Sedimentary diatom and pigment-inferred recent anthropogenic accelerated eutrophication of a Mediterranean lake (Lake Dojran, Republic of North Macedonia / Greece)*
ZHANG Xiaosen , XU Xinyu Jane M. REED
1 Institute of Loess Plateau, Shanxi University, Taiyuan 030006, China
2 Department of Geography, Geology and Environment, University of Hull, Hull HU6 7RX, UK
Abstract Lake eutrophication is recognised as a serious global challenge, and many regional legislative programmes are being made to attempt to relieve nutrient pollution and restore deteriorated lake ecological state. However, it is of primary importance to understand the degradation processes and reference conditions. The palaeolimnological approach allows us to use ecological evidences preserved in lake sediments to track the changes of lake trophic status under human impact. Diatoms, a proxy for ecological and limnological change, and pigments, a proxy for algal production and composition, were analysed on a short sediment sequence from Lake Dojran (Republic of North Macedonia and Greece), and their preservation qualities were evaluated before environmental interpretation. Good diatom preservation is inferred mainly from the consistent co-occurrence of robust, highly-silicifi ed taxa and small taxa throughout the sequence. Pigment evaluation of the comparison between wet sediment samples in dark and cold storage and their corresponding dry sediment samples lyophilized immediately after the recovery reveals that sediment restoration conditions are critical for the accuracy of analysis. We show that the increased chlorophyll and xanthophyll pigment concentrations, particularly the siliceous-algae pigment fucoxanthin and diatoxanthin, together with the distinct increase in diatom concentration, indicate accelerated lake eutrophication and a major ecological shift linked to intensifi ed water abstraction practice and agricultural expansion in the late 18 th to early 19 th century. Evidence of diatom assemblage composition is muted probably by the dominance of widely-tolerant small fragilaroid species in diatom composition and the better competitive ability of cyanobacteria and chlorophytes than diatoms for low light under eutrophic and turbid conditions. This study improves our understanding of recent human-induced environmental change and current ecological restoration target in this lake.
Keyword: diatom, pigment; Lake Dojran; Mediterranean; lake eutrophication; palaeolimnology
1 INTRODUCTION
Anthropogenic nutrient pollution and lake eutrophication have become one of the most widespread, costly and challenging environmental and ecological problems. Nitrogen and phosphorus pollutions have exceeded the earth sustainable boundaries (Rockström et al., 2009; Steff en et al., 2015) and impacted economic growth and human health (Elser and Bennett, 2011; Sutton et al., 2011). Management measures, consumer actions and technological innovation are being made to control both anthropogenic phosphorus and nitrogen diff use and attempt to mitigate harmful algal blooms (Conley et al., 2009; Paerl et al., 2016), and there are now many success stories (Verdonschot et al., 2013), for example, Lake Geneva (France/Switzerland) (Rimet et al., 2009). However, lack of apparent recovery following restricted nutrient loading is mostly common (Bennion et al., 2015), for example, Lake Taihu (China) (Wang et al., 2018; Wan et al., 2019), and more importantly, the target of lake ecological restoration and the timescale to achieve the expectation are still unclear, which is linked to individual unique lake properties and complex lake ecosystems (Bennion et al., 2015). It is thus of primary importance to understand the pathway of human-induced water quality degradation and defi ne the lake reference condition.
Palaeolimnological analysis of lake sediment records can provide insights into tracking humaninduced changes of lake trophic status and defi ning the baseline condition and restoration target (Bennion and Battarbee, 2007; Bennion et al., 2011). Diatoms (Bacillariophyceae) are unicellular algae with a siliceous cell wall, and their silica frustules often preserve well in lake sediments. Due to their sensitivity to a wide range of water chemistry variables, diatoms are recognised as a powerful proxy for past environmental and ecological change (Smol and Stoermer, 2010), and in particular, they off er excellent potential for revealing recent humaninduced lake degradation (Bennion et al., 2010; Hall and Smol, 2010). Algae (diatoms, chlorophytes, cyanobacteria, etc.) are dominant primary producers in lake ecosystems, and as algal preservation in lake sediments can be severely biased by the dissolution of some algal groups while algal pigments can be well preserved, pigments are often taken as a strong proxy for both lake productivity change and the shift of algal structure composition.
The Mediterranean region is a climatically transitional zone under the infl uence of the interaction of the mid-latitude westerlies and the subtropical high-pressure system (i.e. the Azores High), with most of the region controlled by a dry Mediterraneantype climate (Lionello, 2012), and lakes in this region are usually shallow with high conductivity and even saline water (Roberts and Reed, 2009). The potential for palaeolimnological research on recent water quality degradation is largely untapped across the Mediterranean, and the exploration of integrating diatoms and pigments to elucidate recent lake trophic changes also has not been conducted so far in this region. Lake Dojran is a shallow and eutrophic lake in the north-eastern Mediterranean region, and it has been taken as a key site to investigate Mediterranean climate change during the Holocene period (i.e. the past 11 500 years) through palaeolimnological analysis (Francke et al., 2013; Zhang et al., 2014; Masi et al., 2018). Holocene diatom record reveals that both regional climate change and local catchment dynamics aff ect the changes of lake level and trophic status in Lake Dojran (Zhang et al., 2014); however, associated with intensifi ed human impact and increased catchment infl uence during the late Holocene (Athanasiadis et al., 2000; Masi et al., 2018), diatom evidence for major lake ecological shift is weak (Griffi ths et al., 2002; Zhang et al., 2014). Whereas, modern limnological surveys on algal composition indicate human-induced dramatic water level fl uctuation and lake eutrophication in recent decades (Levkov and Stojanovski, 2000-2001; Griffi ths et al., 2002; Sotiria and Petkovski, 2004). This thus emphasises the importance of palaeolimnological analysis in tracking thoroughly the path of human-induced lake trophic change in the past, but also it is essential to combine diatoms and pigments to investigate the change of lake ecological state.
