金属有机框架用于一氧化碳氧化
王 瑞,黄新松,刘天赋,曹 荣
(1.中国科学院福建物质结构研究所,结构化学国家重点实验室,福州 350002;2.中国科学技术大学,合肥 230026)
1 Introduction
Carbon monoxide(CO),a colorless,odorless and tasteless gas,is harmful for humans at a high concentration[1,2].As the primary product or byproduct of inadequate combustion of carbon or carbon-containing compounds,CO is one of the main pollutants ejected from internal combustion engines.Moreover,due to its toxic effect,CO becomes the source of various environmental and health problems[3,4].
Catalytic CO oxidation at lower or ambient temperatures has appealed to a lot of attention.The reaction,during which catalysts convert toxic CO into nontoxic product CO2,can be employed in many practical applications,such as automotive emission control,air purification,gas sensors and fuel cells.It is also one of the most extensive probe reactions in fundamental studies[5,6].
Metal-organic frameworks(MOFs),a new class of porous materials,which consist of metal ions or clusters[also known as secondary building units(SBUs)]and bridging organic linkers,are becoming highly promising catalysts[7—13].Furthermore,compared with traditional materials,such as zeolites and mesoporous silica,MOFs have some unique traits and preponderances[7,14—19],including adjustable structure,multicomponent,various crystalline nature,etc.In recent years,with the increasing studies of MOF materials,many research fellows have focused on effective MOF composite catalysts.Previous reviews based on the similar topic,just briefly presented the different catalytic sites and synthetic methods[20].Herein,we summarize recent progress of MOFs and MOF-based catalysts for CO oxidation sorted by catalytically active sites(further classified by elemental species)and then highlight the factors through the reaction.
2 MOFs and MOF-based Catalysts with Various Active Species/Sites
