达格列净对肥胖2型糖尿病患者脂肪组织、Lp-PLA2、hs-CRP的影响
叶冠伦 罗卓章 梁栋伟
【摘要】 目的:通過观察在糖尿病治疗中加用达格列净后脂肪组织和各种致炎因子的改变情况,探讨达格列净降低动脉粥样硬化性心血管疾病(ASCVD)风险的作用。方法:选取2018年10月-2019年10月在广东省人民医院南海医院就诊的2型糖尿病患者共60例为研究对象,随机将患者分为观察组和对照组,各30例。患者入组前均已进行降糖药物治疗,对照组维持原治疗方案不变,观察组在原治疗方案上加用达格列净。比较两组患者治疗前后的体重、BMI、空腹血糖、空腹C肽、HOMA2-%B、HOMA2-%S、HOMA2-IR、皮下脂肪面积、内脏脂肪面积、心外膜脂肪厚度、腰围、臀围、腰臀比、Lp-PLA2、hs-CRP。结果:对照组治疗前后的内脏脂肪面积、皮下脂肪面积、心外膜脂肪厚度、体重、BMI、腰围、臀围、腰臀比、HbA1c、尿malb/Cr、HOMA2-%B、HOMA2-%S、HOMA2-IR、Lp-PLA2、hs-CRP比较,差异均无统计学意义(P>0.05)。观察组治疗前后的内脏脂肪面积、皮下脂肪面积、心外膜脂肪厚度、体重、BMI、腰围、臀围、腰臀比、HbA1c、HOMA2-%B、HOMA2-%S、HOMA2-IR、Lp-PLA2、尿malb/Cr、hs-CRP比较,差异均有统计学意义(P<0.05)。治疗12周后,观察组患者的腰围、HbA1c、HOMA2-%B、HOMA2-%S、HOMA2-IR、hs-CRP比对照组改善更明显,差异均有统计学意义(P<0.05)。观察组治疗前后HbA1c改变与治疗前HbA1c水平相关。结论:达格列净可有效控制血糖,减少胰岛素抵抗,增强能量平衡调节,减少脂肪组织(尤其是内脏脂肪),可能存在降低ASCVD风险的作用。
【关键词】 达格列净 肥胖 2型糖尿病 脂肪组织 内脏脂肪 皮下脂肪 Lp-PLA2 hs-CRP
[Abstract] Objective: To explore the role of Dapagliflozin in reducing the risk of ASCVD, by observing the changes in adipose tissue and inflammatory factors after the use of Dapagliflozin in the treatment of diabetes. Method: A total of 60 patients with type 2 diabetes in Guangdong Porvincial Peoples Hosptial Nanhai Hosptial from October 2018 to October 2019 were selected as subjects. The patients were randomly divided into observation group and control group, 30 cases in each group. The patients had been treated with hypoglycemic drugs before enrollment. The control group remained unchanged with the original treatment, the observation group was treated with Dapagliflozin on the basis of the original treatment. Weight, BMI, fasting blood glucose, fasting C-peptide, HOMA2-%B, HOMA2-%S, HOMA2-IR, subcutaneous fat area, visceral fat area, epicardial fat thickness, waist circumference, hip circumference, waist-hip ratio, Lp-PLA2, hs-CRP and other data were collected before and after observation. Result: Visceral fat area, subcutaneous fat area, pericardial fat thickness, body weight, BMI, waist circumference, hip circumference, waist-hip ratio, HbA1c, urinary malb/Cr, HOMA2-%B, HOMA2-%S, HOMA2-IR, Lp-PLA2, hs-CRP before and after treatment in the control group showed no statistically significant differences (P>0.05). Visceral fat area, subcutaneous fat area, epicardial fat thickness, body weight, BMI, waist circumference, hip circumference, waist-hip ratio, HbA1c, HOMA2-%B, HOMA2-%S, HOMA2-IR, Lp-PLA2, urinary malb/Cr, and hs-CRP in the observation group before and after treatment were all statistically significant (P<0.05). After 12 weeks of treatment, waist circumference, HbA1c, HOMA2-%B, HOMA2-%S, HOMA2-IR and hs-CRP of the observation group were significantly improved compared with the control group, the differences were statistically significant (P<0.05). The changes of HbA1c before and after treatment were correlated with the level of HbA1c before treatment. Conclusion: Dapagliflozin can effectively control blood glucose, reduce insulin resistance, enhance energy balance regulation, and reduce adipose tissue (especially visceral fat), possibly contributing to ASCVD risk reduction.
