Spatio-temporal variation of subtidal macrobenthic fauna and the ecological assessment of Longkou Artifi cial Island construction in Bohai Sea, China*
LI Xiaojing , CHEN Linlin , ZHOU Zhengquan , LI Baoquan , , LIU Xin , , YANG Lufei, LIU Bo , SONG Bo
1 Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China 2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 University of Yantai, Yantai 264003, China
Abstract To evaluate the status and changes of macrobenthic communities related to the construction of the Longkou Artifi cial Island (LAI) in the Bohai Sea, China, four annual surveys were conducted from 2010 to 2013. Signifi cant changes on environmental variations and macrobenthic communities were observed in 2013 after the LAI construction a couple of years later. The changing environment was primarily presented by lower values of pH, dissolved oxygen (DO), organic material (OM) percentage, and higher salinity, suspended particulate matter (SPM) concentration and oil concentration. The main dominant species shifted from Polychaeta taxa in 2010 to Mollusca taxa in 2013 due to the changes of environmental variables. An apparent miniaturization tendency in body size of macrobenthic species was presented from 2010 to 2013. The biodiversity indices increased yearly from 2010 to 2013. However, inter-site homogenization was observed in both the community structure and health status. Multivariate AZTI Marine Biotic Index (M-AMBI) analysis showed that the health status of stations changed depending on its original status and distance to the LAI. However, no signifi cant diff erences were found in the spatial distribution of either environmental variables or abundance, biomass and biodiversity of macrobenthic communities. All the results will provide a basis for the long-term ecological assessment of reclamation.
Keyword: macrobenthic assemblages; benthic health assessment; M-AMBI index; Longkou Artifi cial Island; Bohai Sea
1 INTRODUCTION
The coastal regions are among the most rapidly urbanizing places worldwide characterized by the rapid development of the economy and the increase of population (Ehrenfeld, 2000). The shortage of land leads to extensive coastal reclamation in these regions. Reclamation brings substantial economic benefi ts and provides more land for living and economic development. In China, this profi t was up 2.7 times the cost on average, along coastal areas (Li et al., 2012). However, this process ignores the damage to the marine ecosystem, especially the loss of marine biotic resources. Reclamation aff ects the marine environment and ecosystem seriously by reducing coastal wetland areas, decreasing biodiversity and destroying the habitats as well as damaging the ecosystem service (Wang et al., 2014).
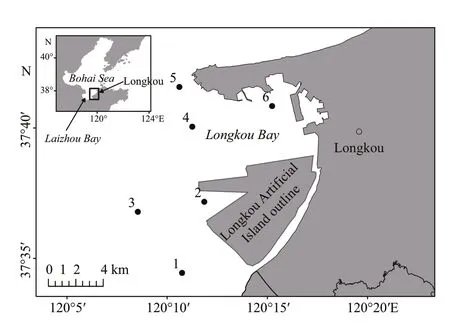
Fig.1 Sampling stations in four surveys on Longkou Artifi cial Island (LAI) (survey stations: 1-6)
Macrobenthic fauna is an ideal indicator for monitoring environmental changes due to their limited mobility and high sensitivity to environmental changes (Bilyard, 1987). Previous investigations have proved that the reclamation activities aff ected the macrobenthic fauna in several ways: disturbed the benthic infauna by decreasing the species richness, density, biomass, and biodiversity (Suo et al., 2015); signifi cantly changed the community structure by decreasing the family number and abundance of macrobenthos (Lu et al., 2002). Meanwhile, the selfrestoration and resistance eff ect in a nature reserve may reduce the infl uence of reclamation over time (Lv et al., 2016).
Longkou Bay is a subsidiary bay in the northeastern corner of Laizhou Bay, Bohai Sea. The water depth of Longkou Bay is less than 10 m in its inner part and 10–20 m in its outer part. The sediment type is mainly composed of silty clay. With the rapid development of local coastal economy in the past years, the shortage of land use in the Longkou district was becoming an imperative issue. To expand more land for living and factories, the Longkou Artifi cial Island (LAI) was constructed in January 2011. More than 6.3×107m3of reclamation projects were completed, forming coff erdams and construction channels of 8.5×104m in one year. Up until June 2012, the length of all coff erdams was 1.2×105m and the total value of the project was up to 1.2×108m3. To minimize the impacts of reclamation on the local marine ecosystem, the LAI project adopted an advanced mode combining off shore artifi cial island construction and block group fi lling technology (An, 2010).
Previous investigations on the impacts of the LAI were focused on the marine environment. An et al. (2010a) found that the current velocity and direction varied signifi cantly due to the construction of the LAI. The composition of surface sediment also changed in the decrease of sand content and increase of clay content (Ren et al., 2016). However, the changes of macrobenthic communities due to the construction of the LAI have not been investigated yet.
The aim of the present study is, (1) to investigate the recent changes of the macrobenthic community before and during the physical disturbance related to the construction of the LAI, (2) to understand the benthic ecological health in the LAI areas, and (3) to provide theoretical guidance for the reclamation construction planning, ecological protection, and sustainable development.
2 MATERIAL AND METHOD
2.1 Study areas and sampling procedure
The sampling area is located on 102.125°E–120.2776°E and 37.575°N–37.678°N. Six sampling stations were set both inside and outside of Longkou Bay to investigate the spatial diff erences of macrobenthic assemblages (Fig.1).
Since the construction activities had not been completed during this survey, the results presented here refl ected only the status of macrobenthic communities before and during the operation. The artifi cial island construction started in January 2011. Hence, four surveys were carried out on September 2010, 2012, and August 2011, 2013. We combined the sampling station and survey year manually for a clearer description, namely, A–D represent the years from 2010 to 2013, respectively; for example, station A1 means that station 1 was sampled in 2010. Eight sediment samples were collected by using a 0.025-m² Van Veen grab at each station and merged into one sample, then sieved through a 0.5-mm mesh and fi xed in 95% ethyl-alcohol. Organisms were identifi ed to the lowest possible taxonomic level, then counted and weighted to an accuracy level of ±0.001 g. The abundance and biomass data were transformed to individuals per square meters for analysis later.
2.2 Environmental variables
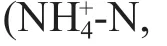
Table 1 Mean values of physico-chemical parameters of sediment and seawater in 4 surveys
2.3 Statistical analysis
Dominant species were calculated with the following equation (Xu and Chen, 1989):
Y=(Ni/N)×fi,
whereNis the total abundance of all the stations;Niis the abundance of the speciesiof all the stations, andfiis the occurrence frequency of the speciesiof all the stations.
Software packages Primer 7 was adopted to analyze the diversity and community structure of macrofauna based on the abundance data.
Benthic health stress level was analyzed by the Multivariate AZTI Marine Biotic Index (M-AMBI) (Borja and Muxika, 2005). M-AMBI was computed by the AZTI’s Marine Biotic Index (AMBI) program (Version 5.0) on the basis of the AMBI guidelines, which were available free online (http://ambi.azti.es). The threshold values for the M-AMBI conditions were as follows: ‘high’ quality>0.77, ‘good’=0.53–0.77, ‘moderate’=0.38–0.53, ‘poor’=0.20–0.38, and ‘bad’<0.20 (Borja and Tunberg, 2011). All of the nonbenthic invertebrate taxa (fi sh and megafauna) were removed (Borja and Muxika, 2005). The reference conditions for M-AMBI in this area followed the method: we chose the lowest AMBI (0) and highest diversityH′ and richness of the four surveys, then increased it by 15% of the highest diversity and richness (Li et al., 2017).
The relationship between environmental variables and dominant species and biodiversity indices were analyzed by the redundancy analysis (RDA) ordination diagram, because the longest gradients in all the detrended correspondence analysis (DCA) was shorter than 3.0 (Ter Braak and Smilauer, 2002). The diff erence in environmental variables, species composition and biodiversity among surveys, and stations, were tested by the two-way ANOVA permutation test due to limited sample data, and the Tukey test was applied for pairwise comparisons to check whether there were any signifi cant diff erences among stations or surveys. Pearson correlation analysis was adopted to analyze the relationship of environmental variables and potential infl uence factors. All the analyses were conducted using software R 3.5.3.