MOFs are constructed from metal nodes and organic linkers connected through coordination bonds.The resulting pore space and unique building patterns endow MOFs and MOFs composites abundant catalytic sites which can be classified into three categories:functionalized metal nodes or coordinatively unsaturated-metal sites(CUSs),functional and modified linkers,and catalytically active guest species[e.g.,metal complexes,metal nanoparticles(MNPs)]incorporated into the cavities of MOFs.Besides using the active sites in the ordered MOFs structure,some researches are focused on pyrolysis of MOF to generate metal or metal oxidecomposites.Since the porosity and long-range structural sequence were partially preserved during this process,this method offers a unique opportunity to achieve the even distribution of active species/sites on porous carbon nanocomposites[7].These catalytic sites could make a difference in CO oxidation reaction.Afterwards,we focus on the element types of active species/sites in recent progress(Table 1).

Table 1 Summary of MOFs and MOF-based catalysts(classified by elements)for CO oxidation
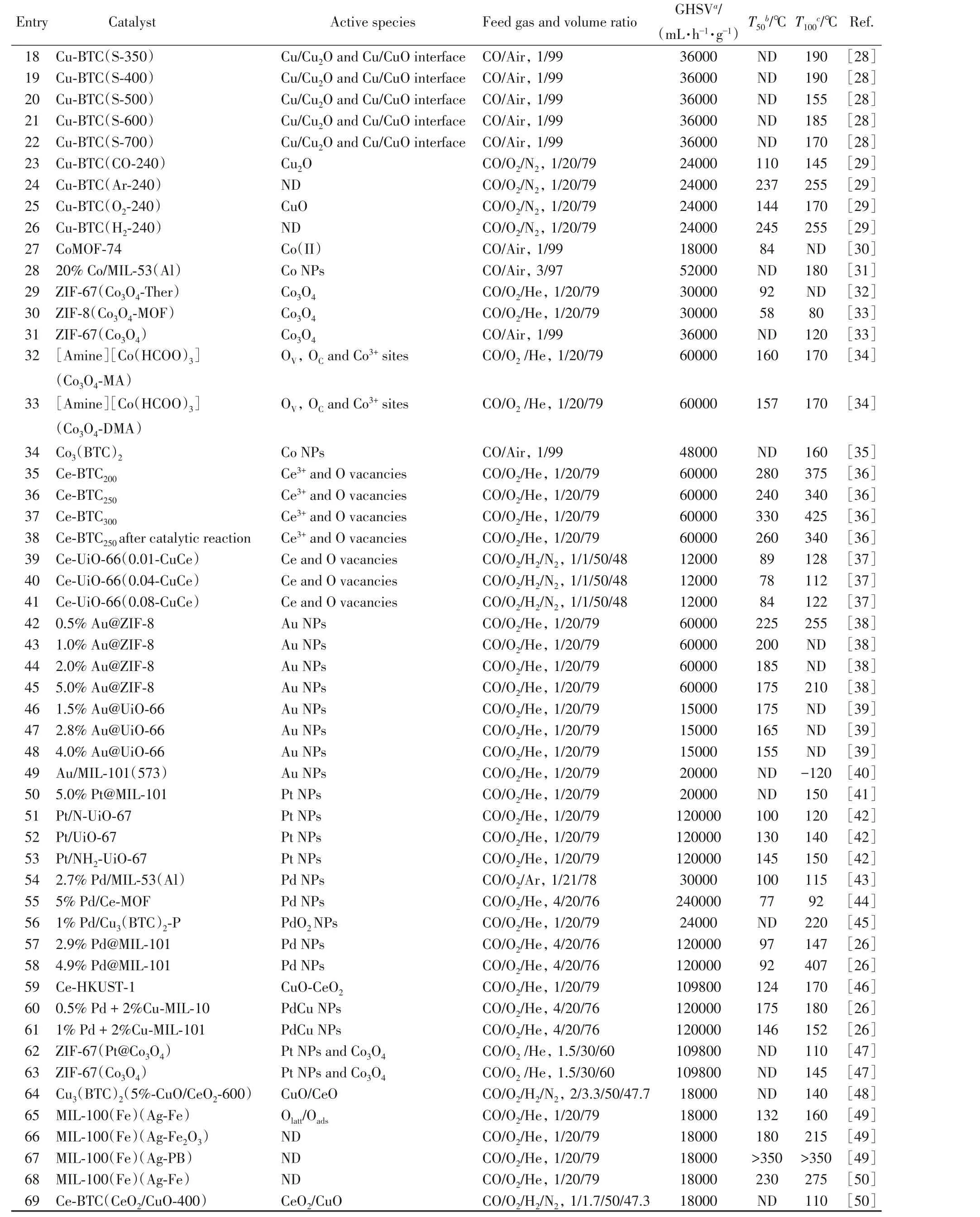
Continued
2.1 Copper(Cu)Species
Cu-MOFs and related substances are frequently studied in CO oxidation.Additionally,in regard to active species,Cu(I),Cu(II),CuO,Cu2O and Cu NPs are researched in many reports(Table 1,Entries 1 to 26).
The first example of using Cu-MOF as a heterogeneous catalyst for the gas-phase oxidation of CO to CO2was reported by Zou et al.[21]in 2007.They synthesized a two-dimensional(2D)porous Cu-based MOF,[Cu(mipt)(H2O)](H2O)2(mipt=5-methylisophthalate),with a paddlewheel Cu-Cu cluster involving open Cu(II)sites.Using12CO and13CO isotopic probe molecules,Zou et al.found active Lewis acid sites on channel walls,which are excellent and stable in catalyzing the CO oxidization(Fig.1).Soon after,a new Cu-containing 3D MOF compound,Cu5(OH)2(nip)4(nip=5-nitroisophthalate),highly active for CO oxidation,was reported by Zhao et al.[22],giving a 100%conversion at 200℃.The high catalytic activity toward the CO oxidation can be ascribed to its high density of coordination-unsaturated Cu(II)sites or Cu(II)active sites.