[Key words] Dapagliflozin Obesity Type 2 diabetes Adipose tissue Visceral fat Subcutaneous fat Lp-PLA2 hs-CRP
First-authors address: Nanhai Hospital of Guangdong Peoples Hospital, Foshan 528200, China
doi:10.3969/j.issn.1674-4985.2020.20.002
2型糖尿病合并肥胖是动脉粥样硬化性心血管病(ASCVD)的危险因素,然而研究显示大部分传统降糖药未显示出在减重以及降低大血管并发症方面有显著获益[1-2]。达格列净是新型降糖药,降糖和减重效果明显;本研究拟通过观察在糖尿病治疗中加用达格列净后脂肪组织和各种致炎因子的改变,探讨达格列净在降低ASCVD风险的作用。现报道如下。
1 资料与方法
1.1 一般资料 选取2018年10月-2019年10月在广东省人民医院南海医院就诊的2型糖尿病患者共60例为研究对象。入组标准:(1)年龄40~70岁;(2)按WHO(1999年)标准确诊2型糖尿病;(3)糖化血红蛋白7%~11%;(4)BMI≥28 kg/m2或腰围男≥90 cm、女≥85 cm。(5)使用胰岛素、磺脲类、格列奈类、双胍类、α糖苷酶类中一种或多种药物降糖3个月以上。排除标准:(1)3个月内曾使用DDP4抑制剂、GLP-1激动剂、他汀类和贝特类等降脂药;(2)有明确心脑血管疾病、周围血管疾病、肝肾疾病、甲状腺疾病病史;(3)有糖尿病急性并发症(糖尿病性酮症酸中毒、糖尿病高渗综合征、乳酸性酸中毒);(4)严重糖尿病慢性并发症。(5)有泌尿生殖系感染。随机将患者分为观察组与对照组,各30例。患者均知情同意,该研究已经医院伦理学委员会批准。
1.2 方法 (1)入组前收集两组资料:性别、年龄、身高、体重、体重指数(BMI)、谷丙转氨酶(ALT)、肌酐(Cr)、总胆固醇(TC)、甘油三酯(TG)、低密度脂蛋白(LDL-C)、高密度脂蛋白(HDL-C)、空腹血糖、空腹C肽、胰岛素分泌功能指数(HOMA2-%B)、胰岛素敏感指数(HOMA2-%S)、胰岛素抵抗指数(HOMA2-IR)、皮下脂肪面积、内脏脂肪面积、心外膜脂肪垫厚度、腰围、臀围、腰臀比、脂蛋白相关脂酶A2(Lp-PLA2)、超敏C反应蛋白(hs-CRP)。(2)患者入组前已进行降糖药物治疗,观察组在原治疗方案基础上加用达格列净(商品名:安达唐,生产厂家:美国阿斯利康公司,批准文号:国药准字J20170040,规格:10 mg)10 mg,1次/d;对照组维持原治疗方案不变。两组均治疗12周。