3 RESULT
3.1 Environmental variables
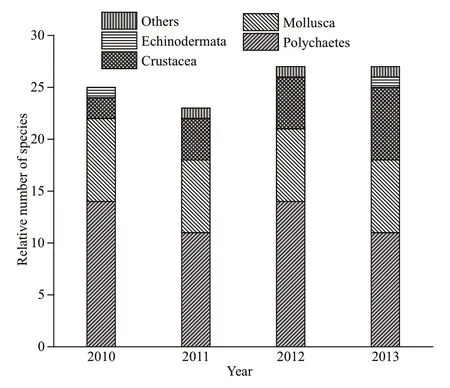
Fig.2 Categories of species of main taxa groups appeared during survey years
The mean annual value of physico-chemical parameters of sediment and seawater are shown in Table 1. The pH (Rmean Sq=0.099,P<0.01, Twoway ANOVA permutation test), salinity (Rmean Sq=18.181,P<0.01, two-way ANOVA permutation test), concentration of DO (Rmean Sq=1.692,P=0.046<0.05, two-way ANOVA permutation test), SPM (Rmean Sq=18 481,P<0.01, two-way ANOVA permutation test) in seawater and concentration of OM (Rmean Sq=0.023,P=0.036<0.05, two-way ANOVA permutation test), oil (Rmean Sq=38278,P=0.004<0.01, two-way ANOVA permutation test) in sediment showed signifi cant temporal diff erences among survey years. Almost all the diff erences were induced by the diff erences between 2013 and other surveys. For example, the mean annual value of pH in seawater decreased about 0.2 unit in 2013 than in other surveys (P<0.01, Tukey test). The salinity increased 2.54–2.76 in 2013 than in other surveys (P<0.01, Tukey test). The concentration of DO in 2013 decreased 1.00 mg/L than in 2011. The concentration of SPM increased 53.12–72.32 mg/L in 2012 and 2013 than in 2010 and 2011 (P<0.01, Tukey test). The concentration of OM in sediment decreased about 0.10 in 2011 and 2013 than in 2010 (P<0.05, Tukey test). The concentration of oil in sediment increased 114.63×10-6to 118.28×10-6in 2013 than in other survey years (P<0.05, Tukey test).
All the parameters of seawater showed no signifi cant spatial diff erences among stations during the survey period. The concentration of the trace element (Cu) (Rmean Sq=39.45,P=0.017<0.05, twoway ANOVA permutation test), and the percentage composition of sand (Rmean Sq=0.023,P=0.036<0.05, two-way ANOVA permutation test) and silt (Rmean Sq=585.64,P<0.01, two-way ANOVA permutation test) in sediment varied signifi cantly among stations. The mean value of the percentage composition of sediment varied among all the stations, with the highest silt value but lowest sand value in station 2, and lowest silt value but highest sand value in station 5. The concentration of Cu in the sediment in station 2 was distinctively higher than that of stations 3, 4, 5, 6. In addition, the concentration of Cu in station 1 was evidently higher than those in station 4.
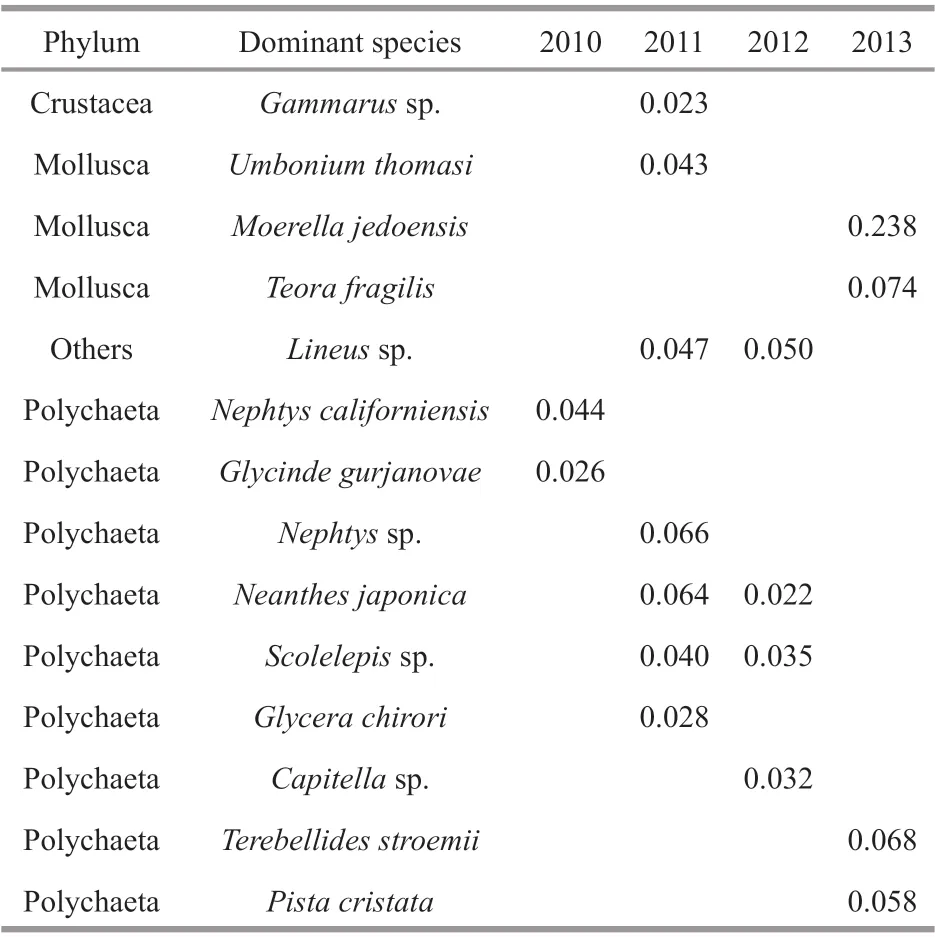
Table 2 Dominant species of the 4 surveys
3.2 Species composition and dominant species
In total, 67 species were identifi ed in 4 surveys: 25 species in 2010, 23 species in 2011, 27 species in 2012, and 27 species in 2013. Polychaeta was the dominant group, which contributed most to the total species (more than 40%), then followed by Mollusca, Crustacea, Echinodermata, or others in all survey years (Fig.2). However, not all the macrobenthic groups were found in every survey. For example, other taxa were not found in 2010 and Echinodermata taxa were not found in 2011 and 2012 in the survey areas. The number of the taxa decreased in 2011 when the reclamation was conducted and then increased in 2012 and 2013, which was eventually higher in 2012 and 2013 than in 2010.
The dominant species changed annually from 2010 to 2013. In 2010, Polychaeta species,N.californiensisandGlycindegurjanovaewere identifi ed as dominant species. More dominant species were presented in the survey areas in 2011 and 2012 after the reclamation. The dominant species changed to 2 Polychaeta taxa and 2 Mollusca taxa in 2013. The dominated group of the community shifted from Polychaeta taxa in 2010 to Mollusca taxa in 2013 (Table 2).
3.3 Abundance and biomass
The average abundance of macrobenthos changed annually from 2010 to 2013, with the total average value of (771.67±104.54) ind./m², (106.67±40.58) ind./m² in 2010, (165.00±59.25) ind./m² in 2011, (155.00±2.20) ind./m² in 2012, and (345±216.20) ind./m² in 2013, respectively. The average biomass changed annually as follows: (37.55±6.35) g/m² of the total average biomass, (6.49±0.95) g/m² in 2010, (16.00±3.67) g/m² in 2011, (13.09±9.67) g/m² in 2012, and (1.96±1.62) g/m² in 2013, respectively. The total abundance (Rmean Sq=149107,P=0.005<0.01, Twoway ANOVA permutation test), abundance of Crustacea (Rmean Sq=3 853.3,P=0.009<0.01, two-way ANOVA permutation test), Mollusca (Rmean Sq=41 255,P=0.025<0.05, two-way ANOVA permutation test), and Echinodermata (Rmean Sq=367.5,P=0.043<0.01, two-way ANOVA permutation test) showed signifi cant diff erences among survey years; however, the diff erences of abundance and biomass among stations were not signifi cant (P>0.05, two-way ANOVA permutation test). Further analysis showed that the total abundance of macrobenthos was signifi cantly higher in 2013 than in 2010 (P=0.012<0.05, Tukey test). The abundance of Crustacea taxa also showed apparent increment (36.67 ind./m²) in 2013 compared to 2010 (P=0.027<0.05, Tukey test).