As mentioned above,MOFs containing Cu(II)active sites are equipped to convert CO to CO2and the catalytic efficiency is decent.Besides,Cu(I)active sites are also known to catalyze the oxidation of CO by the use of valence variation to seize or release oxygen readily[51].Considering this,in 2017,Li and co-workers[25]synthesized a series of new multicomponent MOFs(FDM-3—FDM-7)using trinuclear Cu-based pyrazolate as building blocks.X-Ray photoelectron spectroscopy(XPS) was adopted to examine the valence of Cu in FDM-3—FDM-7.Interestingly,by heating FDM-3—FDM-7 at 200℃ under vacuum for activation,the XPS spectra showed that the percentage of Cu ions existing as Cu(I)in these MOFs increased after the activation,indicating the reduction of Cu(II)to Cu(I).These Cu(I)sites were appropriately responsible for the catalytic reactivity.
Cu based metal-organic frameworks have been identified for efficient CO oxidation at ambient pressure and rising temperatures.Based on the above,the CUSs in the pristine Cu-MOFs act as catalytic centers for the adsorption and activation of CO from the substrates and promote their conversions to CO2.As porous materials,MOFs are able to accommodate metal NPs inside and the resulting substance can largely extend the application of MOFs in catalysis.From thus base,El-Shall et al.[26]incorporated 2—3 nm Cu NPs into MIL-101 using a microwave irradiation method.MIL-101 as a catalyst support had no activity toward CO oxidation under aerobic conditions,which demonstrated that Cu NPs were the only active component in this crystalline material.
Besides encapsulating guest species in MOFs,MOFs pyrolysis is a facile method to obtain MOF derivatives with much enhanced stability and uniform porosity,allowing them suitable for CO oxidation under harsh reaction conditions.Zhang et al.[28]obtained Cu/CuO/Cu2O core with a porous carbon shell structure through direct annealing of a Cu-based metal-organic framework(Cu-BTC)in N2,followed by testing its catalytic activity towards CO oxidation.Comparing with the best activity[T100at 155 ℃ for Cu-BTC(S-500)]in this work,the optimal result for pure Cu-BTC used for CO oxidation in a former report was achieved at T100=170 ℃[24].DFT calculations demonstrated that the significant increase in the electron density on the Cu/Cu2O and Cu/CuO interface plays a pivotal role in the enhancement of CO oxidation.
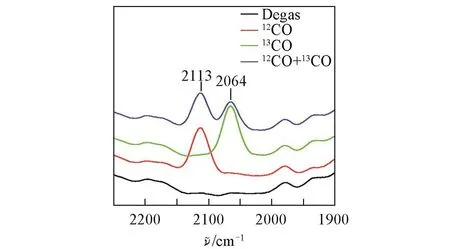
Fig.1 12CO and13CO probe molecules are used to examine active sites[21]
2.2 Cobalt(Co)Species
M-MOF-74[M=Co(II),Fe(II),Ni(II)Cu(II),Mn(II),Mg(II),and Zn(II)]is a well-characterized MOF material that contains high concentrations of coordinatively unsaturated sites.Thus,it has potential applications in transforming CO into CO2.Kim et al.[30]chose isostructural Co-MOF-74,Mg-MOF-74,and Zn-MOF-74 as catalysts for gas-phase CO oxidation.Results showed that only Co-MOF-74 exhibited catalytic activity because of the existence of unexceptionable Lewis acidic CUSs[Co(II)]in the framework and the catalytic activity of Co-MOF-74 was preeminent to those of previously reported MOFs used in catalytic systems.
With respect to Co-based active species,Co3O4species also accounted for a large proportion.In 2011,Wang et al.[32]reported the first example of MOF as host for preparing neat Co3O4nanoparticles by a facile liquid-phase method.The thermolysis of cobalt nitrate was accommodated in the ZIF-8 host to obtain uniform Co3O4particles inside the network.Therefore,the result revealed that in the presents of the Co3O4nanoparticles,the frameworks demonstrated excellent catalytic activities and long-term stability during CO oxidation.
2.3 Cerium(Ce)Species
Cerium,an abundant and inexpensive rare earth metal,as having the access to get different valence state,gives rise to playing a giant role in catalytic field.It is worth mentioning that in recent years there have been many Ce-MOF derivatives used as catalysts in CO oxidation[36,37,48,50].Zhang and co-workers[36]prepared a series of Ce-BTC derivatives through directly calcining Ce-BTC in atmosphere(30%O2/Ar)at different temperatures.The optimal result was achieved on the sample calcined at 250 ℃(Ce-BTC250,Table 1,Entry 36).The improved activity was attributed to the abundant surface Ce3+and oxygen vacancies,benefiting from the unique structural features and huge surface area of the as-prepared Ce-BTC250.