2 SITE DESCRIPTION
Lake Dojran (Fig.1, 41°12′N, 22°44′E, 144 m a.s.l.) is a transboundary lake between the Republic of North Macedonia (i.e. North Macedonia) and the Hellenic Republic (i.e. Greece) in the north-eastern Mediterranean region. The lake receives its water from small rivers, creeks and springs, and loses it through evaporation and groundwater outfl ow today, but surface outfl ow (i.e. the Gjolaja River) was possible at the southern end of the lake in previous phases of high water level, which drained into the Vardar River, a major river in North Macedonia and Greece, and then entered the Aegean Sea (Sotiria and Petkovski, 2004). Maximum lake level was 10.0 m in 1951–1987, decreased to 3.7 m in 2002 due to water abstraction and agricultural practices (Griffi ths et al., 2002), and recovered to 6.7 m in 2010 due to decreased water use and additional water transfer into the lake (Popovska and Bonacci, 2008; Stojov, 2012). Total phosphorus (TP) concentration was 15–130 μg/L in 1953–1960 (Sotiria and Petkovski, 2004), and the lake was consistently eutrophic with the minimum TP value >50 μg/L since 1996 (Temponeras et al., 2000; Lokoska et al., 2006; Tasevska et al., 2010). Due to the infl uence of the typical Mediterranean climate (hot, dry summers and mild, humid winters) and the very simple plate-shaped morphometry of the lake basin, Lake Dojran’s water warms up easily and does not stratify in summer, but it can freeze in winter. Reed beds occupy the fringe of the lake, and submerged plants are common in the littoral zone of the lake. Lake Dojran is currently a eutrophic, shallow and well-mixed lake.
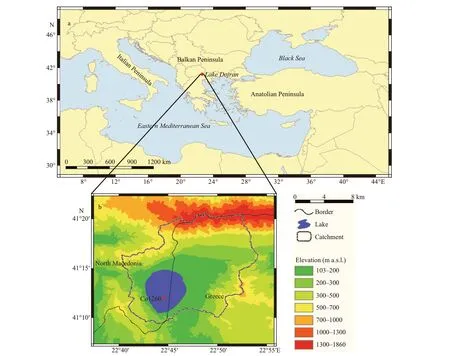
Fig.1 The location of Lake Dojran in the north-eastern Mediterranean region (a) and the catchment of this lake between North Macedonia and Greece and the coring site Co1260 (Francke et al., 2013) (b; modifi ed from Zhang et al., 2014)
3 MATERIAL AND METHOD
The present study is based on the topmost 55 cm section (undisturbed) of a long core Co1260 (Fig.1; Francke et al., 2013; Zhang et al., 2014) in Lake Dojran, which was obtained in June 2011 from the deepest, south-central part of the lake using UWITEC gravity and piston coring equipments. The core was split into two halves in the laboratory. One half was subsampled and freeze-dried, and the other half was sealed airtight and stored in darkness at 4℃. The age model of this core section was estimated separately in this study. As137Cs and210Pb dating methods linked to nuclear bombing apply only to the past several decades, we relied on radiocarbon dating in spite of the uncertainty of14C age calibration for recent samples. Two radiocarbon dates were obtained from the University of Cologne (Germany), of which one from terrestrial plant macrofossil at 53.3 cm depth is 360±7014C BP (1 549±74 cal AD) and the other from bulk organic matter at 16.5 cm depth is 140±3514C BP (1 805±83 cal AD). The14C date from terrestrial plant remains provides reliable age control for the bottom of this core section, and the14C date from bulk organic matter is possibly infl uenced by the carbon reservoir eff ect. We selected to generate a smooth curve between the two dated levels and the sampling date rather than simply using linear interpolation to develop the age model. The age model was established through quadratic polynomial interpolation (the function isy=0.1x2–14.2x+2011) between the two calibrated radiocarbon ages and the year 2011 AD of the surface sediment. We also consider the uncertainty of the age model when interpreting the diatom and pigment data below. The calibration of radiocarbon ages into calendar ages is based on OxCal 4.3 software (Bronk Ramsey, 2009) and IntCal13 calibration curve (Reimer et al., 2013). The age model of this sequence refl ects the sedimentation during the past ca. 450 years (Fig.2).
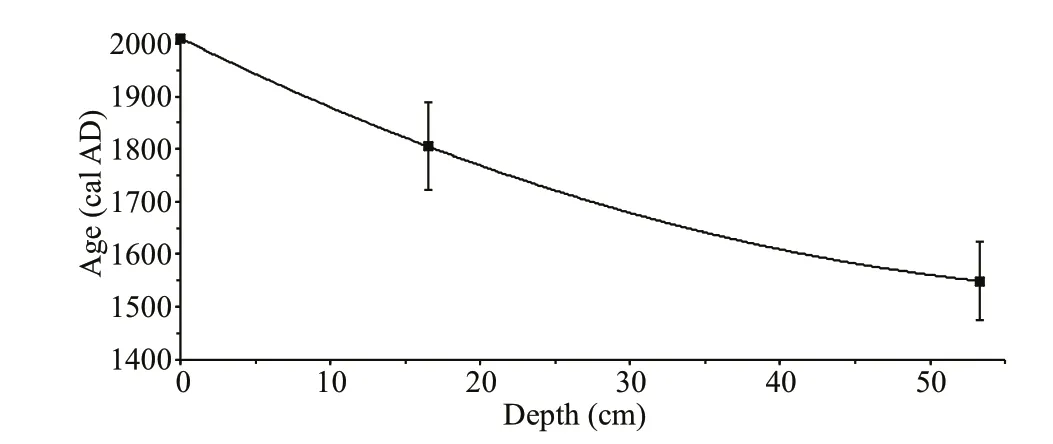
Fig.2 The age-depth model of the 55 cm-long sequence in this study, which is developed through quadratic polynomial interpolation (the function is y=0.1 x 2-14.2 x+2011) between the year 2011 AD of the surface sediment and two calibrated radiocarbon ages
Diatom analysis was carried out on 14 freeze-dried sediment samples taken at 4-cm intervals. Standard techniques in Battarbee et al. (2001) were followed for diatom slide preparation. Approximately 0.1-g dry sediment was heated in 30-mL 30% H2O2to oxidise organic matter, and then a few drops of concentrated HCl were added to remove carbonates (Battarbee et al., 2001). Diatom suspension was diluted to an appropriate concentration, and known quantities of microspheres were added for the calculation of absolute diatom valve concentration. Diatom slides were mounted using Naphrax™. Diatom valves were counted at ×1 000 magnifi cation under oil immersion on an OLYMPUS BX51 light microscope, and more than 300 valves per slide were counted. Diatom fragments were also counted, and were used to potentially assess the quality of diatom preservation. Diatoms were identifi ed mainly following four classic European diatom books published in German language in 1986–1991 (Krammer and Lange-Bertalot, 1986, 1988, 1991a, b), and the identifi cation of diverse benthicNaviculaandCymbellasensu lato species was also based on Lange-Bertalot (2001) and Krammer (2002), respectively. To identify accurately the planktonicStephanodiscusandCyclotellasensu lato species in this study, we referred mainly to recent Václav Houk, Rolf Klee and Hiroyuki Tanaka’s monographs (Houk et al., 2010, 2014).Stephanodiscusminutulus(Kützing) Cleve & Möller andStephanodiscusparvusStoermer & Håkansson are merged intoS.minutulus/parvus.Stephanodiscus
minutulusandS.parvushave similar morphological characteristics and ecological preferences, and it is diffi cult to separate them consistently under the light microscope (Hobbs et al., 2011; Bennion et al., 2012). It is also possible thatS.parvusis a synonym ofS.minutulusthat is a polymorphic taxon (Scheラ er and Morabito, 2003; Cruces et al., 2010). In addition, we referred to Zlatoko Levkov’s taxonomical work in this region (Levkov et al., 2007; Levkov, 2009). We adopted the up-to-date nomenclature from the Catalogue of Diatom Names (Fourtanier and Kociolek, 2011) and the AlgaeBase database (Guiry and Guiry, 2019).
