed to each other, which made it possible to explore therpl16-rps2region of Ofl B2 mutant and WT (Supplementary Sequence S1). The DNA sequences of therpl16-rps2region of Ofl B2 mutant and WT were compared by NCBI BLAST. The results showed that the sequence of Ofl B2 mutant and WT were highly similar with a 1% diff erence, and 51 mutations with 10 deletions/insertions and 41 point mutations existed in Ofl B2 mutant compared to WT. The mismatch rate is higher than the error rate of the highfi delity Taq enzyme (10-6bp/cycle), and thus the PCR error could be excluded in this study. In addition, divergence ofE.longafromE.gracilismay be caused by the gradual excision of small plastid genome fragments under some extreme environments (Bodyl, 1996).
There are 116 genes in the chloroplast genome ofE.gracilis; however, the functions of these genes were rarely studied using molecular techniques. Moreover, streptomycin and Ofl inhibitE.longa growth, suggesting that it requires plastid genes to survive, i.e., its plastid is a functional organelle not just a chloroplast remnant (Gockel et al., 2000). In addition, nuclear encoded plastid genespetJandpsbOwere not transcribed in an Ofl inducedE.gracilismutant in the light (Vacula et al., 2001). Although substantial regulation ofEuglenachloroplast gene expression also occurs at the translational level, the observed patterns in transcriptional levels are possibly signifi cant (Vacula et al., 2001; Idoine et al., 2014; Ebenezer et al., 2017). Our results showed that the expression ofrpl5,5s rRNA,rps14,rpl14, andpsbAgenes in WTE.gracilisunder light-stimulation condition was changed, onlyrpl5gene was signifi cantly down-regulated whereas the other four were signifi cantly up-regulated; this indicates that light stimulation leads to an increase of transcription level of some chloroplast genes ofE.gracilis, showing signifi cant light responses. Unexpectedly, the expression ofrpl5,5s rRNA,rps2,rps8, andpetBgenes in the Ofl B2 mutant were signifi cantly downregulated under light stimulation conditions, suggesting that light stimulation can cause expression change of photosynthetic genes in the nonphotosynthetic Ofl B2 mutant, perhaps with negative eff ects. In WT and Ofl B2 mutant, the same plastid genes responded to light stimulation in diff erent ways. We could not completely remove the residual plastid DNA of bleached mutants after a long-term of 2 years cultivation, proposing that residual plastid DNA is likely to have functions other than photosynthesis, such as synthesis of some certain essential compounds. This is very interesting and the investigation of the Ofl B2 mutant residual plastid genes’ function is thus needed.
5 CONCLUSION
In summary, Ofl B2 bleached mutant retains part of the plastid DNA even after long term (2 years) exposure to Ofl , and the plastid genes respond to light at the transcriptional level. Moreover, chloroplast and plastid genes in WT and Ofl B2 mutant have diff erent responses to light stimulation. Our research will shed light on understanding the functions of residual plastid DNA in non-photosynthetic microorganisms and evolution of the chloroplast in green microalgae.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are included in this published article and in its supplementary information fi les.
7 ACKNOWLEDGMENT
The authors gratefully acknowledge the supports from the Instrumental Analysis Center of Shenzhen University (Xili Campus).
8 AUTHOR CONTRIBUTION
WANG Jiangxin contributed to the conception and design of the study. QIN Huan performed the experiments. LEI Anping, GUO Qingqing, LIU Chenchen, and LI Fenglan performed the statistical analysis. QIN Huan wrote the fi rst draft of the manuscript. WANG Jiangxin, QIN Huan, CHU Zihan, ZHANG Hua, and LEI Anping wrote the sections of the manuscript. WANG Jiangxin, CHU Zihan, and LEI Anping contributed to manuscript revision. All authors read and approved the submitted version.
9 CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or fi nancial relationships that could be construed as a potential confl ict of interest.
References
Ahmadinejad N, Dagan T, Martin W. 2007. Genome history in the symbiotic hybridEuglenagracilis.Gene., 402(1-2): 35-39.