2.4 Noble Metal Species
Noble metal catalysts[gold(Au),platinum(Pt)and palladium(Pd)]are common and efficient in many catalytic reactions.In fact,to reduce specific consumption of noble metals and modify the catalyst,it occurred to researchers that accommodating some MNPs inside the porous MOFs may be a good idea[7,52].Thus,the catalytic oxidation of CO using MNPs@MOF composite catalysts has appealed to scholars.Although MOFs did not show activity for the CO oxidation reaction,the pores of MOFs prevent MNPs from agglomerating and make the particles permanently confined inside the cavities or on the surface.Broadly speaking,compared with pristine MOF catalysts(Table 1),the MNPs@MOFs composite catalysts have shown better manifestation at relatively high temperatures.In 2009,Xu and co-workers[38]did a precursory work about Au NPs deposited to a zeolite-type MOF by a simple solid grinding method.The catalytic performance of Au@ZIF-8 was evaluated in a fixed-bed flow reactor with the increase of Au loading(mass fraction)from 0.5%to 5%.In particular,only the 5%Au@ZIF-8 realized the complete conversion of CO at approximately 210℃.
Recently,Tsumori et al.[40]made a big breakthrough in metal/MOFs composite materials with a thermal transformation method to adjust the pyrolysis products.The obtained MNPs/quasi-MOF composite material not only preserved porous structure,but also realized a strong interaction between the metal NPs(such as Au)and the inorganic nodes(such as Cr—O)of the quasi-MOF,leading to remarkably enhanced catalytic performance in the low-temperature catalytic oxidation of CO.
Besides the Au-based NPs@MOFs,Pt NPs embedded in MOFs also exhibit excellent activity in CO oxidation at lower temperatures.In 2012,Xu and co-workers[41]developed a facile double solvents approach to immobilize Pt NPs within pores of mesoporous MOF-MIL-101 for the oxidation of CO to CO2.The MIL-101 framework had open windows(1.2 and 1.6 nm)smaller than the Pt NPs[mean size,(1.8±0.2)nm],which hindered Pt NPs from aggregating in the MIL-101 framework.The MIL-101 showed no activity in the CO oxidation reaction up to 200℃,while 5%Pt@MIL-101 completely converted CO at 200℃.
3 Variables Influencing the Catalytic Activity
In general,the occurrence of a catalytic reaction is always influenced by many complicated factors.In order to achieve the catalytic oxidation of CO efficiently and sufficiently,we have to take the possible factors into consideration.In this review,we mainly discuss the effects of the variables about chemical structure,loading weight,preparation&pre-treatment techniques and reaction temperature.We believe that understanding these items would be helpful in optimizing the conditions for CO oxidation.
3.1 Chemical Structure
The chemical structure of MOFs-based catalysts has shown pivotal role to the oxidation of CO to CO2.In view of structure,MOFs have much more diversified and periodic networks than other porous materials such as zeolite and mesoporous silica.Moreover,the periodic structure ensures MOFs to disperse active sites uniformly throughout the framework.The porosity promotes the accessibility of active sites and the transport of catalytic substrates/products in MOFs.Besides,the high surface area supports the adsorption and improvement of substrate molecules around the active sites,further strengthening the subsequent activation and catalytic conversion[7].Furthermore,MOFs as supports also play an important role in MNP@MOFs materials due to their unique structural natures.
3.1.1Chemical Structure of Pristine MOFsCu based MOFs have been proved to possess catalytic activity in many reports,such as Cu5(OH)2(nip)4(nip=5-nitroisophthalate)[22].It has shown high catalytic reactivity toward CO oxidation.In the view of Cu5(OH)2(nip)4,six unsaturated coordination sites in each SBUs can be generated readily when removing the six coordinated H2O molecules,which resulted in higher density of unsa-turated copper sites(4.4 Cu2+per nm3).Likewise,in another typical Cu-MOFs,Cu(mip)(3.1 Cu2+per nm3)[21],two uncoordinated sites can be achieved after the removal of two apical H2O molecules in each building unit.These two MOFs demonstrated high catalytic performance in the CO oxidization reaction owing to the high density of Cu(II)active sites.