The total abundance and biomass of macrobenthos fl uctuated irregularly from 2010 to 2013. However, the higher abundance value and lower biomass value of main taxa groups in 2013 were clearly shown compared to other survey years (Fig.3).
3.4 Diversity indices
The number of species (S) (Rmean Sq=67.5,P<0.01, Two-way ANOVA permutation test), Shannon-Wiener diversity (H′) (Rmean Sq=3.113,P=0.001<0.01, Two-way ANOVA permutation test) and Margalef’s index (d) (Rmean Sq=1.348,P<0.01, Two-way ANOVA permutation test) showed a distinctly increasing trend from 2010 to 2013 (Fig.4). The results of the pairwise test revealed that the number of species, Shannon-Wiener diversity (H′) and Margalef’s index (d) in 2013 increased signifi cantly by 4.67, 1.04, 0.63 than those in 2010 (P<0.01, Tukey test). The increment of Margalef’s index (d) was also signifi cant in 2012 compared to 2010 (P=0.013<0.05, Tukey test). However, none of these indices diff ered signifi cantly among stations (P>0.05, Two-way ANOVA permutation test).
3.5 Community structure
Five groups were signifi cantly clustered based on the abundance data by the non-metric multidimensional scaling (nMDS) ordination (P<0.05) (Fig.5). The detailed stations in 5 groups are the following: all of the stations in 2013 and station 6 in 2010; most stations in 2010; most stations in 2011, and 2012; only two stations, respectively.
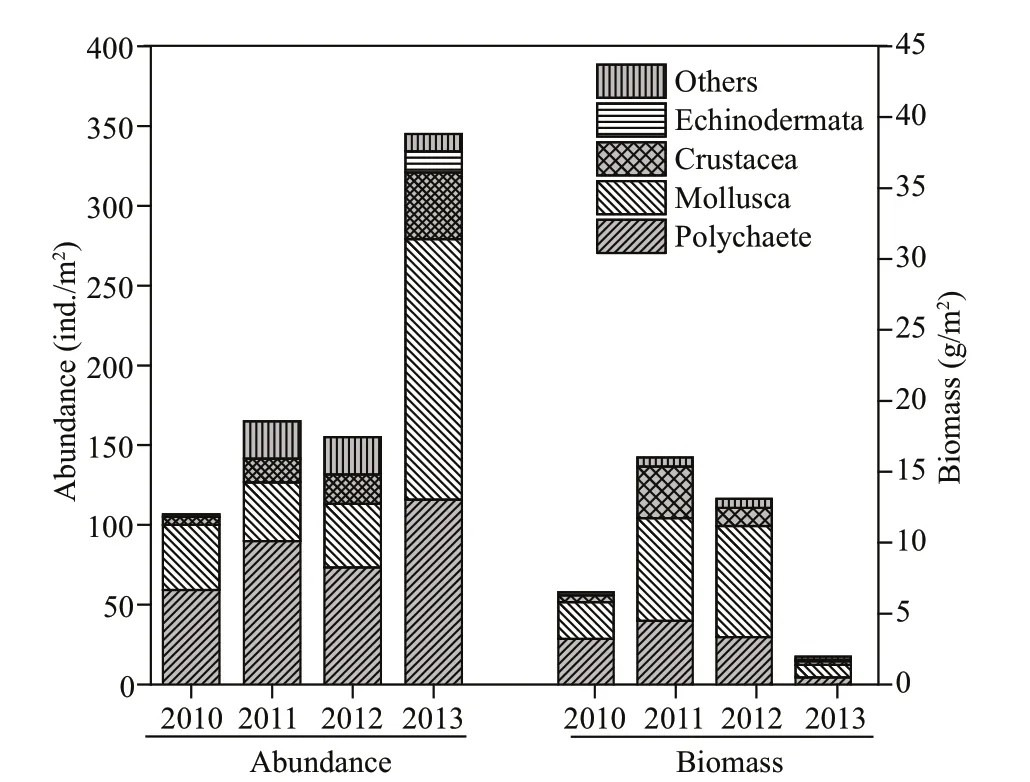
Fig.3 Abundance and biomass of main taxa groups of macrobenthos in 4 surveys
Two-way analysis of similarities (ANOSIM) indicated signifi cant temporal diff erences (R=0.528,P<0.01) but no spatial diff erences (R=0.095,P>0.05). The pairwise test showed that the community structure of 2013 was signifi cantly diff erent from the other 3 years, 2010 (R=0.456,P<0.01), 2011 (R=0.849,P<0.01), and 2012 (R=0.709,P<0.01). The community structure of 2011 was also evidently diff erent from 2010 (R=0.309,P<0.05).
Three groups were divided objectively by the survey years based on the results of MDS ordinations to see the diff erence in macrobenthic communities among survey years. Group 2010 included all the stations in 2010; Group 2011–2012 included all the stations in 2011 and 2012; and Group 2013 included all the stations in 2013. SIMPER analysis revealed that the average similarity increased among 3 groups by years. The average similarity of Group 2010 was 7.24, with the main contributors ofMoerellajedoensis,NephtyscaliforniensisandGlycindegurjanovae. The average similarity of Group 2011–2012 was 22.86, with the dominant species ofNeanthesjaponica,Nephtyssp.,Glycerachirori,Scolelepissp.,Umboniumthomasi,Gammarussp., and Capitellidae. The average similarity of Group 2013 was 36.51, with the primary contributors beingMoerellajedoensis,Terebellidesstroemii,Teorafragilis, andPistacristata.
3.6 Assessment of benthic health
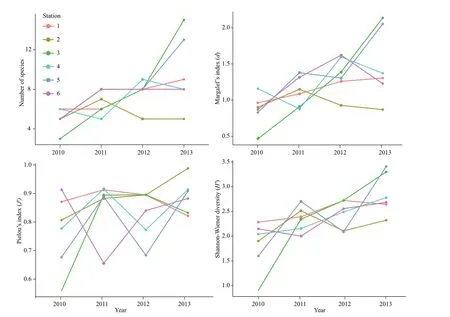
Fig.4 Species number and three biodiversity indices in all survey years and stations
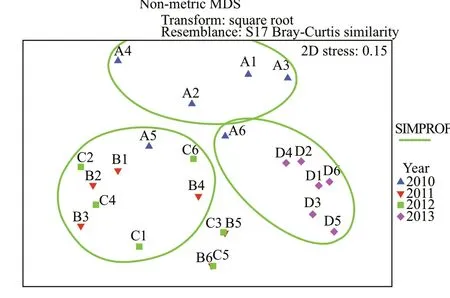
Fig.5 Non-metric MDS analysis of macrobenthic community structure for all sampling stations from 2010 to 2013
Overall, the ecological status (ES) of most sampling stations was in ‘good’ to ‘high’ ES and only station 2 was in ‘moderate’ ES in 2012 (Fig.6). Two stations were in ‘high’ ES in 2010 and then only one station was in ‘high’ ES from 2011 to 2013. The ES of station 3 that was relatively far from the LAI was getting better from 2010 to 2013. However, the ES of station 2 that was close to the LAI worsened compared to other stations from 2010 to 2012, although it appeared to have some improvement in 2013. The ES of stations 1 and 4 were also getting worse in 2011 and then improved in 2012 and 2013 but they did not recover to the ‘good’ status of 2010. Station 5 was better in 2011 than in the other three years. However, station 6 did not show any distinctive changes from 2010 to 2013. It can be concluded that the construction of LAI imposed diff erent impacts on this area, namely negative eff ects on the ES of stations 1, 2 and 4; positive eff ects on the ES of stations 3 and 5, and no eff ects on station 6.