Pigment analysis was based on 14 wet sediment samples taken at 4-cm intervals, and for comparison with diatom data, these wet samples are taken from the same depths of the core section as the dry samples above for diatom analysis. They were stored in darkness at 4°C after core recovery and freeze-dried shortly before analysis. Five dry sediment samples, taken at 12 cm intervals and corresponding to No. 1, 4, 7, 10 and 13 of the 14 wet pigment samples, were also analysed for pigments, and similar to diatom samples, they were freeze-dried immediately after core recovery and stored at room temperature. The pigment analysis of these fi ve dry sediment samples is used to evaluate the degradation of pigments in referring to the results of the corresponding wet sediments. Approximately 0.2-g freeze-dried sediment was soaked in 5-mL extraction solvent (a mixture of 80% acetone, 15% methanol and 5% water) overnight for pigment extraction (McGowan, 2013). The solvent pigment extracts were dried under N2gas, re-dissolved in 500-μL injection solvent (a 70:25:5 mixture of acetone, ion-pairing reagent and methanol), and then separated through the highperformance liquid chromatography (HPLC) based on pigment polarity and mass (McGowan, 2013). Pigments were identifi ed according to their spectral characteristics and retention times, by referring to a range of standard phytoplankton pigment data and graphics (Jeff rey et al., 1997; Egeland et al., 2011). Pigment quantifi cation is based on the conversion of the chromatogram peak area to the concentration, and pigment concentration is expressed as nanomoles per g total organic carbon (nmol/g TOC).
4 RESULT AND DISCUSSION
4.1 Diatom dissolution and preservation
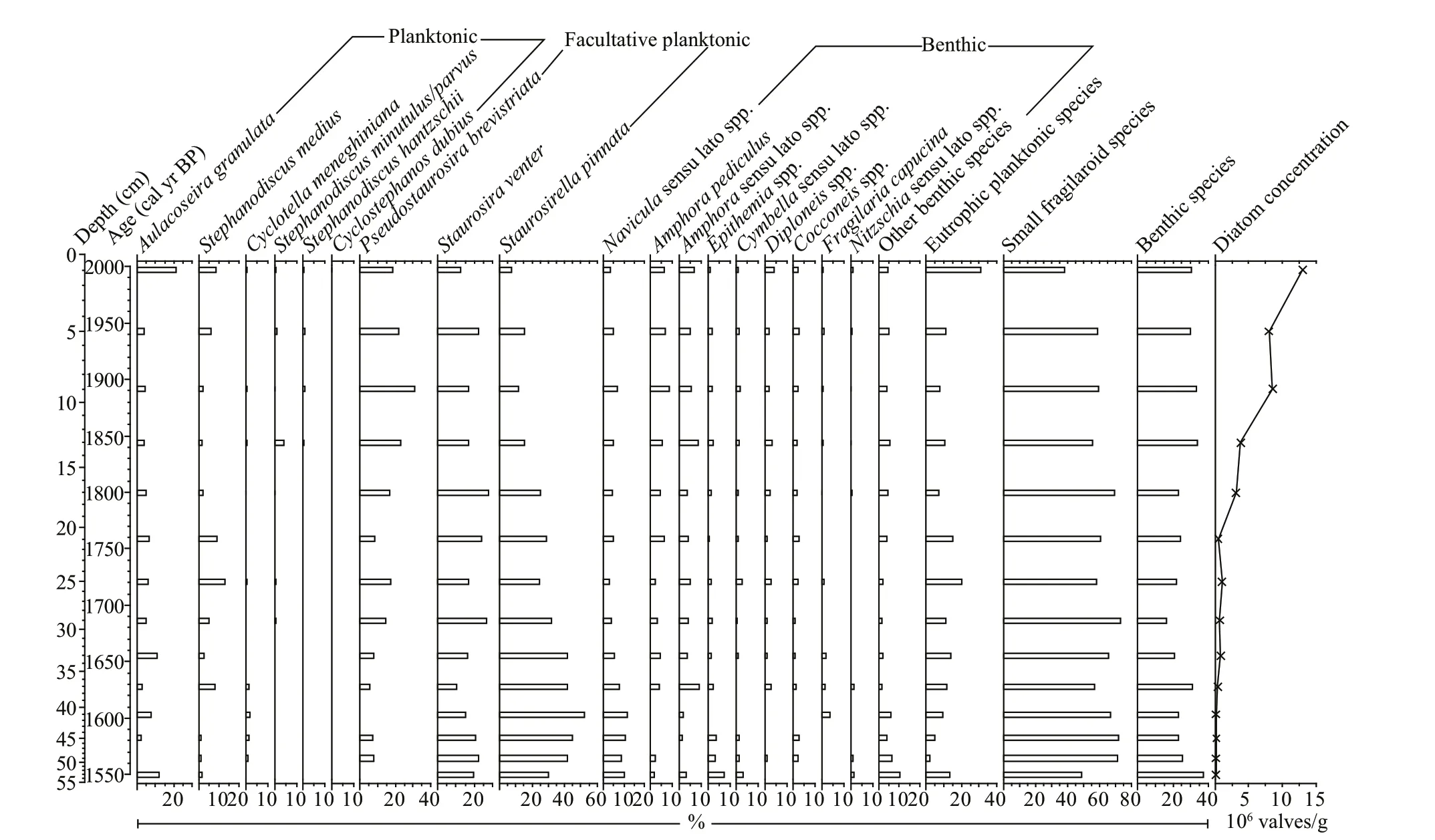
Fig.3 Summary diagram of sedimentary diatom assemblage composition with the groups of eutrophic planktonic species, small fragilaroid species and benthic species, and diatom concentration in this study
More than 300 diatom valves are counted per sample, and diatom valve concentration is >105/g throughout the sequence. Small fragilaroid species, comprisingPseudostaurosirabrevistriata(Grunow) Williams & Round,Staurosiraventer(Ehrenberg) Cleve & Möller andStaurosirellapinnata(Ehrenberg) Williams & Round, are consistently abundant throughout the sequence, and benthic species are diverse and also consistently at relatively high abundance (Fig.3). This is coherent with the currently shallow water state and extensively distributed reed beds of the lake. Planktonic taxa comprise mainly typical eutrophic species, and eutrophicAulacoseiragranulata(Ehrenberg) Simonsen,StephanodiscusmediusHåkansson,CyclotellameneghinianaKützing,Stephanodiscusminutulus/parvus,StephanodiscushantzschiiGrunow andCyclostephanosdubius(Hustedt) Round occur in this sequence (Fig.3). The common occurrence of eutrophic planktonic diatom taxa is consistent with the currently eutrophic, shallow and well-mixed lake water state. Thus, the observed diatom assemblage composition in the lake sediment is closely linked with the limnological and ecological states of the lake, and it is possible that diatom dissolution is minor and diatoms can be considered to be well preserved in this sequence. A simple diatom dissolution index (Findex; Ryves et al., 2001), i.e. the ratio of pristine valves to total valves, is often used to assess the preservation of diatoms, but this index is deemed inappropriate for this sequence, due to the shifts of main taxa and their diff erent degrees of dissolution, i.e. taphonomic bias, and the fragmentation in some degree during the process of diatom slide preparation. However, both robust, highly-silicifi ed taxa (A.granulata,S.medius,Epithemiaspecies, etc.) and small taxa (P.brevistriata,S.venter,Amphorapediculus(Kützing) Grunow, etc.) are consistently observed throughout the sequence (Fig.3). Thus, although diatom dissolution cannot be assessed quantitatively, empirical observation results show that the diatom data of this sequence can be reliably used as an indicator for recent environmental change.