1.3 觀察指标 (1)内脏脂肪及皮下脂肪检测采用日本欧姆龙脂肪检测仪(型号:HDS-2000),采用生物电阻抗分析法测定,该法是在脐和后背之间给一个固定电压,利用电阻大的地方等势线密集电压大的原理,测量腹部侧面的电压,得到内脏脂肪面积占腹部横截面的比值。(2)心外膜脂肪垫厚度检测采用美国GE彩超机(型号:VividE95),检测标准为取胸骨旁长轴切面,RV中点垂直于AO环,测定心包壁层、脏层之间脂肪层心脏收缩末期厚度,取三次心搏平均值。(3)Lp-PLA2试剂盒来自天津康尔克生物科技有限公司,检测方法为酶联免疫法。
1.4 统计学处理 采用统计软件包SPSS 21.0对入组患者数据进行统计学分析。计量资料采用(x±s)描述,组间比较采用t检验,组内比较采用配对
t检验;计数资料以率(%)表示,比较采用字2检验;观察组治疗前后HbA1c改变(△HbA1c)相关因素分析采用多元线性回归分析。以P<0.05为差异有统计学意义。
2 结果
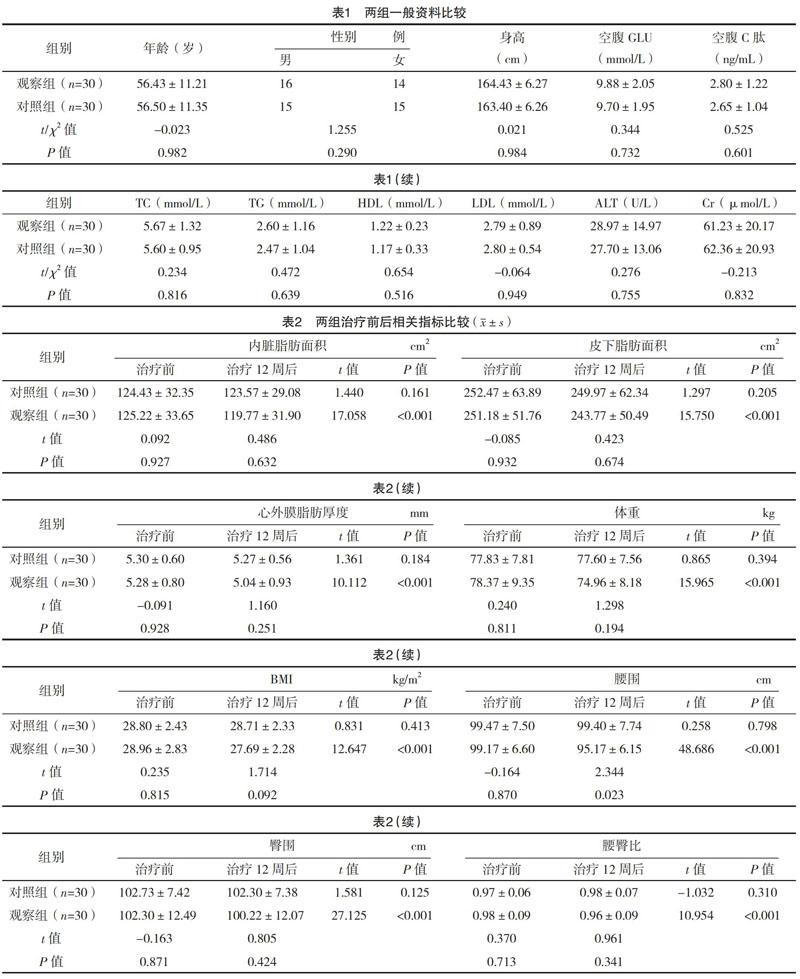
2.1 两组一般资料比较 两组一般资料比较,差异均无统计学意义(P>0.05),具有可比性,见表1。
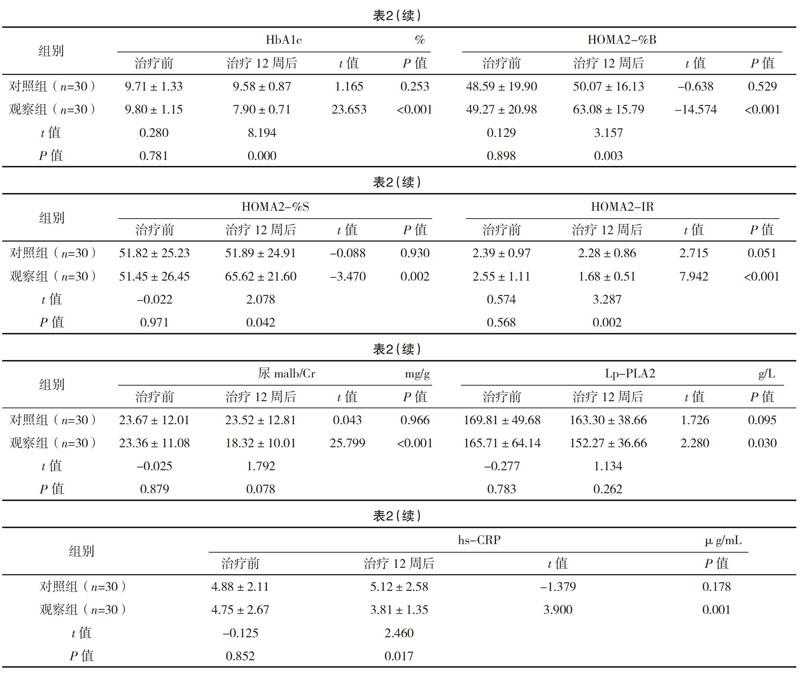
2.2 两组治疗前后相关指标比较 对照组治疗前后的内脏脂肪面积、皮下脂肪面积、心外膜脂肪厚度、体重、BMI、腰围、臀围、腰臀比、HbA1c、尿malb/Cr、HOMA2-%B、HOMA2-%S、HOMA2-IR、Lp-PLA2、hs-CRP比较,差异均无统计学意义(P>0.05)。观察组治疗前后的内脏脂肪面积、皮下脂肪面积、心外膜脂肪厚度、体重、BMI、腰围、臀围、腰臀比、HbA1c、HOMA2-%B、HOMA2-%S、HOMA2-IR、Lp-PLA2、尿malb/Cr、hs-CRP比较,差异均有统计学意义(P<0.05)。治疗12周后,观察组患者的腰围、HbA1c、HOMA2-%B、HOMA2-%S、HOMA2-IR、hs-CRP比对照组改善更明显,差异均有统计学意义(P<0.05)。见表2。