3.7 The relationship of macrobenthos and environmental variables
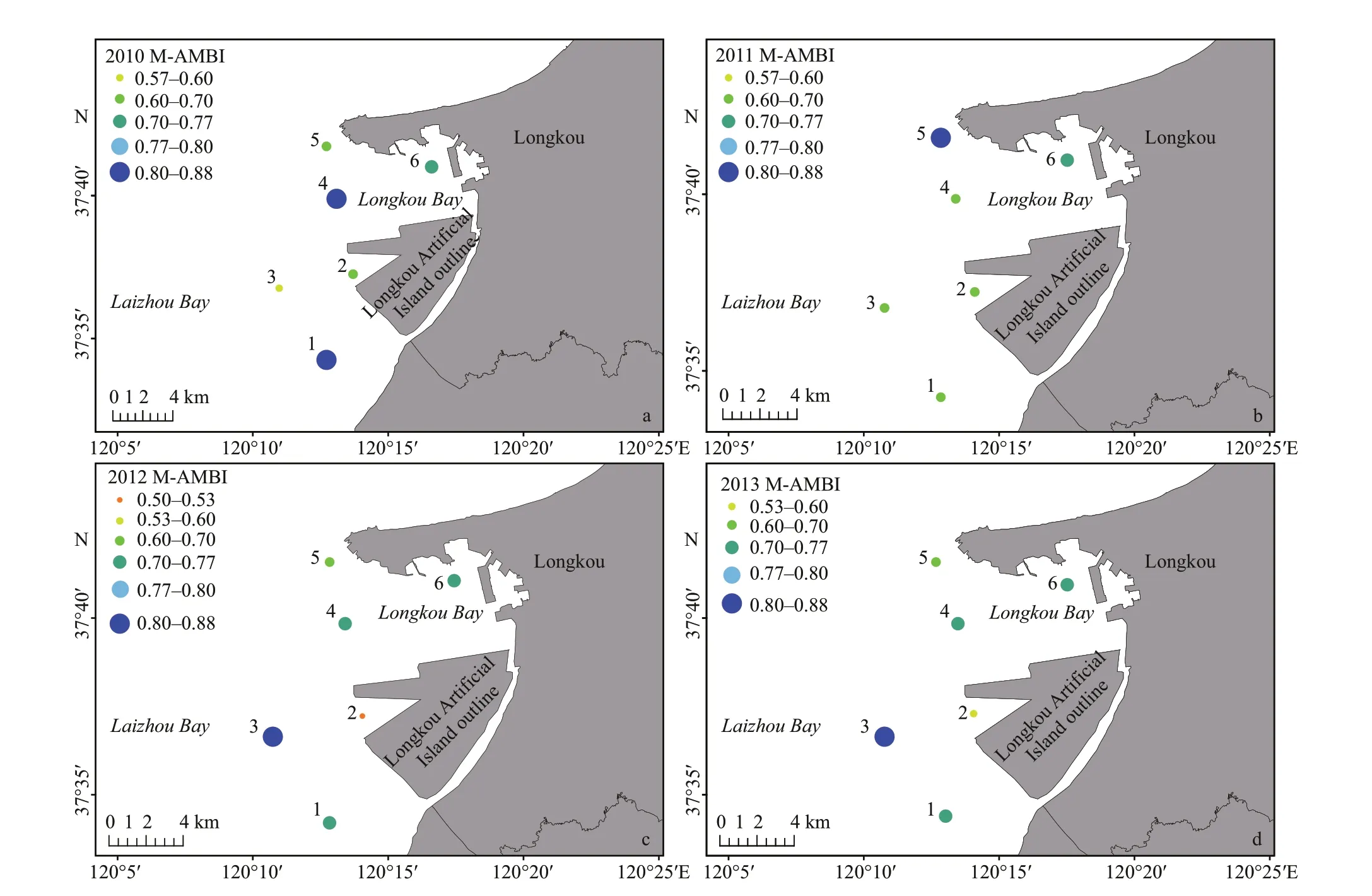
Fig.6 M-AMBI analysis of all the sampling stations from 2010 to 2013
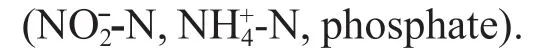
Permutation tests showed that the environmental variables, concentration of Pb, Cd, Cr, oil percentage of the sediment, and pH, salinity, and depth of seawater, signifi cantly infl uenced the abundance of dominant species and were signifi cantly correlated with RDA1 and RDA2 (P<0.05). The dominant species in 2013 were correlative positively to the salinity and SPM concentration in seawater, and oil concentration in sediment. The species (Scolelepissp.,Capitellasp.,Umboniumthomasi, andNeanthesjaponica) that were tolerant to contamination were related positively to most trace metal in sediment, and these species were mainly dominant in station 1 and 2 in 2011 and 2012. The dominant species,GlycindegurjanovaeandNephtyscaliforniensis, in 2010 andGlycerachirori,Nephtyssp.,Gammarussp., andLineussp. in 2011 and 2012 were positively correlative with pH, DO, depth and Cr, and Pb concentration in the sediment. These species were mainly found in stations 4, 5, and 6 from 2010 to 2012 (Fig.8).
3.8 The potential infl uential factors of environmental variables besides those of reclamation
Some infl uential factors refl ected climate change and human disturbance in Longkou district (Table 3), which might be closely associated with the detected important environmental variables to macrobenthic communities. The average temperature increased by 0.7°C from 2010 to 2013. The average rainfall was especially higher in 2013 than it was in other years. The production of sea fi shing decreased by years from 2010 to 2013, and the production of mariculture was evidently higher in 2010 and decreased in 2011 and 2012, then increased in 2013. The volume of water supply and cargo handling capacity of Longkou Port increased year by years. Since 2011, 12×107m3projects have been completed in 2 years (Table 3).
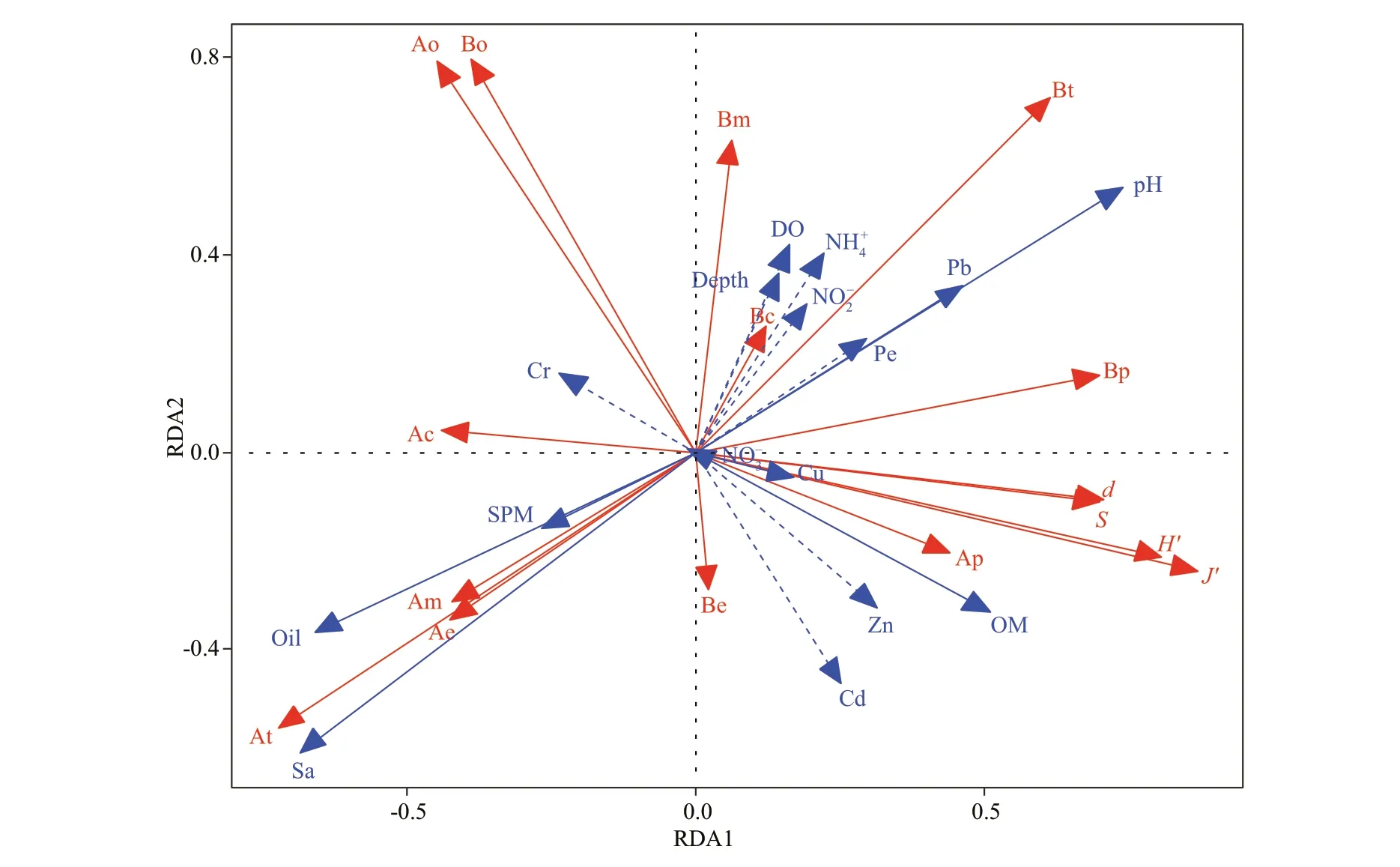
Fig.7 RDA ordination diagram of macrobenthic variables and environmental variables in Laizhou Bay
Fig.8 RDA ordination diagram of dominant species and environmental variables in Laizhou Bay
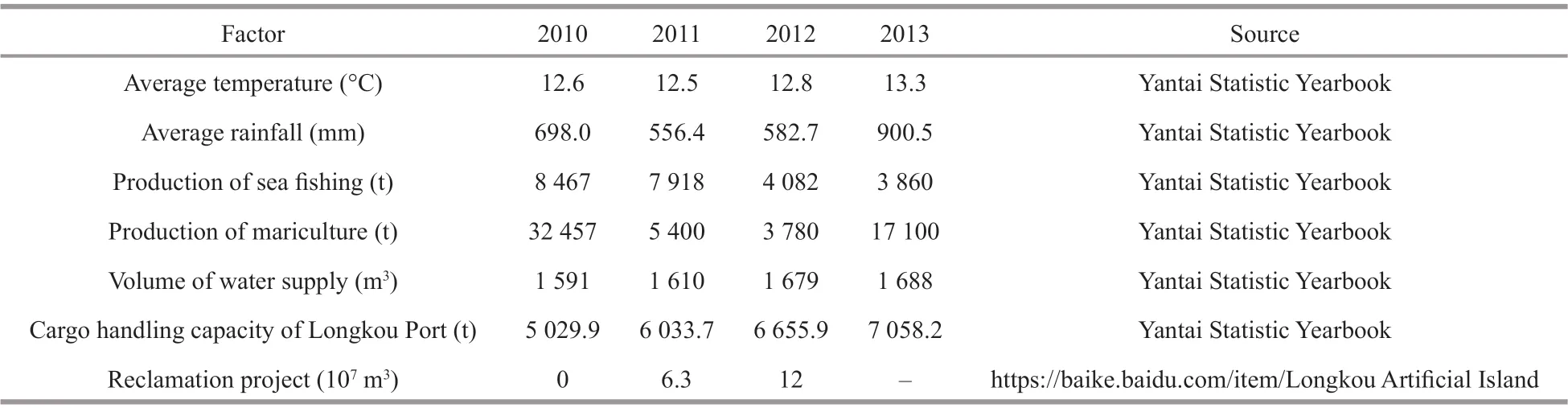
Table 3 The potential infl uential factors of environmental variables in Longkou district from 2010 to 2013
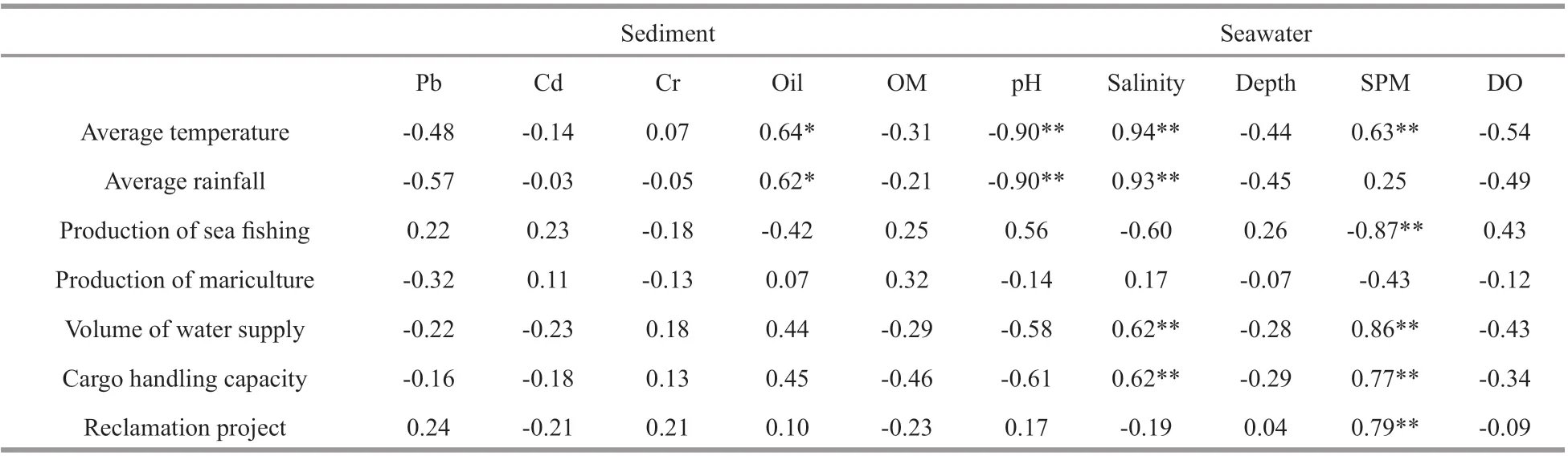
Table 4 Pearson correlation results of environmental variables and potential infl uential factors