4.2 Pigment degradation and preservation
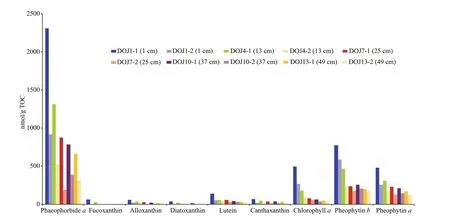
Fig.4 Comparison of pigment concentrations between fi ve wet sediment samples in dark and cold storage before analysis (DOJ*-1) and their corresponding dry sediment samples lyophilized immediately after the recovery (DOJ*-2)
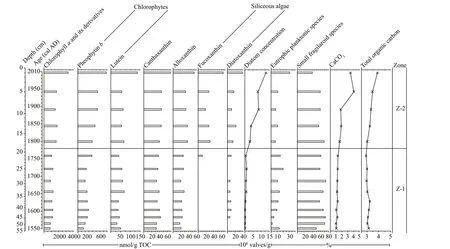
Fig.5 Summary diagram of sedimentary chlorophyll and xanthophyll pigment concentrations in this study and comparison with diatom, carbonate and organic matter content data from the same sequence
Totally nine pigments are identifi ed in the chromatograms of these samples, and they are phaeophorbidea, fucoxanthin, alloxanthin, diatoxanthin, lutein, canthaxanthin, chlorophylla, pheophytinband pheophytina(Figs.4 & 5), which are listed here according to their retention times. Pigment concentrations are expressed in nanomoles per gram total organic carbon (nmol/g TOC) based on their peak areas in the chromatograms. The degradation of pigments is evaluated through the comparison of pigment concentrations in the fi ve dry sediment samples, lyophilized immediately after core recovery and stored under ambient temperature, with their corresponding wet sediment samples that are kept in cold storage until the analysis. Compared with the wet sediment samples, no new pigment derivative occurs in the fi ve dry samples, and the nine pigments exhibit diff erent degrees of degradation with fucoxanthin and diatoxanthin declining even below the limit of detection (Fig.4). Although this is related with the initial concentrations of pigments as in the wet samples, this shows clearly the diff erent levels of stability of pigments and confi rms the important role of sediment storage conditions in pigment analysis (Reuss and Conley, 2005). Exposure to heat, light and oxygen enhances pigment degradation, with the ambient condition increasing exposure to heat and light and the powdery texture of dry sediment maximizing exposure to oxygen (Leavitt and Hodgson, 2001), and Reuss and Conley (2005) suggested that freeze-dried sediments should be also stored in freezer. Fucoxanthin contains an epoxy group, which makes the pigment more susceptible to degradation (Reuss and Conley, 2005). Whereas, diatoxanthin, lutein, canthaxanthin, and alloxanthin do not have such an epoxy group and thus are more stable than fucoxanthin, although their degradation also occurs. This study shows that lutein is the most stable one of the fi ve pigments above (Fig.4). Carotenoids contain a long chain of alternating double bonds which makes them susceptible to degradation, and their breakdown products are colourless and undetectable; whereas, chlorophylls contain a tetrapyrrole ring, and their degradation products of diff erent types (i.e. oxidation, loss of Mg and loss of phytol chain) are still coloured and can be detected (McGowan, 2013). Chlorophyllais unstable and its degradation product pheophytinais quite stable (Reuss and Conley, 2005). This study confi rms the high stability of pheophytinaand shows that phaeophorbideais a relatively unstable degradation product of chlorophylla(Fig.4). Veuger and van Oevelen (2011) suggested that a simple index, i.e. the ratio of chlorophylladerivatives to chlorophylla, could be used to indicate pigment preservation; however, due to the diff erent levels of stability of phaeophorbideaand pheophytina, this index is not suitable in this study. Chlorophyllbis not detected in this study and shows high instability in most cases, while its derivative pheophytinbis highly stable. Thus, sediment storage conditions are key factors for the accuracy of pigment analysis (Reuss and Conley, 2005), and the wet sediment samples freeze-dried just before the analysis in this study can be used as a proxy for recent environmental change.
4.3 Human-induced lake ecological change during the past ca. 450 years
Based on the evaluations of diatom dissolution/preservation and pigment degradation/preservation above, accurate and unbiased results about the change of lake ecological status can be obtained from this sequence. This sequence can be distinctly divided into two zones with a major ecological shift occurring at around 1770 cal AD based on pigment concentration data, which is consistent with the changes of diatom concentration and geochemical proxies (i.e. the contents of carbonates and organic matter in the sediments) (Fig.5). Although there is also a clear shift fromS.pinnatatoP.brevistriatain the diatom assemblage composition (Fig.3), it is well known that the two species have very wide nutrient tolerances and similar habitat preferences and thus the diatom composition data do not show a tipping point of ecological state in this lake during the past ca. 450 years.