Bennett M S, Triemer R E. 2015. Chloroplast genome evolution in the Euglenaceae.J.Eukaryot.Microbiol., 62(6): 773-785.
Bodyl A. 1996. Is the origin ofAstasialongaan example of the inheritance of acquired characteristics?ActaProtozool., 35(2): 87-94.
Buetow D E. 1982. The Biology ofEuglena, Vol. III. Academic Press, New York. p.157-195.
Ebenezer T E, Carrington M, Lebert M, Kelly S, Field M C. 2017.Euglenagracilisgenome and transcriptome: organelles, nuclear genome assembly strategies and initial features.In: Schwartzbach S D, Shigeoka S eds. Euglena: Biochemistry, Cell and Molecular Biology. Springer, Cham. p.125-140.
Eberhard S, Drapier D, Wollman F A. 2002. Searching limiting steps in the expression of chloroplast-encoded proteins: relations between gene copy number, transcription, transcript abundance and translation rate in the chloroplast ofChlamydomonasreinhardtii.PlantJ., 31(2): 149-160.
Gockel G, Hachtel W, Baier S, Fliss C, Henke M. 1994. Genes for components of the chloroplast translational apparatus are conserved in the reduced 73-kb plastid DNA of the nonphotosynthetic euglenoid fl agellateAstasialonga.Curr.Genet, 26(3): 256-262.
Gockel G, Hachtel W, Michael M. 2000. Complete gene map of the plastid genome of the nonphotosynthetic euglenoid fl agellateAstasialonga.Protist, 151(4): 347-351.
Hadariová L, Vesteg M, Birčák E, Schwartzbach S D, Krajčovič J. 2017. An intact plastid genome is essential for the survival of colorlessEuglenalongabut notEuglenagracilis.Curr.Genet, 63(2): 331-341.
Hallick R B, Hong L, Drager R G, Favreau M R, Monfort A, Orsat B, Spielmann A, Stutz E. 1993. Complete sequence ofEuglenagracilischloroplast DNA.NucleicAcidsRes., 21(15): 3 537-3 544.
Heizmann P, Doly J, Hussein Y, Nicolas P, Nigon V, Bernardi G. 1981. The chloroplast genome of bleached mutants ofEuglenagracilis.Biochim.Biophys.Acta, 653(3): 412-415.
Heizmann P, Ravel-Chapuis P, Nigon V. 1982. Minicircular DNA having sequence homologies with chloroplast DNA in a bleached mutant ofEuglenagracilis.Curr.Genet, 6(2): 119-122.
Heizmann P, Salvador G F, Nigon V. 1976. Occurrence of plastidial rRNAs and plastidial structures in bleached mutants ofEuglenagracilis.Exp.CellRes., 99(2): 253-260.
Hussein Y, Heizmann P, Nicolas P, Nigon V. 1982. Quantitative estimations of chloroplast DNA in bleached mutants ofEuglenagracilis.Curr.Genet, 6(2): 111-117.
Idoine A D, Boulouis A, Rupprecht J, Bock R. 2014. The diurnal logic of the expression of the chloroplast genome inChlamydomonasreinhardtii.PLoSOne, 9(10): e108760.
Kivic P A, Vesk M. 1972. Structure and function in the euglenoid eyespot apparatus: the fi ne structure, and response to environmental changes.Planta, 105(1): 1-14.
Kivic P A, Vesk M. 1974. An electron microscope search for plastids in bleachedEuglenagracilisandinAstasialonga.Can.J.Bot., 52(4): 695-699.
Krajčovič J, Ebringer L, Polόnyi J. 1989. Quinolones and coumarins eliminate chloroplasts fromEuglenagracilis.Antimicrob.AgentsChemother., 33(11): 1 883-1 889.
Krinsky N I, Goldsmith T H. 1960. The carotenoids of the fl agellated alga,Euglenagracilis.Arch.Biochem.Biophys., 91(2): 271-279.
Križková L, Nagy M, Polόnyi J, Ebringer L. 1998. The eff ect of fl avonoids on ofl oxacin-induced mutagenicity inEuglenagracilis.Mutat.Res., 416(1-2): 85-92.