2.3 观察组△HbA1c多元线性回归分析 以△HbA1c为因变量,治疗前内脏脂肪面积、皮下脂肪面积、心外膜脂肪厚度、身高、体重、BMI、腰围、臀围、腰臀比、空腹GLU、空腹C肽、HbA1c、尿malb/Cr、HOMA2-%B、HOMA2-%S、HOMA2-IR、Lp-PLA2、hs-CRP、TC、TG、HDL-C、LDL-C、ALT、Cr自变量,行多元线性回归分析,发现只有HbA1c差异有统计学意义(P=0.000)。观察组治疗前后△HbA1c(因变量)与治疗前HbA1c(自变量)相关性回归方程为:△HbA1c=-4.899+0.693×HbA1c。
3 讨论
2型糖尿病占糖尿病患者90%以上,2型糖尿病患者腹部肥胖的发生率高达45.4%[3]。肥胖被认为是慢性炎症性疾病,可能导致胰岛素抵抗、血压异常、血脂异常,这些都是引起动脉粥样硬化的因素。肥胖患者显著特点是脂肪组织增多,脂肪组织可分为皮下脂肪和内脏脂肪。与皮下脂肪相比,内脏脂肪和血液供应和神经分布更为丰富,脂代谢更活跃,对人体交感神经的反应更灵敏,最终导致代谢风险增加[4]。内脏脂肪的存在与胰岛素抵抗相关[5]。胰岛素抵抗加重血管内皮损伤,引起血管重塑并最终导致高血压[6]。有研究表明,内脏脂肪积累是2型糖尿病患者长期心血管事件的独立危险因素[7]。心外膜脂肪是内脏脂肪一种,具有独特的分子结构和生物学特性,不仅是脂质储存单元,还参与心肌脂质和能量平衡,释放和合成游离脂肪酸能力更强。在生理条件下,心外膜脂肪通过抗动脉粥样硬化和抗炎作用,以及较高的游离脂肪酸释放和摄取发挥心脏保护作用;异常升高的心外膜脂肪可分泌多种生物活性物质和过量的游离脂肪酸,导致全身炎症、胰岛素抵抗、血脂异常,最终导致代谢综合征和动脉粥样硬化[8-11]。
Lp-PLA2主要由成熟的巨噬细胞和淋巴细胞产生和分泌。研究发现Lp-PLA2能将促炎因子血小板活化因子(PAF)水解成不活跃的lyso-PAF;PAF能促进血小板聚集、中性粒细胞和单核细胞趋化、白三烯等炎症介质和释放、血栓形成和炎症反应;Lp-PLA2通过水解PAF,能减少炎症和血栓,有抗炎和抗动脉粥样硬化作用[12]。同时,证据表明Lp-PLA2在動脉粥样硬化损伤病变中以较高浓度表达[13]。Lp-PLA2还能水解血管内膜中的氧化卵磷脂,产生溶血磷脂酰胆碱和氧化游离脂肪酸,这两种产物均为促炎产物,它能刺激和产生黏附分子和细胞因子,进一步促进单核细胞从腔到内膜的聚集运动。巨噬细胞来源于内膜中单核细胞的聚集,然后通过吞噬氧化低密度脂蛋白而转化为泡沫细胞,并最终聚集成AS斑块。释放的细胞因子和蛋白酶,降解平滑肌细胞和纤维帽的胶原基质,导致斑块的脆性增加和破裂进一步引起血栓的形成和心血管事件的发生[14-17]。