Chlorophyllais a widely distributed pigment that originates from all algae and aquatic plants as well as riverine terrestrial plant detritus (Leavitt and Hodgson, 2001). However, due to its exposure to oxygen at the soil surface, chlorophyllais poorly preserved in the terrestrial detrital material (Lami et al., 2000), particularly under the infl uence of human-induced deforestation and agricultural development in the catchment of this lake (Athanasiadis et al., 2000; Masi et al., 2018). Thus, the concentration of chlorophyllaand its derivatives indicates primarily total algal production and can be used as a proxy to infer the change of lake productivity (Leavitt and Hodgson, 2001; McGowan, 2013). In Fig.5, the lower concentration of chlorophyllaand its derivatives in Zone Z-1 (55–19 cm, ca. 1550–1770 cal AD) is largely attributed to lower lake productivity rather than higher pigment degradation, and their higher concentration in Zone Z-2 (19–0 cm, ca. 1770–2010 cal AD), particularly the higher concentration of phaeophorbideathat derives mainly from planktonic algae under the condition of zooplankton grazing (Guilizzoni and Lami, 2002), indicates higher lake productivity. The increase of lake productivity is probably linked to a human-induced dramatic lakelevel decline rather than climate change. The maximum lake level was approximately 15 m at the end of the 18thcentury, and in the year 1808, a channel was dug at the southern end of the lake to connect the Gjolaja River. This is the most infl uential water abstraction practice in this lake during the past 450 years, and in order to reduce the lake area for larger agricultural land and take more water for downstream irrigation, this channel was deepened gradually since then and the maximum lake level declined to less than 4 m in 2002 (Popovska et al., 2005; Stojov, 2012). The lake littoral zone and its macrophytic vegetation also reduced (Griffi ths et al., 2002; Sotiria and Petkovski, 2004). Although recent climate warming can promote chlorophyllaconcentration and lake algal production (Smol, 2019 and references therein), this rapid climate warming arose in the 20thcentury (IPCC, 2013) and is much later than the occurrence of the ecological shift in this lake. The slight chronological diff erence between the reconstructed lake ecological breakpoint and the documented causal human activity could be attributed to the possible carbon reservoir eff ect of the bulk organic matter date and the uncertainty of the age model. In all, the increase in the concentration of chlorophyllaand its derivatives is linked with increased algal blooms and accelerated lake eutrophication since the 19thcentury under the infl uence of intensifi ed anthropogenic water reduction and agricultural nutrient diff use.
Most xanthophylls are used as indicators for specifi c algal classes or functional groups (Leavitt and Hodgson, 2001). In Fig.5, higher lutein and pheophytinbconcentrations in Zone Z-2 (19–0 cm, ca. 1770–2010 cal AD) indicate increased chlorophyte production (McGowan et al., 2012). The slightly higher concentrations of canthaxanthin and alloxanthin in this zone indicate increased production of cyanobacteria and planktonic cryptophytes, respectively (Leavitt and Hodgson, 2001; Guilizzoni and Lami, 2002; McGowan, 2013). Distinctly increased fucoxanthin and diatoxanthin concentrations result from increased production of siliceous algae, particularly diatom production, rather than decreased diatom dissolution, and this is consistent with distinctly increasing diatom concentration in this zone. There is no clear changing trend of the relative abundances of individual eutrophic planktonic diatom species as well as the sum of these taxa in this study (Fig.3), and another palaeolimnoligical analysis in this lake conducted by Griffi ths et al. (2002) also does not show the response of diatom assemblage composition to the accelerated lake eutrophication. This can be probably attributed to the dominance of facultative planktonic fragilaroid taxa rather than planktonic taxa in the diatom assemblage composition and the wide environmental tolerances of the fragilaroid species. Small fragilaroid species can tolerate a wide range of trophic states and survive in a variety of benthic habitats consisting of lake sediments, submerged plants and the base of emergent plants (Sayer, 2001). Because non-planktonic diatoms can derive nutrients from sediments and macrophytes, they respond more directly and sensitively to habitat availability rather than water-column nutrient enrichment (Bennion et al., 2010; Hall and Smol, 2010). Although lake littoral zone and its macrophytic vegetation reduced under human agricultural activities as mentioned above, the decline of both lake area and water level would make the previous pelagic zone become shallow and available for the fragilaroid species to attach to the sediments, due to the simple plate-shaped morphometry of the lake basin. This is a possible reason that the fragilaroid species are consistently at high relative abundance and dominate the diatom assemblage composition in this sequence.
The chlorophyll and xanthophyll pigment concentration data and diatom concentration data show an increase in the production of diatoms, chlorophytes, cyanobacteria and planktonic cryptophytes, indicating increased algal biomass and lake productivity, which is linked to water abstraction practices and more intensive agricultural activities since the 19thcentury. This is also supported by increasing organic matter content (i.e. total organic carbon (TOC)) and distinctly increasing carbonate content from the same sequence, both of which are mainly derived from the in-lake authigenic origin (Francke et al., 2013). Although diatom assemblage composition does not show evidence for the accelerated lake eutrophication, other types of algae could respond and/or new toxic algal species could occur in such a nutrient-enriched environment, which is supported by the algal investigations in recent decades (Sotiria and Petkovski, 2004; Lešoski et al., 2010). Nutrient-polluted lakes often suff er from harmful cyanobacterial blooms and nuisance weeds. It is possible that chlorophytes and/or cyanobacteria override diatoms and become the most important primary producers in this lake at a higher trophic level, because chlorophytes and cyanobacteria are better competitors for low light and turbid status than diatoms under eutrophic, well-mixed water conditions (Tilman et al., 1986) and thus infl uence the response of diatoms. However, the increased production of chlorophytes and cyanobacteria does not restrain diatom growth through competition and/or allelopathy and diatoms can co-exist with other types of algae, because diatom concentration and diatom specifi c pigments (i.e. fucoxanthin and diatoxanthin) show a clear increasing trend (Figs.3 & 5). Thus, the combined diatom and pigment analyses in this study provide clear evidence for recent human-induced accelerated lake eutrophication and a major ecological shift, and improves understanding of the predisturbance condition for current ecological restoration in this lake.