The status of seawater was especially infl uenced by human activities and climate change. The pH was negatively correlated to average temperature and rainfall of Longkou (P<0.01). Salinity was positively related to average temperature, average rainfall, volume of water supply, and cargo handling capacity of Longkou Port (P<0.01). The concentration of SPM was positively correlated to average temperature, the reclamation project, the volume of water supply, and the cargo handling capacity of Longkou Port, but was negatively related to the production of sea fi shing (P<0.01). In the sediment, the oil concentration had a signifi cantly positive correlation with average temperature and rainfall of the Longkou district (P<0.05) (Table 4).
4 DISCUSSION
4.1 The changes of environmental variables and macrobenthic community structure related to the reclamation in Laizhou Bay
The environmental variables and macrobenthic community did not show any distinctive changes from 2010 to 2011 when the construction started in January 2011. Further, they were absolutely abnormal in 2013 and showed signifi cant diff erences compared with those in other surveys, which might be explained by the time lag eff ect of reclamation activities on the adjacent marine environment and macrobenthic community. Otherwise, the average rainfall, production of mariculture and cargo handling capacity of Longkou Port was absolutely higher in 2013, which might also cause the distinctive changes in environmental variables and communities in 2013. The signifi cant correlation between environmental variables, pH, salinity, and SPM concentration, and other human activities besides reclamation could further testify to our hypothesis (Table 4).
Although the infl uencing factors were diverse, and the mechanism of modifi cation of environmental variables and communities were complicated, the eff ect of reclamation was distinct because LAI suddenly appeared in 2011 but was completed in 2016 due to its original plan and some other factors are leading to continuous damage to the environment and biological communities all the time. Besides, it has been found that the reclamation could cause serious problems: damage to coastal ecosystems, and deterioration of marine environment, such as increasing water turbidity, sediment deposition, and variation of the hydrodynamic environment (Dugan et al., 2008; Yan et al., 2015; Duan et al., 2016). Hence, the sudden disturbance of the LAI project will inevitably lead to changes on the biological communities in Longkou Bay.
