胃癌细胞系中CD44+胃癌干细胞的表达和意义
任芳 陈毅德 冯丽华 郑志高 范鑫 林雨标 冯水土

摘要:目的 探讨胃癌细胞系中CD44+胃癌干细胞的基因表达及其意义。方法 采用无血清培养基获得漂浮球,在胃腺癌细胞系MKN-45中用FACS检测肿瘤干细胞(CSC)表面标志物CD44的表达,采用FACS分离CD44+亚群,通过体外培养鉴定两种不同细胞群CD44+和CD44-中的致瘤、自我更新、分化特性,采用实时RT-PCR评估CD44+和CD44-中干细胞特异性基因的表达。结果 MKN-45和NCI-N87在无血清培养基中形成胃癌微球体,而KATOⅢ和AGS不能形成胃癌微球体,MKN-45细胞系经FACS分选CD44+和CD44-中细胞,80%MKN-45细胞高表达CD44;CD44+细胞在无血清培养基中显示出更高的微球体形成、分化特性;参与Wnt2、Bmi1、Oct3/4、Notch1、Sox2、Nanog和其他基因的“干细胞”基因的表达水平在CD44+亞群中高于CD44-亚群。结论 人胃癌细胞中的CD44+亚群可能含有胃癌干细胞样细胞,可为胃癌的诊断、治疗提供参考。
关键词:胃癌;CD44;胃癌干细胞;基因表达
中图分类号:R735.2 文献标识码:A DOI:10.3969/j.issn.1006-1959.2020.20.013
文章编号:1006-1959(2020)20-0045-06
Expression and Significance of CD44+ Gastric Cancer Stem Cells in Gastric Cancer Cell Lines
REN Fang,CHEN Yi-de,FENG Li-hua,ZHENG Zhi-gao,FAN Xin,LIN Yu-biao,FENG Shui-tu
(Department of Oncology and Hematology,Xiamen Haicang Hospital,Xiamen 361026,Fujian,China)
Abstract:Objective To investigate the gene expression and significance of CD44+ gastric cancer stem cells in gastric cancer cell lines.Methods Floating spheres were obtained with serum-free medium. FACS was used to detect the expression of tumor stem cell (CSC) surface marker CD44 in gastric adenocarcinoma cell line MKN-45, CD44+ subgroups were separated by FACS, and two different cell populations were identified by in vitro culture The tumorigenicity, self-renewal, and differentiation characteristics of CD44+ and CD44-. Real-time RT-PCR was used to evaluate the expression of stem cell-specific genes in CD44+ and CD44-.Results MKN-45 and NCI-N87 formed gastric cancer microspheres in serum-free medium, while KATOⅢ and AGS could not form gastric cancer microspheres. The MKN-45 cell line was sorted by FACS for CD44+ and CD44- cells, 80% MKN-45 cells highly express CD44; CD44+ cells show higher microsphere formation and differentiation characteristics in serum-free medium; the expression level of "stem cell" genes involved in Wnt2, Bmi1, Oct3/4, Notch1, Sox2, Nanog and other genes CD44+ subgroup was higher than CD44- subgroup.Conclusion The CD44+ subset of human gastric cancer cells might contain gastric cancer stem cell-like cells, which could provide references for the diagnosis and treatment of gastric cancer.