5 CONCLUSION
Lake Dojran, a shallow and eutrophic lake across the Republic of North Macedonia and Greece, is a key site for understanding the environmental change in the Mediterranean region. This study conducted the diatom and pigment analyses of the Lake Dojran sediment sequence that spans the past ca. 450 years, and their preservation qualities were evaluated prior to palaeolimnological interpretation. For diatoms, good preservation is inferred from the co-existence of robust, highly-silicifi ed taxa and small taxa throughout the sequence and also from the correspondence of the consistent diatom composition with the current limnological conditions. For pigment preservation, our comparison of wet sediment samples, which were in dark and cold storage until the analysis, with the corresponding dry sediment samples, which were lyophilized immediately after coring, reveals that sediment restoration conditions exert large infl uence on the accuracy of pigment analysis. In the palaeolimnological interpretation, the chlorophyll and xanthophyll pigment concentration and diatom concentration data provide clear evidence for accelerated lake eutrophication and ecological shift linked to intensifi ed anthropogenic water reduction and agricultural nutrient diff use in the late 18thto early 19thcentury; whereas, diatom assemblage composition does not show evidence, probably because widely-tolerant small fragilaroid species dominate the diatom composition and/or lightcompetitive chlorophytes and cyanobacteria override diatoms under eutrophic and turbid conditions. This study improves our understanding of recent humaninduced environmental change and current ecological restoration target in this lake.
6 DATA AVAILABILITY STATEMENT
The data that support the fi ndings of this study are available in the Supplementary Information File.
7 ACKNOWLEDGMENT
We would especially like to thank Suzanne McGowan (University of Nottingham, UK) for providing help for pigment analysis. Many thanks are
also due to Bernd Wagner (University of Cologne, Germany) and Alexander Francke (University of Wollongong, Australia) for providing samples for analysis. Two anonymous reviewers are also thanked for their detailed and constructive comments, suggestions and improvements on the manuscript.
References
Athanasiadis N, Tonkov S, Atanassova J, Bozilova E. 2000. Palynological study of Holocene sediments from Lake Doirani in northern Greece.JournalofPaleolimnology, 24(3): 331-342, https://doi.org/10.1023/A:1008161819212.
Battarbee R W, Jones V J, Flower R J, Cameron N G, Bennion H, Carvalho L, Juggins S. 2001. Diatoms.In: Smol J P, Birks H J B, Last W M eds. Tracking Environmental Change using Lake Sediments. Volume 3: Terrestrial, Algal, and Siliceous Indicators. Kluwer Academic Publishers, Dordrecht. p.155-202.
Bennion H, Battarbee R W, Sayer C D, Simpson G L, Davidson T A. 2011. Defi ning reference conditions and restoration targets for lake ecosystems using palaeolimnology: a synthesis.JournalofPaleolimnology, 45(4): 533-544, https://doi.org/10.1007/s10933-010-9419-3.
Bennion H, Battarbee R. 2007. The European Union Water Framework Directive: opportunities for palaeolimnology.JournalofPaleolimnology, 38(2): 285-295, https://doi.org/10.1007/s10933-007-9108-z.
Bennion H, Carvalho L, Sayer C D, Simpson G L, Wischnewski J. 2012. Identifying from recent sediment records the eff ects of nutrients and climate on diatom dynamics in Loch Leven.FreshwaterBiology, 57(10): 2 015-2 029, https://doi.org/10.1111/j.1365-2427.2011.02651.x.
Bennion H, Sayer C D, Tibby J, Carrick H J. 2010. Diatoms as indicators of environmental change in shallow lakes.In: Smol J P, Stoermer E F eds. The Diatoms: Applications for the Environmental and Earth Sciences. 2ndedn. Cambridge University Press, Cambridge. p.152-173.
Bennion H, Simpson G L, Goldsmith B J. 2015. Assessing degradation and recovery pathways in lakes impacted by eutrophication using the sediment record.FrontiersinEcologyandEvolution, 3: 94, https://doi.org/10.3389/fevo.2015.00094.
Bronk Ramsey C. 2009. Bayesian analysis of radiocarbon dates.Radiocarbon, 51(1): 337-360, https://doi.org/10. 1017/S0033822200033865.
Conley D J, Paerl H W, Howarth R W, Boesch D F, Seitzinger S P, Havens K E, Lancelot C, Likens G E. 2009. Controlling eutrophication: nitrogen and phosphorus.Science, 323(5917): 1 014-1 015, https://doi.org/10.1126/science.1167755.
Cruces F, Rivera P, Urrutia R. 2010. Observations and comments on the diatomStephanodiscusminutulus(Kützing) Cleve & Möller (Bacillariophyceae) found for the fi rst time in Chile from bottom sediments collected in Lake Laja.GayanaBotánica, 67(1): 12-18. http://doi.org/10.4067/S0717-66432010000100002.
Egeland E S, Garrido J L, Clementson L, Andresen K, Thomas C S, Zapata M, Airs R, Llewellyn C A, Newman G L, Rodríguez F, Roy S. 2011. Data sheets aiding identifi cation of phytoplankton carotenoids and chlorophylls.In: Roy S, Llewellyn C A, Egeland E S, Johnsen G eds. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography. Cambridge University Press, Cambridge. p.675-822.
Elser J, Bennett E. 2011. A broken biogeochemical cycle.Nature, 478(7367): 29-31, https://doi.org/10.1038/478029a.
Fourtanier E, Kociolek J P. 2011. Catalogue of Diatom Names, On-line Version, updated 19 Sep. 2011. http://researcharchive.calacademy.org/research/diatoms/names/index.asp.
Francke A, Wagner B, Leng M J, Rethemeyer J. 2013. A Late Glacial to Holocene record of environmental change from Lake Dojran (Macedonia, Greece).ClimateofthePast, 9(1): 481-498, https://doi.org/10.5194/cp-9-481-2013.
Griffi ths H I, Reed J M, Leng M J, Ryan S, Petkovski S. 2002. The recent palaeoecology and conservation status of Balkan Lake Dojran.BiologicalConservation, 104(1): 35-49, https://doi.org/10.1016/S0006-3207(01)00152-5.
Guilizzoni P, Lami A. 2002. Paleolimnology: use of algal pigments as indicators.In: Bitton G ed. Encyclopedia of Environmental Microbiology. John Wiley & Sons, New York. p.2 306-2 317.
Guiry M D, Guiry G M. 2019. AlgaeBase. World-Wide Electronic Publication, National University of Ireland. http://www.algaebase.org.
Hall R I, Smol J P. 2010. Diatoms as indicators of lake eutrophication.In: Smol J P, Stoermer E F eds. The Diatoms: Applications for the Environmental and Earth Sciences. 2ndedn. Cambridge University Press, Cambridge. p.122-151.