In our surveys, more dominant-species appeared when the construction started in 2011 and the biodiversity increased each year. However, these changes do not indicate a good status of macrobenthic communities, which could be explained by the Intermediate Disturbance Hypothesis (IDH). The theory of IDH states that the biodiversity of biocommunities will be kept at a high level under intermediate frequency or intensity of disturbances (Wilkinson, 1999; Gerwing et al., 2017). The reclamation project changed the benthic habitat and decreased the absolute dominant status of preexisting dominant species, which off er more chances for other species to survive and spread. This phenomenon was also reported in other surveys. Lu et al. (2002) indicated that the family number and abundance of macrobenthos increased with distance from the reclaimed areas. Yan et al. (2015) also pointed out that a low-intensity reclamation could increase species diversity and biomass.
Simultaneously, the miniaturization of species and homogenization of the structure of communities have happened in the survey areas. The phenomena were indicated by the increasing average similarity among stations over time and the high discrepancy between total abundance and total biomass in 2013, separately. The miniaturization happened continuously in Laizhou Bay for a long time. In the 1980s, the macrobenthic community of Laizhou Bay was dominated by large-body sized MolluscaMusculus
senhouseiand EchinodermataEchinocardium
cordatum(Sun and Liu, 1991). However, the dominant species shifted to smaller body size individuals of Polychaeta, Bivalve, and Crustacea since the 1990s (Zhou et al., 2007; Liu et al., 2014). The trigger factors leading to this scenario might be comprehensive, due to the complication of physicochemical and biological factors (Pearson and Rosenberg, 1978; Dong et al., 2016). The construction of the LAI would certainly aggravate this process. As for the homogenizing eff ects, it can be seen from the health status of macrobenthic communities. The health status of station 2 was deteriorating after reclamation while station 3 changed from the worst status to the best status among all the stations. The eff ects of reclamation may be dependent on both the distance from the reclamation areas and the original status of the station.
No remarkable spatial heterogeneities were observed for the abundance, biomass, and biodiversity of macrobenthic communities and environmental variables of seawater, whereas, signifi cant temporal changes occurred due to the synchronous changes of climate and anthropic perturbation. The main reasons can be explained as follows: the areas we surveyed were relatively on a small-scale, which were only situated inside and outside of Longkou Bay in Laizhou Bay, Bohai Sea. The fl uidity of seawater could cause the similarity of environmental variables of seawater in such a small-scale area. For the immobile sediment, the percentage of silt and sand was signifi cantly diff erent among stations. The main taxa groups of biological communities are highly consistent in this small-scale area. The abundance, biomass, and biodiversity were based on the data of all species in the taxa groups or macrobenthic communities, so these indices may fail to show the detailed distinction of each species in communities.
The environmental status deteriorated by time with lower values of pH, DO, OM percentage and higher values of salinity, SPM concentration, and oil concentration after the construction of LAI. Besides, the macrobenthic communities also showed changes with lower ecological status of dominant species and miniaturization of species. However, the health status of the stations was in “good” to “high” levels except station 2 among survey periods. This discrepancy was explicable because the health status was based on the ecological status of every species in the station and it could not be infl uenced signifi cantly by only a few dominant species and the body size of species. What is more, the benthic health could not respond as quickly as the environmental status to the perturbation. Although the benthic health status of most stations was good in our research period, station 2 has shown moderate disturbance trends due to the LAI construction. It will be hard to keep this good status in the future due to the deteriorated environment besides the lasting dramatic project disturbance.
The direct and subsequent impact from reclamation on the marine ecosystem might last for a short time or quite a long period. The recovery of the community from the impacts of dredging activities caused by coastal reclamation may last 2 to 10 years (Newell et al., 1998). Here, we also emphasize our limitations in this present work. Due to the limited surveys before reclamation and because no compatible reports were found to fi ll this gap, we could not draw a clear conclusion on the succession of bio-communities and the representative status of macrobenthos before reclamation. Additionally, the construction phase had not been completed yet during our surveys, so the overall infl uence of reclamation might have been seriously underestimated.
4.2 The relationship between macrobenthic communities and environmental variables in Laizhou Bay
The RDA ordination analysis showed that the environmental variables, depth, salinity, and pH in seawater and oil, and trace metals (Pb, Cr, Cd) in sediment, signifi cantly infl uenced the macrobenthic communities.
Depth and salinity were important factors regulating the distribution pattern of macrofauna (Hagberg et al., 2003; Zhang et al., 2016; Xu et al., 2018). Usually, the deeper regions have lower temperature and higher salinity (Liu et al., 1986). It seems that the depth was correlated negatively with salinity in our study, which may be caused by the complicated disturbance in the semi-closed bay. Station 2 was situated in the semi-closed area created by LAI where signifi cant deposition occurred (An et al., 2010) and the depth here was changed from 9.8 m in 2010 to 5.5 m in 2013. This may also be the cause of the worse health status of station 2 compared to other stations.
Acidifi cation generally has a negative eff ect on calcifi cation of species, such as Mollusca, sea urchin, Crustacea (Byrne and Przeslawski, 2013). Therefore, the CrustaceaGammarussp. and MolluscaUmboniumthomasiwere distributed mainly in areas with high pH. Obviously, the biomass was correlated positively to pH in our study because the Mollusca and Crustacea were the main contributions to biomass.
The sediment contaminants, oil, and trace metal are sub-toxic to marine biology. They will be cumulated and transferred by macrobenthic species via the food cycle (Gesteira and Dauvin, 2005; Rabaoui et al., 2015). Some small-size Polychaeta species (Scolelepissp.,Capitellasp.,Glycindegurjanovae,Nephtyscaliforniensis) with high tolerance to contaminants were positively correlated with trace metal in sediment. The dominant species changed from Polychaeta (carnivorous group, trophic level IV) in 2010 to Bivalve (planktophagous group, trophic level II) and Polychaeta (detritivorous group, trophic level II) in 2013. These lower trophic level species might have been more adaptive to the new habitat with higher salinity, and oil concentration in 2013. The defi nition of functional groups was referred to Li et al. (2013).
It is unreasonable to attribute the cause of the observed variation to the measured variables because other unmeasured variables may be more responsible (Zhou et al., 2007). The environmental variables measured could only partially explain the variation of the macrobenthic communities. Sediment type is also an important factor determining the distribution of species by infl uencing the availability of foods and habitat heterogeneity for macrobenthos (Shou et al., 2018). Unfortunately, the sediment composition was not included in RDA analysis due to missing values in 2013. In the present work, Echinodermata taxa were not found but the Nemertinea appeared in 2011 and 2012 after the construction of the LAI, which may be related to the sediment type. Li et al. (2013) analyzed the benthic functional group in the Laizhou Bay and found the ratio of Echinodermata in total macrobenthic biomass decreased signifi cantly compared to Echinodermata data in 1988. He concluded that this was most possibly attributed to the changing of local habitat, especially the changing of sand grain size. EchinodermataAmphiuravadicolaprefers to live in the sand substrate with a grain size of MdΦ0.36–0.78 mm (Lu et al., 2008). The reclamation of the LAI modifi ed the grain size of sediment by increased clay content (MdΦ<4 mm) (Ren et al., 2016). For this reason, the changing habitat was no longer suitable for the survival ofA.vadicola. On the contrary, Nemertinea speciesLineussp. was not found in 2010, but then reoccurred from 2011 to 2013. The new forming habitat by reclamation activities might be suitable for these animals’ survival and growth. The health status of station 2 was getting worse after the construction of the LAI, which can also be related to its higher silt percentage and concentration of Cu in sediment. Station 2, closest to the LAI, had the worse status, which was caused notably by the reclamation construction.
The eff ects of climate change and anthropic activities are complicated and synergistic on ecological systems. In the correlation analysis, we found that the pH, oil concentration, and salinity were signifi cantly related to average temperature and rainfall in the Longkou district. The substantial changes in the environmental variables were also related to climate change. Water depth in the Longkou Bay could be aff ected by the reclamation projects and dredging of the Longkou Port (An et al., 2010). The SPM was created by the construction of the LAI (You and Chen, 2019). Oil, as the main pollutant was also reported in 2010 due to the vessel leakage in Longkou Port (Han et al., 2010). The oil concentration that increased signifi cantly in 2013 was most possibly caused by the increase of fi shing vessels in Longkou Port (Table 4) and the release of it by the construction equipment, such as excavators, loaders, bulldozers, dump trucks, and rollers, during the reclamation activities.