Key words:Gastric cancer;CD44;Gastric cancer stem cells;Gene expression
尽管胃癌(gastric cancer,GC)的发病率和死亡率一直下降,但它仍然是世界上第5大最常见的恶性肿瘤,2012年大约有100万新增病例,尤其普遍在东亚(主要在中国)。同时,胃癌是全球癌症死亡的第3大原因 [1]。大多数患者早期无症状或存在非特异性症状,确诊时常为晚期,日本和韩国使用钡光荧光检查或内窥镜检查,更有利于早诊断、早治疗[2]。GC被认为是一种预后不良的恶性疾病,5年生存率<30%[3]。目前GC研究的主要焦点是通过寻找新工具和技术来实现早发现、早诊断、早治疗[4]。因此,如何识别且清除早期胃癌细胞是胃癌患者早诊断、早治疗的关键。癌症干细胞(CSC)可以为胃癌诊断和治疗提供新方法。研究表明,CSCs是肿瘤起始、侵袭、远处转移和抗癌药物耐药的原因[5],胃CSC可能在胃癌早期发挥重要作用并促进肿瘤发生进展。目前有学者推测可通过特定表面标志物的鉴定CSC [6],其中CD44首先被確定是乳腺癌的特异性CSC标志物[7],也已被证明是其他许多实体瘤的CSC标志物[8],也有学者认为CD44可能也是胃CSC标志物[9]。本研究从人MKN-45胃腺癌细胞系中分离、鉴定、并培养胃CSC细胞,观察CD44+细胞的特性,评估其作为胃癌细胞表面标志物的价值。
1材料与方法
1.1细胞培养 人胃癌细胞系MKN-45、NCI-N87、KATOⅢ、AGS购自ATCC公司,置于含10%胎牛血清的1640培养基(HyClone)中培养;加入100 U/ml青霉素和100 μg/ml链霉素(Gibco);在37 ℃、5%CO2的细胞培养箱中,培养基每2 d更换1次。将细胞以80%汇合传代,并以20%汇合接种,保持最佳培养条件。
1.2胃癌微球体细胞培养 从贴壁细胞(1.0×104/ml)中提取胃癌微球体细胞,置于无血清的PRMI-1640培养基(HyClone)中,培养基含2%B27、20 ng/ml EGF(R&D)、10 ng/ml人FGF-2(R&D)和10 mM HEPES(Invitrogen)。 7~10 d后观察6孔板中的胃癌微球体细胞,并使用倒置显微镜(Olympus)以40和200放大倍数进行定量。胃癌微球体通过胰蛋白酶-EDTA溶液(GIBCO)消化,40-μm筛(BD Biosciences)过滤后获得单细胞悬液,并再次将单细胞接种。最后去除生长因子,添加10%FBS后,从胃癌微球体中获得分化细胞。
1.3流式细胞术分析和分选 CD44可作为原发性肿瘤和胃癌CSC鉴别标记[9,11-13]。本实验通过使用FACS分析人MKN-45胃腺癌细胞系的CD44表达模式。通过FACS分选培养CD44+胃癌细胞,诱导肿瘤微球体形成。培养基中加入10%FBS代替EGF和FGF-2,观测CD44的表达及其形态。步骤如下:蛋白酶消化MKN-45细胞,用PBS洗涤两次并离心,将1×106个细胞沉淀重悬于含2%FBS的PBS中,使用100倍稀释的抗人CD44-FITC小鼠单克隆抗体(克隆G44-26,BD Pharmingen)于室温下,孵育30 min后,在FACS Calibur(Becton-Dickinson)上分析样品。收集(5~10)×106个细胞并染色,并使用FACS Aria(Becton-Dickinson)分选。选染色最显著的前10%细胞和最模糊的10%细胞作为阳性和阴性对照组。FACS分选后,使用台盼蓝染色鉴别细胞活性,分选细胞含量预计高于96%。使用CD44小鼠单克隆抗体分析胃癌微球体细胞和分化细胞的CD44表达。