Hobbs W O, Fritz S C, Stone J R, Donovan J J, Grimm E C, Almendinger J E. 2011. Environmental history of a closed-basin lake in the US Great Plains: diatom response to variations in groundwater fl ow regimes over the last 8500 cal. yr BP.Holocene, 21(8): 1 203-1 216, https://doi.org/10.1177/0959683611405242.
Houk V, Klee R, Tanaka H. 2010. Atlas of freshwater centric diatoms with a brief key and descriptions. Part III. Stephanodiscaceae A:Cyclotella,Tertiarius,Discostella.Fottea, 10(Supplement): 1-498.
Houk V, Klee R, Tanaka H. 2014. Atlas of freshwater centric diatoms with a brief key and descriptions. Part IV. Stephanodiscaceae B:Stephanodiscus,Cyclostephanos,Pliocaenicus,Hemistephanos,Stephanocostis,Mesodictyon&Spicaticribra.Fottea, 14(Supplement): 1-530.
IPCC. 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge and New York. 1 535p.
Jeff rey S W, Mantoura R F C, Bjørnland T. 1997. Data for the identifi cation of 47 key phytoplankton pigments.In: Jeff rey S W, Mantoura R F C, Wright S W eds. Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods. UNESCO Publishing, Paris. p.447-560.
Krammer K, Lange-Bertalot H. 1986. Süsswasserfl ora von Mitteleuropa. Band 2/1: Bacillariophyceae. Teil 1: Naviculaceae. Gustav Fischer Verlag, Stuttgart. 876p.
Krammer K, Lange-Bertalot H. 1988. Süsswasserfl ora von Mitteleuropa. Band 2/2: Bacillariophyceae. Teil 2: Epithemiaceae, Bacillariaceae, Surirellaceae. Gustav Fischer Verlag, Stuttgart. 596p.
Krammer K, Lange-Bertalot H. 1991a. Süsswasserfl ora von Mitteleuropa. Band 2/3: Bacillariophyceae. Teil 3: Centrales, Fragilariaceae, Eunotiaceae. Gustav Fischer Verlag, Stuttgart. 576p.
Krammer K, Lange-Bertalot H. 1991b. Süsswasserfl ora von Mitteleuropa. Band 2/4: Bacillariophyceae. Teil 4: Achnanthaceae. Gustav Fischer Verlag, Stuttgart. 437p.
Krammer K. 2002. Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats. Volume 3:Cymbella. Gantner Verlag, Ruggell. 584p.
Lami A N, Guilizzoni P, Marchetto A. 2000. High resolution analysis of fossil pigments, carbon, nitrogen and sulphur in the sediment of eight European Alpine lakes: the MOLAR project.JournalofLimnology, 59(S1): 15-28, https://doi.org/10.4081/jlimnol.2000.s1.15.
Lange-Bertalot H. 2001. Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats. Volume 2:NaviculaSensu Stricto, 10 Genera Separated fromNaviculaSensu Lato,Frustulia. Gantner Verlag, Ruggell. 526p.
Leavitt P R, Hodgson D A. 2001. Sedimentary pigments.In: Smol J P, Birks H J B, Last W M eds. Tracking Environmental Change using Lake Sediments. Volume 3: Terrestrial, Algal, and Siliceous Indicators. Kluwer Academic Publishers, Dordrecht. p.295-325.
Lešoski J, Zdraveski N, Kristić S. 2010. Preliminary results on cyanobacterial survey on Dojran Lake—the beginning of revealing of the ultimate truth about the lake’s water quality.In: Conference on Balkan Water Observation and Information System for Decision Support (BALWOIS). Ohrid.
Levkov Z, Krstic S, Metzeltin D, Nakov T. 2007. Iconographia Diatomologica. Volume 16: Diatoms of Lakes Prespa and Ohrid. Gantner Verlag, Ruggell. 613p.
Levkov Z, Stojanovski P. 2000-2001. Changes in Doiran Lake’s diatom fl ora, a 13 years study. Godisen zbornik Biologija, 53-54: 22-38.
Levkov Z. 2009. Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats. Volume 5:AmphoraSensu Lato. Gantner Verlag, Ruggell. 842p.
Lionello P. 2012. The Climate of the Mediterranean Region: From the Past to the Future. Elsevier, Amsterdam. 592p.
Lokoska L, Jordanoski M, Veljanoska-Sarafi loska E, Tasevska O. 2006. Water quality of Lake Dojran from biological and physical-chemical aspects.In: Conference on Balkan Water Observation and Information System for Decision Support (BALWOIS). Ohrid.
Masi A, Francke A, Pepe C, Thienemann M, Wagner B, Sadori L. 2018. Vegetation history and paleoclimate at Lake Dojran (FYROM/Greece) during the Late Glacial and Holocene.ClimateofthePast, 14(3): 351-367, https://doi.org/10.5194/cp-14-351-2018.
McGowan S, Barker P, Haworth E Y, Leavitt P R, Maberly S C, Pates J. 2012. Humans and climate as drivers of algal community change in Windermere since 1850.FreshwaterBiology, 57(2): 260-277, https://doi.org/10.1111/j. 1365-2427.2011.02689.x.
McGowan S. 2013. Palaeolimnology: pigment studies.In: Elias S A, Mock C J eds. Encyclopedia of Quaternary Science. 2ndedn. Elsevier, Amsterdam. p.326-338.
Paerl H W, Scott J T, McCarthy M J, Newell S E, Gardner W S, Havens K E, Hoff man D K, Wilhelm S W, Wurtsbaugh W A. 2016. It takes two to tango: when and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems.EnvironmentalScience&Technology, 50(20): 10 805-10 813, https://doi.org/10. 1021/acs.est.6b02575.
Popovska C, Bonacci O. 2008. Ecohydrology of Dojran Lake.In: Hlavinek P, Bonacci O, Marsalek J, Mahrikova I, eds. Dangerous Pollutants (Xenobiotics) in Urban Water Cycle. Springer, Dordrecht. p.151-160.
Popovska C, Gesovska V, Ivanoski D. 2005. Ecological and hydrological state of Dojran Lake.Vodoprivreda, 37(216-218): 175-180.
Reimer P J, Bard E, Bayliss A, Beck J W, Blackwell P G, Ramsey C B, Buck C E, Cheng H, Edwards R L, Friedrich M, Grootes P M, Guilderson T P, Hafl idason H, Hajdas I, Hatté C, Heaton T J, Hoff mann D L, Hogg A G, Hughen K A, Kaiser K F, Kromer B, Manning S W, Niu M, Reimer R W, Richards D A, Scott E M, Southon J R, Staff R A, Turney C S M, van der Plicht J. 2013. IntCal13 and Marine13 radiocarbon age calibration curves 0-50,000 years cal BP.Radiocarbon, 55(4): 1 869-1 887, https://doi.org/10.2458/azu_js_rc.55.16947.