4.3 Recommendations on coastal reclamation
Our fi ndings agree well with previous investigations on the coastal reclamation. The coastal reclamation has a great impact on the environment and the ecosystem, including water turbidity, sediment deposition and the grain size of sediment, hydrodynamic environment, and macrobenthic assemblages (An et al., 2013; Yan et al., 2013; Shen et al., 2015). The local government has realized the damaging eff ects of reclamation on the ecological environment and implemented the most stringent control measures in 2018. However, some projects are still carried out in Bohai Sea. Minimizing the damage of the projects, environmental protection and recovery are still urgently needed. Some suggested recommendations are as follows:
1) the reclamation construction can lead to signifi cant increase of SPM concentration, which will aff ect the survival of some key species seriously, especially in their early development stage that is sensitive to SPM, and will aff ect the population recruitment later. Therefore, the site selection of reclamation should be more discreet, avoiding the spawning period and the spawning grounds;
2) echinodermata taxa are more sensitive to coastal reclamation, thus, great attention should be paid to these coastal reclamation areas;
3) long-term surveys on macrobenthic communities are necessary for the assessment of impacts of reclamation on marine ecosystems due to the time lag phenomenon of eff ects from reclamation construction.
4.4 Limitations of our study
It will be hard to achieve any interesting fi nding when analyzing the eff ect of the mechanism of reclamation on macrobenthic communities without suffi cient sampling. However, our survey focused on the temporary changes of macrobenthic communities under the eff ects of the LAI projects but not the mechanism of the eff ects, although it is very important. The mechanism of the eff ects on macrobenthos is complicated and our limited data cannot make it clear at all. The sample collection and species identifi cations are time and labor consuming. The data cannot be made available in a timely and easy fashion. Longterm analysis is meaningful for the macrobenthic changes and eff ects analysis of human activities such as reclamation. However, the periodical survey and reports on biological communities could give timely information about the changes in the biological health status. We hope our research will provide timely information of the temporal status and changes of environmental variables and macrobenthic communities due to the construction of the LAI and then be a basis for further analysis of the eff ects of reclamation on marine ecological systems.
5 CONCLUSION
We investigated the changes of the environment and macrobenthic assemblages related to the construction of the LAI in Laizhou Bay. The construction of the LAI had complicated eff ects on the environment and the macrobenthic community. Our fi ndings could provide a basis for the long-term ecological assessment in the reclamation areas, as well as a guideline for the management of reclamation projects and a sustainable development strategy. The main conclusions are followed:
1) the body of water and the sediment environment were getting worse every year with lower values of pH, DO, OM percentage and higher values of salinity, SPM concentration, and oil concentration. These changes will aff ect the community in turn. For example, higher abundance of some dominant species in 2013 was related to higher salinity, SPM concentration in seawater, and oil concentration in sediment;
2) the species composition of the macrobenthic community changed signifi cantly, which was evidenced by the shift of the dominant species from Polychaeta taxa in 2010 to Mollusca taxa in 2013. More taxa of dominant species were found in 2011 when the construction started;
3) the average abundance and biomass of macrobenthos also changed during the LAI construction, represented by the increase of one to two times the value of abundance and decreased two to seven times the value of the biomass in 2013 compared to other surveys. An apparent miniaturization tendency in body size of macrobenthic species occurred from 2010 to 2013;
4) we found a spatial homogenization of adjacent habitat and community structure in survey areas during the construction of the LAI Artifi cial Island, indicated by the increased average similarity of biocommunities each year from 2010 to 2013;
5) the benthic ecological health changed due to the distance from the LAI. The ES of station 3 that was relatively far from the LAI was improving while the ES of station 2 that was close to the LAI was getting worse.
6 DATA AVAILABILITY STATEMENT
The data that support the fi ndings of this study are available upon reasonable request from the corresponding author.
7 ACKNOWLEDGMENT
We also thank Dr. WANG Quanchao for his help in sampling and species identifi cation. We would like to thank anonymous reviewers for their suggestions and comments on earlier versions of the manuscript.
References
An Y N, Wu J Z, Zhu L H, Hu R J, Yue N N. 2010. Response of erosion-deposition pattern to artifi cial islands construction in Longkou Bay.MarineGeologyLetters, 26(10): 24-30, https://doi.org/10.16028/j.1009-2722.2010.10.002. (in Chinese with English abstract)
An Y N, Yang K, Wang Y, Li J. 2013. Eff ect on trend of coastal geomorphological evolution after construction of artifi cial islands in Longkou Bay.AdvancedMaterialsResearch, 726-731: 3 308-3 312, https://doi.org/10.4028/www.scientifi c.net/AMR.726-731.3308.
An Y N. 2010. Study on Impacts of Off shore Artifi cial Islands Cluster’s Construction on Scouring-Deposition Features in Longkou Bay. Ocean University of China, Qingdao. (in Chinese with English abstract)
Bilyard G R. 1987. The value of benthic infauna in marine pollution monitoring studies.MarinePollutionBulletin, 18(11): 581-585.
Borja A, Muxika H. 2005. Guidelines for the use of AMBI (AZTI's Marine Biotic Index) in the assessment of the benthic ecological quality.MarinePollutionBulletin, 50(7): 787-789, https://doi.org/10.1016/j.marpolbul.2005. 04.040.
Borja A, Tunberg B G. 2011. Assessing benthic health in stressed subtropical estuaries, eastern Florida, USA using AMBI and M-AMBI.EcologicalIndicators, 11(2): 295-303, https://doi.org/10.1016/j.ecolind.2010.05.007.
Byrne M, Przeslawski R. 2013. Multistressor impacts of warming and acidifi cation of the ocean on marine invertebrates’ life histories.IntegrativeandComparativeBiology, 53(4): 582-596, https://doi.org/10.1093/icb/ict049.
Dong Y W, Huang X W, Wang W, Li Y, Wang J. 2016. The marine ‘great wall’ of China: local-and broad-scale ecological impacts of coastal infrastructure on intertidal macrobenthic communities.DiversityandDistributions, 22(7): 731-744.
Duan H B, Zhang H, Huang Q F, Zhang Y K, Hu M W, Niu Y N, Zhu J S. 2016. Characterization and environmental impact analysis of sea land reclamation activities in China.Ocean&CoastalManagement, 130: 128-137, https://doi.org/10.1016/j.ocecoaman.2016.06.006.
Dugan J E, Hubbard D M, Rodil I F, Revell D L, Schroeter S. 2008. Ecological eff ects of coastal armoring on sandy beaches.MarineEcology, 29(S1): 160-170, https://doi.org/10.1111/j.1439-0485.2008.00231.x
Ehrenfeld J G. 2000. Evaluating wetlands within an urban context.EcologicalEngineering, 15(3-4): 253-265, https://doi.org/10.1016/s0925-8574(00)00080-x.
Gerwing T G, Gerwing A M A, Macdonald T, Cox K, Juanes F, Dudas S E. 2017. Intertidal soft-sediment community does not respond to disturbance as postulated by the intermediate disturbance hypothesis.JournalofSeaResearch, 129: 22-28, https://doi.org/10.1016/j.seares. 2017.09.001.
Gesteira J G, Dauvin J C. 2005. Impact of theAegeanSea oil spill on the subtidal fi ne sand macrobenthic community of the Ares-Betanzos Ria (Northwest Spain).MarineEnvironmentalResearch, 60(3): 289-316, https://doi.org/10.1016/j.marenvres.2004.11.001.
Hagberg J, Jonzén N, Lundberg P, Ripa J. 2003. Uncertain biotic and abiotic interactions in benthic communities.Oikos, 100(2): 353-361, https://doi.org/10.1034/j.1600- 0706.2003.12138.x.