1.4 RT-PCR分析CD44+和CD44-胃癌细胞参与干细胞相关途径的基因表达情况 按照试剂盒说明书,使用Trizol试剂(Invitrogen)从CD44+和CD44-细胞中提取总细胞RNA。取1 μg总RNA,通过oligo(dT)18引物,反转合成cDNA,按照 RevertAid First Strand cDNA Synthesis Kit(Fermentas)试剂盒说明书进行。反应体系包含:0.1 μM正向和反向引物以及SYBR Green PCR混合物(Applied Biosystems)进行RT-qPCR。具体引物序列如下:CD44正向引物:TCCAGGCAACTCCTAGTAGTA,反向引物:CTGTCCCTGTTGTCGAAT;Wnt2正向引物:ACTCTCAGGACATGCTGGCT,反向引物:ACGAGGTCATTTTTCGTTGG;Bmi1正向引物:TGGAGAAGGAATGGTCCACTTC,反向引物:GTGAGGAAACTGTGGATGAGGA;Notch1正向引物:CCTGAGGGCTTCAAAGTGTC,反向引物:CGGAACTTCTTGGTCTCCAG;Oct4正向引物:CTGGAGAAGGAGAAGCTGGA,反向引物:CAAATTGCTCGAGTTCTTTCTG;Sox2正向引物:TGCGAGCGCTGCACAT,反向引物:CGGGCAGCGTGTACTTATCC;Nanog正向引物:CAACCAGACCCAGAACATCC,反向引物:TTCCAAAGCAGCCTCCAAG;C-myc正向引物:TCAAGAGGCGAACACACAAC,反向引物:GGCCTTTTCATTGTTTTCCA;ABCG2正向引物:TGGCTTAGACTCAAGCACAGC,反向引物:TCGTCCCTGCTTAGACATCC;CXCR4正向引物:GAGTGGCCGACCTCCTCTT,反向引物:ACATGGACTGCCTTGCATAGG;GAPDH正向引物:ACCCACTCCTCCACCTTTGA,反向引物:CTGTTGCTGTAGCCAAATTCGT。具体操作如下:在95 ℃变性,5 min后,反应在95 ℃下进行40个延伸、扩增循环。通过7900HT快速实时PCR系统(Applied Biosystems)进行信号检测。以GAPDH基因作为内参,根据2-△△CT法计算目的基因相对表达量。每组重复实验 3 次,使用Microsoft Excel计算平均值和标准差。
1.5统计学方法 应用SPSS 23.0软件对所有的实验数据进行统计学分析,计量资料以(x±s)表示,组间比较采用t检验;采用双侧检验,P<0.05 为差异有统计学意义。
2结果
2.1胃泌素形成不同的人胃癌细胞系 MKN-45和NCI-N87细胞显示直径约100μm的经典球状集落,KATOⅢ细胞形成少量集落,而AGS细胞系不能形成球状集落并死亡,见图1。
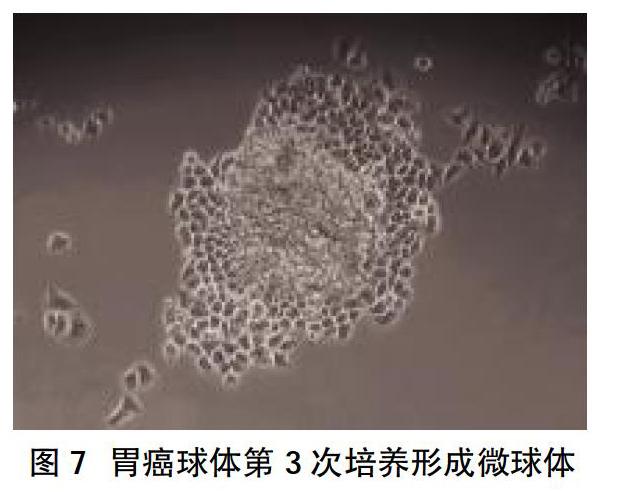
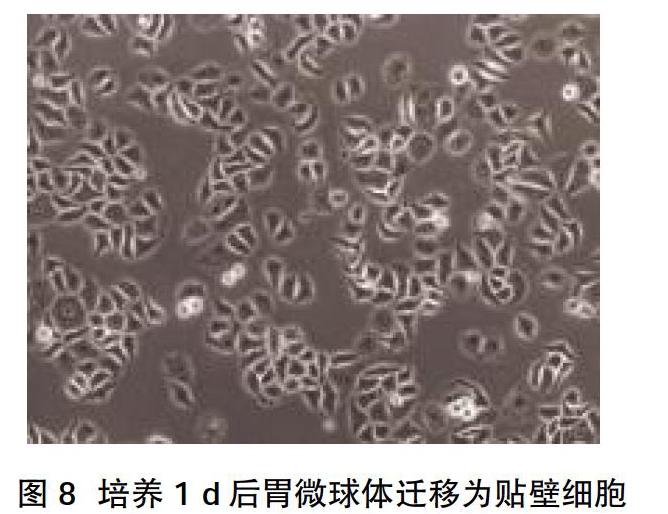
2.2 CD44+干细胞相关基因的表达 MKN-45细胞显示出高水平的CD44表达,高达82%细胞表达CD44;随后通过FACS从MKN-45细胞系中分选出CD44+和CD44-细胞,分选的细胞的纯度大于96%,见图2。“肿瘤干性”基因包括CD44、Wnt2、Bmi1、Notch1、Oct4、Sox2、Nanog、C-myc、转运蛋白和运动基因(ABCG2、CXCR4),在CD44+中表达高于CD44-细胞亚群,差异有统计学意义(P<0.05),见图3。相对于CD44-细胞,CD44+细胞形成更多、更大的微球体,差异有统计学意义(P<0.05),见图4、图5。CD44+ MKN-45细胞在无血清细胞培养基中培养,以维持未分化状态,培养10~14 d后,通过倒置相差显微镜观察形成漂浮的微球体,见图6。此外,胃癌球体在第3次细胞培养传代后生长为更大、更紧凑的微球体,见图7。培养1 d后,漂浮的胃微球体迁移为贴壁细胞,贴壁细胞获得与其亲本MKN-45非常相似的多边形形态,见图8。流式细胞术分析胃癌微球体和分化的贴壁细胞表明,在分化过程中CD44表达从98%降低至85%,见图9。
3讨论