Reuss N, Conley D J. 2005. Eff ects of sediment storage conditions on pigment analyses.LimnologyandOceanography:Methods, 3(10): 477-487, https://doi.org/10.4319/lom.2005.3.477.
Rimet F, Druart J C, Anneville O. 2009. Exploring the dynamics of plankton diatom communities in Lake Geneva using emergent self-organizing maps (1974-2007).EcologicalInformatics, 4(2): 99-110, https://doi.org/10.1016/j.ecoinf.2009.01.006.
Roberts N, Reed J M. 2009. Lakes, wetlands, and Holocene environmental change.In: Woodward J C ed. The Physical Geography of the Mediterranean. Oxford University Press, Oxford. p.255-286.
Rockström J, Steff en W, Noone K, Persson Å, Chapin F S, Lambin E F, Lenton T M, Scheff er M, Folke C, Schellnhuber H J, Nykvist B, de Wit C A, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder P K, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell R W, Fabry V J, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley J A. 2009. A safe operating space for humanity.Nature, 461(7263): 472-475, https://doi.org/10.1038/461472a.
Ryves D B, Juggins S, Fritz S C, Battarbee R W. 2001. Experimental diatom dissolution and the quantifi cation of microfossil preservation in sediments.Palaeogeography,Palaeoclimatology,Palaeoecology, 172(1-2): 99-113, https://doi.org/10.1016/S0031-0182(01)00273-5.
Sayer C D. 2001. Problems with the application of diatomtotal phosphorus transfer functions: examples from a shallow English lake.FreshwaterBiology, 46(6): 743-757, https://doi.org/10.1046/j.1365-2427.2001.00714.x.
Scheラ er W, Morabito G. 2003. Topical observations on centric diatoms (Bacillariophyceae, Centrales) of Lake Como (N. Italy).JournalofLimnology, 62(1): 47-60, https://doi.org/10.4081/jlimnol.2003.47.
Smol J P, Stoermer E F. 2010. The Diatoms: Applications for the Environmental and Earth Sciences. 2ndedn. Cambridge University Press, Cambridge. 686p.
Smol J P. 2019. Under the radar: long-term perspectives on ecological changes in lakes.ProceedingsoftheRoyalSocietyB:BiologicalSciences, 286: 20190834, https://doi.org/10.1098/rspb.2019.0834.
Sotiria K, Petkovski S. 2004. Lake Dojran—An overview of the current situation. Greek Biotope/Wetland Centre (EKBY) and Society for the Investigation and Conservation of Biodiversity and the Sustainable Development of Natural Ecosystems (BIOECO), Thermi. 117p.
Steff en W, Richardson K, Rockström J, Cornell S E, Fetzer I, Bennett E M, Biggs R, Carpenter S R, de Vries W, de Wit C A, Folke C, Gerten D, Heinke J, Mace G M, Persson L M, Ramanathan V, Reyers B, Sorlin S. 2015. Planetary boundaries: guiding human development on a changing planet.Science, 347(6223): 1259855, https://doi.org/10. 1126/science.1259855.
Stojov V. 2012. Hydrological state of Dojran Lake related to tectonic, climatic and human impacts.In: Conference on Balkan Water Observation and Information System for Decision Support (BALWOIS). Ohrid.
Sutton M A, Oenema O, Erisman J W, Leip A, van Grinsven H, Winiwarter W. 2011. Too much of a good thing.Nature, 472(7342): 159-161, https://doi.org/10.1038/472159a.
Tasevska O, Kostoski G, Guseska D. 2010. Rotifers based assessment of the Lake Dojran water quality.In: Conference on Balkan Water Observation and Information System for Decision Support (BALWOIS). Ohrid.
Temponeras M, Kristiansen J, Moustaka-Gouni M. 2000. Seasonal variation in phytoplankton composition and physical-chemical features of the shallow Lake Doïrani, Macedonia, Greece.Hydrobiologia, 424(1): 109-122, https://doi.org/10.1023/A:1003909229980.
Tilman D, Kiesling R, Sterner R, Kilham S S, Johnson F A. 1986. Green, bluegreen and diatom algae: taxonomic diff erences in competitive ability for phosphorus, silicon and nitrogen.ArchivfürHydrobiologie, 106(4): 473-485.
Verdonschot P F M, Spears B M, Feld C K, Brucet S, Keizer-Vlek H, Borja A, Elliott M, Kernan M, Johnson R K. 2013. A comparative review of recovery processes in rivers, lakes, estuarine and coastal waters.Hydrobiologia, 704(1): 453-474, https://doi.org/10.1007/s10750-012-1294-7.
Veuger B, van Oevelen D. 2011. Long-term pigment dynamics and diatom survival in dark sediment.LimnologyandOceanography, 56(3): 1 065-1 074, https://doi.org/10. 4319/lo.2011.56.3.1065.
Wan L L, Chen X Y, Deng Q H, Yang L, Li X W, Zhang J Y, Song C L, Zhou Y Y, Cao X Y. 2019. Phosphorus strategy in bloom-forming cyanobacteria (DolichospermumandMicrocystis) and its role in their succession.HarmfulAlgae, 84: 46-55, https://doi.org/10.1016/j.hal.2019.02. 007.
Wang S Y, Xiao J, Wan L L, Zhou Z J, Wang Z C, Song C L, Zhou Y Y, Cao X Y. 2018. Mutual dependence of nitrogen and phosphorus as key nutrient elements: one facilitatesDolichospermumfl os-aquaeto overcome the limitations of the other.EnvironmentalScience&Technology, 52(10): 5 653-5 661, https://doi.org/10.1021/acs.est.7b04992.
Zhang X S, Reed J M, Wagner B, Francke A, Levkov Z. 2014. Lateglacial and Holocene climate and environmental change in the northeastern Mediterranean region: diatom evidence from Lake Dojran (Republic of Macedonia/Greece).QuaternaryScienceReviews, 103: 51-66, https://doi.org/10.1016/j.quascirev.2014.09.004.
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Exploring sensitive area in the tropical Indian Ocean for El Niño prediction: implication for targeted observation*
- Analysis of the typhoon wave distribution simulated in WAVEWATCH-III model in the context of Kuroshio and wind-induced current*
- Characterizing the capability of mesoscale eddies to carry drifters in the northwest Pacifi c*
- Observation system simulation experiments using an ensemble-based method in the northeastern South China Sea*
- Statistical analysis of intensity variations in tropical cyclones in the East China Sea passing over the Kuroshio*