The above analysis has proved that pristine Cu based MOFs containing abundant active sites possess high activities in CO oxidation.Therefore,can we draw a conclusion that MOFs with the above characteristics show remarkable performance? In 2015,Kimet al.[30]reported a CO oxidation catalyst based on M-MOF-74(M=Co2+,Mg2+,and Zn2+).They found that only Co-MOF-74 showed catalytic activity,and 87%conversion was achieved at 130℃.The results revealed that the high energy of the 3dorbital in Mg2+and fully occupied set of 3dorbitals in Zn2+do not provide good overlap forσ-donation from CO.Therefore,both metal-CO interactions therein were predominantly a result of ion-induced dipole interactions.
However,the partially filled 3dorbital and its appropriate energy level of the 3dorbital in Co2+can lead to efficientσ-donation andπback-donation between CO and Co2+.Consequently,the prominent performance of Co-MOF-74 compared to that of Mg-MOF-74 and Zn-MOF-74 was attributed to the relatively dominant Lewis acidic character of Co2+,and CO molecule was readily activated on the Co2+CUSs in the structure.Besides,theT50value for Co-MOF-74 was 84 ℃,which was significantly lower than that of Cu(nip)(155 ℃)[22].The high activity of Co-MOF-74 was attributed to the high density of CUSs(4.51 Co2+per nm3)[53],high porosity and its surface area.
In short,pristine MOFs as splendid catalysts for CO catalytic oxidation should have the following characteristics:(1)abundant CUSs,(2)high porosity and surface area,(3)suitable metal center and(4)particular stability.
3.1.2Chemical Structure of MOFs as SupportsAs described previously,MOFs can be a platform to support noble metal nanoparticles used for converting CO to CO2.Reported articles exhibited that Au@ZIF-8[38],Au@UIO-66[39][Fig.2(A)]and Pt@MIL-101[41][Fig.2(B)]showed excellent catalytic performance.It should be noted that some of the MOFs could not become supports for these MNPs for catalysis.Specific MOFs must have their unique natures such as stability and porosity.ZIF-8 possessed an intersecting three-dimensional structural feature,high thermal stability(over 500 ℃),large pore size(diameter of 1.16 nm),and large surface area(SBET=1413 m2/g),which was appropriate for depositing small Au NPs[54].UIO-66 has been chosen as support because of their high thermal stability(up to 450 ℃ in air)and strong resistance towards water,organic solvents and other chemicals[55—57].MIL-101,a chromium-based MOF,was chosen for this strategy because of high stability in water,large surface area,and two hydrophilic zeolite type cavities with free diameters of ca.2.9 and 3.4 nm accessible through two pore windows of ca.1.2 and 1.6 nm in diameter[58].