癌症干细胞为具有无限增殖、分裂、多向分化潜能的细胞亚群[14]。最早在急性髓性白血病中发现了CSC,其中CD34+ CD38-亚群具有干细胞增殖分化能力[15],越来越多的证据表明CSCs存在于各种实体肿瘤中,包括乳腺[7]、结肠直肠[16]、肺[17]、头颈部[18]、胰腺[19]、肝[20]、胃癌[9]、胶质瘤[21]、前列腺[22]等。因此,癌症干细胞的检测对癌症患者的准确诊断和有效治疗具有重要意义。寻找细胞表面标志物是鉴定和分离CSC最重要方法,流式细胞术或磁性细胞分选已在许多实体恶性肿瘤中成功分离CSC。然而,实体瘤CSC表面识别标志物的确定无疑是CSC领域最困难的挑战[6]。大多数用于分选的标记物是经验性,且源自正常干细胞。最近已有报道分离、鉴定胃CSC的各种技术和细胞表面标志物[13,23-26],其中CD44是用于胃CSC最具有潜力标记物之一。CD44是Ⅰ类跨膜糖蛋白,可以作为细胞外基质如透明质酸的受体。CD44因其在介导细胞-基质相互作用以及与恶性过程相关的重要功能,尤其是癌症转移中的重要作用而备受关注[27]。HA-CD44在细胞外结构域中的相互作用激活了多种信号通路,涉及CSC自我更新、克隆形成、抗凋亡/存活,放化疗抗性等[28]。 CD44单独或与其他标记物组合鉴定CSC已应用于許多肿瘤中,如乳腺癌[7]、胰腺[29]、结肠直肠[30]、肺[31]、肝细胞[32]、卵巢[33]、头颈部[34]等。Takaishi等通过分析一组人类GC细胞系,在CD44+细胞亚群中鉴定出CSC[9]。与CSC的定义标准一样,从5个GC细胞系中的3个细胞系中分选出CD44+细胞,在悬浮状态下形成球状集落,以及在SCID小鼠的胃和皮肤中形成异种移植肿瘤。因此,本研究试图通过CD44富集MKN45细胞中收集可能存在的CSC。许多研究人员使用荧光激活细胞分选来鉴定和分离CSCs,确定其在特定的无血清培养基中形成球体。本课题组通过FACS从MKN-45细胞系中分选出CD44+和CD44-细胞,发现CD44+细胞在体外显示出更高胃癌微球体形成效率,与相关报道结果一致[9]。此外,CD44+细胞能够在无血清培养基中作为胃癌微球体增殖,并在10%FBS存在下分化,表明它们保留了自我更新能力和分化潜能。
目前研究已在多个癌症中检测到干细胞相关基因(也称为“肿瘤干性基因”),其可解释CSCs的自我增殖、分化特性[35]。因此,开发有效的诊断、治疗方法首先需确定CSC中有活性、特异的分子标志物。据报道,这些基因的失调及其信号通路的变化参与了胃癌的发生发展。通过RT-PCR(qRT-PCR)技术可分析CD44+和CD44-胃癌细胞之间“干性基因”的mRNA表达差异。本研究发现,与CD44-亚群相比,CD44+胃癌细胞群高表达的“干性基因”包括CD44、Wnt2、Bmi1、Notch1、Oct4、Sox2、Nanog、C-myc、转运蛋白和运动基因,如ABCG2和CXCR4[36-39]。研发能够特异阻断这些与CSC增殖、分化和转移相关基因的药物将增强肿瘤的治疗效果并改善预后。同时对这些基因进行深入研究,进一步阐明它们对CSCs的确切作用以及其与胃癌的关系具有重要意义。然而,一些胃癌细胞系本身含有高比例的CD44+癌细胞,这与当前的CSC模型不一致,可能与CSC尚未完全纯化,且标志物特异性不足有关[40]。本研究中,MKN-45细胞显示出高水平的CD44表达,高达80%。此外,肿瘤块内可能存在多个CSC亚群,可能需要多个标记物的组合来识别完整的CSC群体[41]。最近已报道LGR5 +(GPR49+)干细胞位于胃窦区域,持续分化为所有胃窦细胞[42]。本研究还分析了干细胞相关基因LGR5在胃癌细胞系中的表达,但并未发现LGR5在CD44+和CD44-肿瘤细胞中表达的差异。因此,LGR5可能不是胃癌CSC标记。
总之,MKN-45细胞中存在CSC,FACS是分离和鉴定胃癌CSC的有效手段。然而,由于胃CSC的特异性表面标志物未知,异种移植肿瘤模型是鉴定CSCs的金标准,因此需要进一步的DNA高通量测序和体内实验来鉴定潜在的胃癌干细胞标志物,这可能有助于早期诊断和更有效的胃癌靶向治疗。
參考文献:
[1]Jacques F,Isabelle S,Rajesh D,et al.Cancer incidence and mortality worldwide:Sources,methods and major patterns in GLOBOCAN 2012[J].International Journal of Cancer,2015,136(5):E359-E386.