C反应蛋白(CRP)是在炎症或损伤过程中,由巨噬细胞释放IL-6、IL-1,刺激肝脏迅速合成的一种急性时相蛋白,它能敏感地反映机体炎症反应的存在。hs-CRP能引血管内皮细胞功能障碍,引起动脉粥斑块形成,此外还有促血栓形成作用[18]。动脉粥样硬化的炎症反应可能与血浆Lp-PLA2和hs-CRP的协同作用有关。Lp-PLA2在动脉粥样硬化过程中与hs-CRP有不同的生理病理机制,扮演不同角色,hs-CRP可能是急性非特异性炎症反应的产物,而Lp-PLA2可能在心血管炎症中起着更为特殊的作用[19]。
本研究发现对照组病例在未改变治疗方案时HbA1c等各项提标均无明显改变。观察组原治疗方案基础上加用达格列净治疗12周后各项指标均明显改善。血糖控制指标HbA1c从(9.80±1.15)%下降至(7.90±0.71)%,降幅为19.38%,降糖效果显著,与达格列净区别于原降糖药物的独特降糖机制有关。达格列净通过抑制近曲小管SGLT-2受体,抑制葡萄糖的重吸收,使尿糖排出增多,达到降糖目的[20]。本研究通过多元线性回归分析发现,HbA1c的降幅与治疗前HbA1c水平有关,血糖越高,降糖幅度越大。通过对观察组HOMA2-%B、HOMA2-%S、HOMA2-IR的分析,发现治疗后胰岛素分泌功能改善、胰岛素敏感性提高,胰岛素抵抗减少,可能与治疗后糖毒性消除有关。体重控制指标中的体重、BMI均下降;脂肪指标中的腰围、臀围、腰臀比、内脏脂肪面积、皮下脂肪面积、心外膜脂肪厚度均下降,腰围降幅大于臀围,内脏脂肪面积下降大于皮下脂肪;以上改变可能与排糖同时改善体内能量平衡有关。反映ASCVD指标Lp-PLA2稍下降,提示可能用药后存在动脉粥样硬化损伤病变改善。hs-CRP未见明显统计学意义改变,考虑为CRP为非特异性炎症指标,观察过程中不排除感染或非感性炎症因素干扰,且样本量少导致未能得出明显阳性结果。
总而言之,达格列净通过有效的血糖控制、减少胰岛素抵抗、增强能量平衡调节、减少脂肪组织(尤其是内脏脂肪),可能存在降低ASCVD风险作用。
参考文献
[1] Lau D C,Teoh H.Impact of Current and Emerging Glucose-Lowering Drugs on Body Weight in Type 2 Diabetes[J].Canadian Journal of Diabetes,2015,39(5):148-154.
[2] Eriksson J,Laine M.Diabetes Drugs and Body Weight[J].Duodecim,2013,129(1):73-78.