Han B, Song Z L, Cao L, Yu F, Chen A P, Wang X R. 2010. Survey and assessment of coastal seawater quality in Longkou Bay.AdvancesinMarineScience, 28(2): 186-192. (in Chinese with English abstract)
Li B Q, Li X J, Bouma T J, Soissons L M, Cozzoli F, Wang Q C, Zhou Z Q, Chen L L. 2017. Analysis of macrobenthic assemblages and ecological health of Yellow River Delta, China, using AMBI & M-AMBI assessment method.MarinePollutionBulletin, 119(2): 23-32, https://doi.org/10.1016/j.marpolbul.2017.03.044.
Li J M, Sun C, Xie E N. 2012. An empirical analysis of economic driving forces on land reclamation.ChineseFisheriesEconomics, 30(6): 61-68. (in Chinese with English abstract)
Li S W, Liu Y J, Li F, Zhang Y, Xu Z F, Lv Z B, Wang T T, Zhang A B. 2013. Macrobenthic functional groups in Laizhou Bay, East China.ChineseJournalofEcology, 32(2): 380-388, https://doi.org/10.13292/j.1000-4890. 2013.0143. (in Chinese with English abstract)
Liu R Y, Cui Y H, Xu F S, Tang Z C. 1986. Ecological characteristics of macrobenthos of the Yellow Sea and the East China Sea.StudiaMarinaSinica, 27: 153-173.
Liu X S, Zhao R, Hua E, Lu L, Zhang Z N. 2014. Macrofaunal community structure in the Laizhou Bay in summer and the comparison with historical data.MarineScienceBulletin, 33(3): 283-292. (in Chinese with English abstract)
Lu H, Shen C, Feng B. 2008. Distribution of Branchiostoma belcheri and Amphiura vadicola in relation to bottom grain size in Dafangji waters, Maoming.PeriodicalofOceanUniversityofChina, 38(2): 297-302, https://doi.org/10.16441/j.cnki.hdxb.2008.02.023.
Lu L, Goh B P L, Chou L M. 2002. Eff ects of coastal reclamation on riverine macrobenthic infauna (Sungei Punggol) in Singapore.JournalofAquaticEcosystemStressandRecovery, 9(2): 127-135.
Lv W W, Liu Z Q, Yang Y, Huang Y H, Fan B, Jiang Q C, Zhao Y L. 2016. Loss and self-restoration of macrobenthic diversity in reclamation habitats of estuarine islands in Yangtze Estuary, China.MarinePollutionBulletin, 103(1-2): 128-136, https://doi.org/10.1016/j.marpolbul.2015. 12.030.
Newell R C, Seiderer L J, Hitchcock D R. 1998. The impact of dredging works in coastal waters: a review of the sensitivity to disturbance and subsequent recovery of biological resources on the sea bed.OceanographyandMarineBiology:AnAnnualReview, 36(1): 127-178.
Pearson T H, Rosenberg R. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment.OceanographyandMarineBiologyAnnualReview, 16: 229-311.
Rabaoui L, El Zrelli R, Mansour M B, Balti R, Mansour L, Tlig-Zouari S, Guerfel M. 2015. On the relationship between the diversity and structure of benthic macroinvertebrate communities and sediment enrichment with heavy metals in Gabes Gulf, Tunisia.JournaloftheMarineBiologicalAssociationoftheUnitedKingdom, 95(2): 233-245, https://doi.org/10.1017/S0025315414001489.
Ren P, Sun Z G, Wang C Y, Zhao Q S, Zhu H. 2016. Impacts of construction of artifi cial islands on the fl ow-sediment regulation scheme on grain and clay compositions in the Longkou Bay.AdvancesinMarineScience, 34(4): 578-587. (in Chinese with English abstract)
Shen C C, Shi H H, Zheng W, Li F, Peng S T, Ding D W. 2015. Study on the cumulative impact of reclamation activities on ecosystem health in coastal waters.MarinePollutionBulletin, 103(1-2): 144-150, https://doi.org/10.1016/j.marpolbul.2015.12.028.
Shou L, Liao Y B, Tang Y B, Chen J F, Jiang Z B, Gao A G, Chen Q Z. 2018. Seasonal distribution of macrobenthos and its relationship with environmental factors in Yellow Sea and East China Sea.JournalofOceanologyandLimnology, 36(3): 772-782, https://doi.org/10.1007/s00343-018-6271-1.
Sun D Y, Liu Y C. 1991. Species composition and quantitative distributions of biomass and density of the macrobenthic infauna in the Bohai Sea.JournalofOceanographyofHuanghai&BohaiSea, 9(1): 42-50. (in Chinese with English abstract)
Suo A N, Cao K, Zhao J H, Lin Y. 2015. Study on impacts of sea reclamation on fi sh community in adjacent waters: a case in Caofeidian, North China.JournalofCoastalResearch, 73(sp1): 183-188, https://doi.org/10.2112/SI73-032.1.
Ter Braak C J F, Šmilauer P. 2002. CANOCO Reference Manual and CanoDraw for Windows User's Guide: Software for Canonical Community Ordination (version 4. 5). Ithaca NY, USA, www.canoco.com. FAO.
Wang W, Liu H, Li Y Q, Su J L. 2014. Development and management of land reclamation in China.Ocean&CoastalManagement, 102: 415-425, https://doi.org/10. 1016/j.ocecoaman.2014.03.009.
Wilkinson D M. 1999. The disturbing history of intermediate disturbance.Oikos, 84(1): 145-147.
Xu Y, Yu F, Li X Z, Ma L, Dong D, Kou Q, Sui J X, Gan Z B, Gong L, Yang M, Wang Y Y, Sun Y, Wang J B, Wang H F. 2018. Spatiotemporal patterns of the macrofaunal community structure in the East China Sea, off the coast of Zhejiang, China, and the impact of the Kuroshio Branch Current.PLoSOne, 13(1): e0192023, https://doi.org/10.1371/journal.pone.0192023.
Xu Z L, Chen Y Q. 1989. Aggregated intensity of dominant species of zooplankton in autumn in the East China Sea and Yellow Sea.ChineseJournalofEcology, 8(4): 13-15, 19. (in Chinese with English abstract)
Yan H K, Wang N, Yu T L, Fu Q, Liang C. 2013. Comparing eff ects of land reclamation techniques on water pollution and fi shery loss for a large-scale off shore airport island in Jinzhou Bay, Bohai Sea, China.MarinePollutionBulletin, 71(1-2): 29-40, https://doi.org/10.1016/j.marpolbul.2013.03.040.
Yan J G, Cui B S, Zheng J J, Xie T, Wang Q, Li S Z. 2015. Quantifi cation of intensive hybrid coastal reclamation for revealing its impacts on macrozoobenthos.EnvironmentalResearchLetters, 10(1): 014004, https://doi.org/10. 1088/1748-9326/10/1/014004.
Yantai Statistic Yearbook. 2011-2014, http://tjj.yantai.gov.cn/col/col118/index.html. Accessed on 2018-3-29.
You Z J, Chen C. 2019. Coastal dynamics and sediment resuspension in Laizhou bay.In: Wang X H ed. Sediment Dynamics of Chinese Muddy Coasts and Estuaries: Physics, Biology and their Interactions. Elsevier, Amsterdam. p.123-142.
Zhang J L, Xiao N, Zhang S P, Xu F S, Zhang S Q. 2016. A comparative study on the macrobenthic community over a half century in the Yellow Sea, China.JournalofOceanography, 72(2): 189-205, https://doi.org/10.1007/s10872-015-0319-z.
Zhou H, Zhang Z N, Liu X S, Tu L H, Yu Z S. 2007. Changes in the shelf macrobenthic community over large temporal and spatial scales in the Bohai Sea, China.JournalofMarineSystems, 67(3-4): 312-321, http://dx.doi.org/10. 1016/j.jmarsys.2006.04.018.
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Exploring sensitive area in the tropical Indian Ocean for El Niño prediction: implication for targeted observation*
- Analysis of the typhoon wave distribution simulated in WAVEWATCH-III model in the context of Kuroshio and wind-induced current*
- Characterizing the capability of mesoscale eddies to carry drifters in the northwest Pacifi c*
- Observation system simulation experiments using an ensemble-based method in the northeastern South China Sea*
- Statistical analysis of intensity variations in tropical cyclones in the East China Sea passing over the Kuroshio*