[2]Yun SS,Han KY.Screening and Early Detection of Gastric Cancer:East Versus West[J].Surgical Clinics of North America,2015,95(5):1053-1066.
[3]Siegel RL,Fedewa SA,Miller KD,et al.Cancer statistics for Hispanics/Latinos,2015[J].CA:A Cancer Journal for Clinicians,2015.
[4]Beeharry MK,Liu WT,Yan M,et al.New blood markers detection technology: A leap in the diagnosis of gastric cancer[J].World Journal of Gastroenterology,2016,22(3):12.
[5]Eaves CJ.Cancer stem cells:Here,there,everywhere[J].Nature,2008,456(7222):581-582.
[6]Woodward WA,Sulman EP.Cancer stem cells: markers or biomarkers[J]Cancer&Metastasis Reviews,2008,27(3):459-470.
[7]Al-Hajj M,Wicha MS,Benito-Hernandez A,et al.Prospective identification of tumorigenic breast cancer cells[J].Proceedings of the National Academy of Sciences,2003,100(7):3983-3988.
[8]Thapa R,Wilson GD.The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer[J].Stem Cells International,2016(2016):2087204.
[9]Shigeo T,Tomoyuki O,Shuiping T,et al.Identification of gastric cancer stem cells using the cell surface marker CD44[J].Stem Cells,2010,27(5):1006-1020.
[10]Weiswald LB,Bellet D,Dangles-Marie V.Spherical Cancer Models in Tumor Biology[J].Neoplasia,2015,17(1):1-15.
[11]Zhang C,Li C,He F,et al.Identification of CD44+CD24+ gastric cancer stem cells[J].J Cancer Res Clin Oncol,2011,137(11):1679-1686.
[12]Yoon C,Park DJ,Schmidt B,et al.CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance[J].Clinical Cancer Research,2014,20(15):3974-3988.
[13]Yu D,Shin HS,Choi G,et al.Proteomic analysis of CD44(+)and CD44 gastric cancer cells[J].Molecular&Cellular Biochemistry,2014,396(1-2):213-220.
[14]O'Connor,Michael L,Xiang D,et al.Cancer stem cells:A contentious hypothesis now moving forward[J].Cancer Letters,2014,344(2):180-187.
[15]Bonnet D,Dick JE.Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell[J].Nat Med,1997,3(7):730-737.
[16]Daisuke I,Takatsugu I,Keisuke M,et al.Colorectal Cancer Stem Cells Acquire Chemoresistance Through the Upregulation of F-Box/WD Repeat-Containing Protein 7 and the Consequent Degradation of c-Myc[J].Stem Cells,2017,35(9):2027-2036.
[17]Alamgeer M,Peacock CD,Matsui W,et al.Cancer stem cells in lung cancer:Evidence and controversies[J].Respirology,2013,18(5):757-764.
[18]Jing H,Toshio F,Syed RH,et al.Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma[J].BMC Cancer,2014(14):173.
[19]Li C,Heidt DG,Dalerba P,et al.Identification of pancreatic cancer stem cells[J].Cancer Res,2007,67(3):1030-1037.
[20]Taro Y,Xin WW.Cancer stem cells in the development of liver cancer[J].Journal of Clinical Investigation,2013,123(5):1911-1918.