[3] Xu Y,Wang L,He J,et al.Prevalence and Control of Diabetes in Chinese Adults[J].JAMA,2013,310(9):948-959.
[4] Sang-A C,Joon J H,Jae-Young C,et al.Visceral Fat Area and Serum Adiponectin Level Predict the Development of Metabolic Syndrome in a Community-Based Asymptomatic Population[J].PLoS One,2017,12(1):e0169289.
[5] Moon H U,Ha K H,Han S J,et al.The Association of Adiponectin and Visceral Fat with Insulin Resistance and β-Cell Dysfunction[J].Journal of Korean Medical Science,2019,34(1):e7.
[6] Paolo T,Monica V,Lucia P,et al.Decreased homocysteine trans-sulfuration in hypertension with hyperhomocysteinemia: relationship with insulin resistance[J].The Journal of Clinical Endocrinology and Metabolism,2018,103(1):56-63.
[7] Kwon S H,Han A L.The Correlation between the Ratio of Visceral Fat Area to Subcutaneous Fat Area on Computed Tomography and Lipid Accumulation Product as Indexes of Cardiovascular Risk[J].Journal of Obesity & Metabolic Syndrome,2019,28(3):186-193.
[8] Levelt E,Pavlides M,Banerjee R,et al.Ectopic and Visceral Fat Deposition in Lean and Obese Patients with Type 2 Diabetes[J].Journal of the American College of Cardiology,2016,68(1):53-63.
[9] Packer M.Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium[J].Journal of the American College of Cardiology,2018,71(20):2360-2372.
[10] Chakravarthy B K,Arun M,Kumar R P,et al.Effect of aerobic exercise on echocardiographic epicardial adipose tissue thickness in overweight individuals[J].Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy,2018,11(11):303-312.
[11] Christensen Re H,Scholten B J V,Hansen C S,et al.
Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes[J].Cardiovascular Diabetology,2019,18(1):e114.
[12] Stefano A D,Mannucci L,Tamburi F,et al.Lp-PLA2, a new biomarker of vascular disorders in metabolic diseases[J].International Journal of Immunopathology and Pharmacology,2019,33:1-4.
[13] Schanstra J P,Luong T T D,Makridakis M,et al.Systems biology identifies cytosolic PLA2 as a target in vascular calcification treatment[J].JCI Insight,2019,4(10):e125638.
[14] Pokharel Y,Sun Wensheng,Polfus L,et al.Lipoprotein associated phospholipase A2 activity, apolipoprotein C3 loss-of-function variants and cardiovascular disease: The Atherosclerosis Risk In Communities Study[J].Atherosclerosis,2015,241(2):641-648.
[15] Wang Danchen,Guo Xiuzhi,Hou Lian,et al.Measuring lipoprotein-associated phospholipase A2 activity in China: Protocol comparison and recalibration[J].Journal of Clinical Laboratory Analysis,2019,33(1):e22628
[16] Huamin Liu,Yan Yao,Youxin Wang,et al.Association between high-sensitivity C-reactive protein,lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A cross-sectional study[J].J Cell Mol Med. 2018(22):5145-5150.
[17] Alkuraishy H M,Al-Gareeb A I,Waheed H J.Lipoprotein-Associated Phospholipase A2 is Linked with Poor Cardio-Metabolic Profile in Patients with Ischemic Stroke: A Study of Effects of Statins[J].Journal of Neurosciences in Rural Practice,2018,9(4):496-503.
[18] Ebrahimi M,Heidari-Bakavoli A R,Shoeibi S,et al.
Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors[J].Journal of Clinical Laboratory Analysis,2016,30(5):672-676.
[19] Liu Huamin,Yao Yan,Wang Youxin,et al.Association between high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A cross-sectional study[J].Journal of Cellular and Molecular Medicine,2018,22(10):5145-5150.
[20] Feng Miao,Lv Haihong,Xu Xia,et al.Efficacy and safety of dapagliflozin as monotherapy in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials[J].Medicine,2019,98(30):e16575.
(收稿日期:2019-11-18) (本文編辑:张爽)