Krnáčová K, Rýdlová I, Vinarčíková M, Krajčovič J, Vesteg M, Horváth A. 2015. Characterization of oxidative phosphorylation enzymes inEuglenagracilisand its white mutant strainWgmZOfl L.FEBSLett., 589(6): 687-694.
Oldenburg D J, Bendich A J. 2016. The linear plastid chromosomes of maize: terminal sequences, structures, and implications for DNA replication.Curr.Genet, 62(2): 431-442.
Qin H W, Chen L F, Lu N, Zhao Y H, Yuan X. 2012. Toxic eff ects of enrofl oxacin onScenedesmusobliquus.Front.Environ.Sci.Eng., 6(1): 107-116.
Schwartzbach S D, Schiff J A. 1974. Chloroplast and cytoplasmic ribosomes ofEuglena: selective binding of dihydrostreptomycin to chloroplast ribosomes.J.Bacteriol., 120(1): 334-341.
Siemeister G, Hachtel W. 1989. A circular 73 kb DNA from the colourless fl agellateAstasialongathat resembles the chloroplast DNA ofEuglena: restriction and gene map.Curr.Genet, 15(6): 435-441.
Sulli C, Fang Z W, Muchhal U, Schwartzbach S D. 1999. Topology ofEuglenachloroplast protein precursors within endoplasmic reticulum to Golgi to chloroplast transport vesicles.J.Biol.Chem., 274(1): 457-463.
Thomas E J, Ortiz W. 1995. Loss of chloroplast transcripts for proteins associated with photosystem II: an early event during heat-bleaching inEuglenagracilis.PlantMol.Biol., 27(2): 317-325.
Tucci S, Vacula R, Krajčovič J, Proksch P, Martin W. 2010. Variability of wax ester fermentation in natural and bleachedEuglenagracilisstrains in response to oxygen and the elongase inhibitor fl ufenacet.J.Eukaryot.Microbiol., 57(1): 63-69.
Vacula R, Steiner J M, Krajčovič J, Ebringer L, Löff elhardt W. 2001. Plastid state- and light-dependent regulation of the expression of nucleus-encoded genes for chloroplast proteins in the fl agellateEuglenagracilis.FoliaMicrobiol., 46(5): 433-441.
Vesteg M, Vacula R, Burey S, Löff elhardt W, Drahovská H, Martin W, Krajčovič J. 2009. Expression of nucleusencoded genes for chloroplast proteins in the fl agellateEuglenagracilis.J.Eukaryot.Microbiol., 56(2): 159-166.
Wang J X, Shi Z X, Xu X D. 2002. Chloroplast-less mutants of two species ofEuglena.ActaHydrobiol.Sin., 26(2): 175-179.
Wang J X, Shi Z X, Xu X D. 2004. Residual plastids of bleached mutants ofEuglenagracilisand their eff ects on the expression of nucleus-encoded genes.Prog.Nat.Sci., 14(3): 213-217.
Yoshida Y, Tomiyama T, Maruta T, Tomita M, Ishikawa T, Arakawa K. 2016. De novo assembly and comparative transcriptome analysis ofEuglenagracilisin response to anaerobic conditions.BMCGenomics, 17: 182.
Zakryś B, Milanowski R, Karnkowska A. 2017. Evolutionary origin ofEuglena.In: Schwartzbach S D, Shigeoka S eds. Euglena: Biochemistry, Cell and Molecular Biology. Springer, Cham. p.3-17.
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Exploring sensitive area in the tropical Indian Ocean for El Niño prediction: implication for targeted observation*
- Analysis of the typhoon wave distribution simulated in WAVEWATCH-III model in the context of Kuroshio and wind-induced current*
- Characterizing the capability of mesoscale eddies to carry drifters in the northwest Pacifi c*
- Observation system simulation experiments using an ensemble-based method in the northeastern South China Sea*
- Statistical analysis of intensity variations in tropical cyclones in the East China Sea passing over the Kuroshio*