[21]Singh SK,Clarke ID,Terasaki M,et al.Identification of a cancer stem cell in human brain tumors[J].Cancer Res,2003,63(18):5821-5828.
[22]Li T,Su Y,Mei Y,et al.ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients' outcome.[J].Laboratory investigation; A Journal of Technical Methods and Pathology,2010,90(2):234-244.
[23]Ishimoto T,Oshima H,Oshima M,et al.CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis[J].Cancer Science,2010,101(3):673-678.
[24]Lau WM,Teng E,Chong HS,et al.CD44v8-10 Is a Cancer-Specific Marker for Gastric Cancer Stem Cells[J].Cancer Research,2014,74(9):2630-2641.
[25]Fukamachi H,Shimada S,Ito K,et al.CD133 is a marker of gland-forming cells in gastric tumors and Sox17 is involved in its regulation[J].Cancer ence,2011,102(7):1313-1321.
[26]Nishikawa S,Konno M,Hamabe A,et al.Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy[J].International Journal of Oncology,2013,42(4):1437-1442.
[27]Negi LM,Talegaonkar S,Jaggi M,et al.Role of CD44 in tumour progression and strategies for targeting[J].Journal of Drug Targeting,2012,20(7):561.
[28]Bourguignon LY,Shiina M,Li JJ.Hyaluronan-CD44 interaction promotes oncogenic signaling,microRNA functions,chemoresistance,and radiation resistance in cancer stem cells leading to tumor progression[J].Advances in Cancer Research,2014,123(22):255-275.
[29]Ling L,Xinbao H,Jun Q,et al.Antibody Against CD44s Inhibits Pancreatic Tumor Initiation and Postradiation Recurrence in Mice[J].Gastroenterology,2014,6(10):165.
[30]Dotse E,Bian Y.Isolation of colorectal cancer stem-like cells[J].Cytotechnology,2016,68(4):609-619.
[31]Leung EL,Fiscus RR,Tung JW,et al.Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties[J].PLoS One,2010,5(11):e14062.
[32]Michishita M,Ezaki S,Ogihara K,et al.Identification of tumor-initiating cells in a canine hepatocellular carcinoma cell line[J].Research in Veterinary ence,2014,96(2):315-322.
[33]Meng E,Long B,Sullivan P,et al.CD44+/CD24 ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival[J].Clinical & Experimental Metastasis,2012,29(8):939-948.
[34]Luciana A,Douglas G,Cristiane S,et al.Profiling the Behavior of Distinct Populations of Head and Neck Cancer Stem Cells[J].Cancers,2016,8(1):7.
[35]Bhardwaj A,Arora S,Prajapati VK,et al.Cancer"stemness"-regulating microRNAs:role,mechanisms and therapeutic potential[J].Current Drug Targets,2013,14(10):1175.
[36]Borah A,Raveendran S,Rochani A,et al.Targeting self-renewal pathways in cancer stem cells:clinical implications for cancer therapy[J].Oncogenesis,2015,4(11):e177.
[37] Es-Haghi M,Soltanian S,Dehghani H.Perspective:Cooperation of Nanog,NF-κΒ, and CXCR4 in a regulatory network for directed migration of cancer stem cells[J].Tumor Biology,2015,37(2):1559-1565.
[38]Matsuoka J,Yashiro M,Sakurai K,et al.Role of the Stemness Factors Sox2,Oct3/4,and Nanog in Gastric Carcinoma[J].Journal of Surgical Research,2012,174(1):130-135.
[39]Siddique HR,Saleem M.Role of BMI1,a stem cell factor,in cancer recurrence and chemoresistance:preclinical and clinical evidences[J].Stem Cells,2012,30(3):372-378.
[40]Rocco A,Compare D,Nardone G.Cancer stem cell hypothesis and gastric carcinogenesis:Experimental evidence and unsolved questions[J].World Journal of Gastrointestinal Oncology,2012,4(3):54-59.
[41]Brungs D,Aghmesheh M,Vine KL,et al.Gastric cancer stem cells:evidence,potential markers,and clinical implications[J].Journal of Gastroenterology,2016,51(4):313-326.
[42]Wu C,Xie Y,Gao F,et al.Lgr5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markers[J].Gene,2013,525(1):18-25.
收稿日期:2020-09-02;修回日期:2020-09-12
編辑/成森