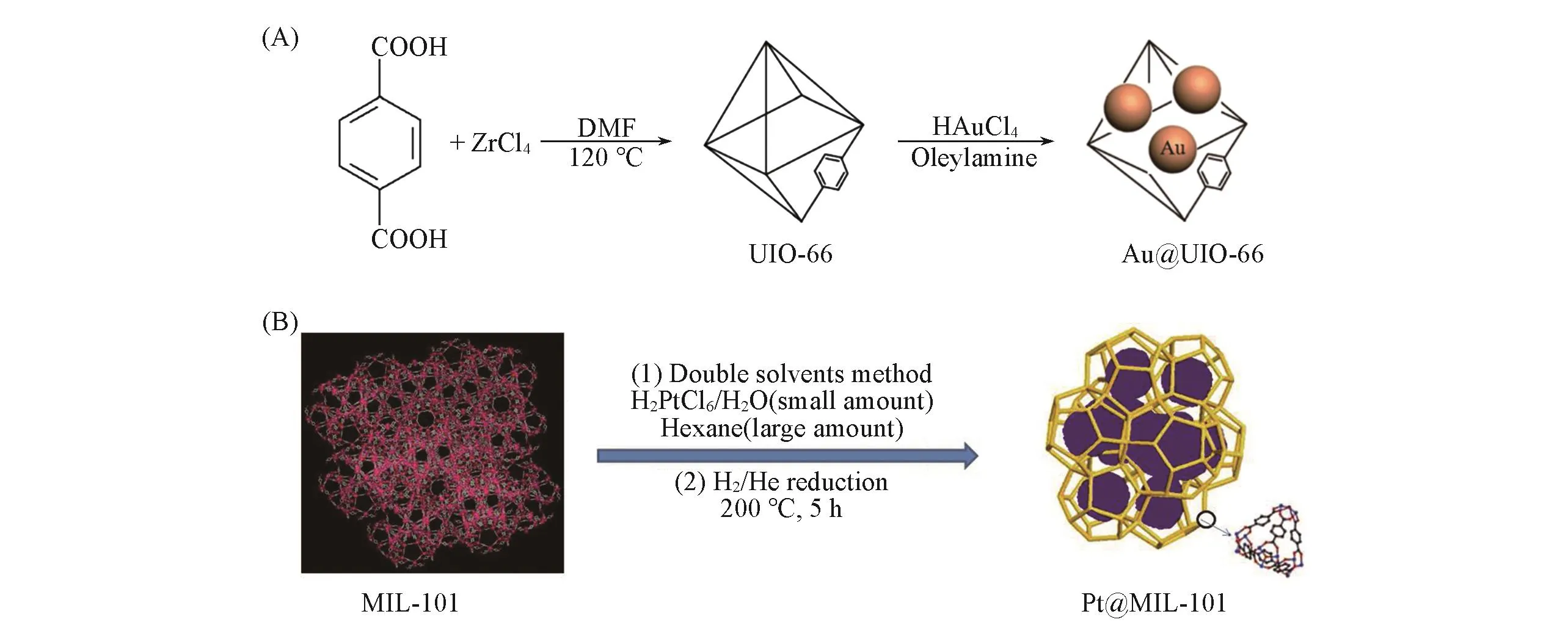
Fig.2 MNPs@MOF for CO oxidation
Through these examples,it can be concluded that the high stability,large surface area and proper cavities are necessary features for becoming supports in immobilizing MNPs and avoiding aggregation.El-Shall et al.[44]developed a microwave irradiation method for the incorporation of Pd nanoparticles within Ce-MOF and finally got Pd/Ce-MOF as a unique catalyst that was capable of CO oxidation.The catalytic activity of this material toward CO oxidation was significantly higher than those of other reported metal nanoparticles supported on MOFs.For example,5%Pd/Ce-MOF showed 50%CO conversion at 77℃ in comparison with 170℃for 5%Au@ZIF-8[38].The author pointed out that the high activity of the Pd/Ce-MOF catalyst is due to the presence of Ce(III)and Ce(IV)ions within the metal-organic framework support.In addition to the oxygen stored on the Ce(III)/Ce(IV)centers,Pd nanoparticles supported on the Ce-MOF can store oxygen in the form of a thin palladium oxide layer at the nanoparticle-support interface.From this,we can draw a conclusion that in order to achieve low temperature oxidation,the MOFs supports should be capable of activating substrates(CO or O2)and providing synergistic effects with MNPs.
3.2 Loading Weight
Generally,different loading weights of MNPs is bound to having corresponding impacts on catalysis.In view of this,MNPs@MOFs catalysts also face the same problem.The increasing content of Au loadings(mass fraction)on MOFs supports can enhance the activities in some ways.For example,the catalytic activities of 0.5%,1.0%,2.0%,5.0%Au@ZIF-8[38]and 1.5%,2.8%,4.0%Au@Uio-66[39]improved in direct proportion with the increasing of Au loading.The whole tendency still remained positive when the loading weight was high,even if a light aggregation of Au appeared.
Not all material exhibited the above phenomenon that the catalytic activities improved in direct proportion with the increasing loading weight.El-Shall et al.[44]obtained 3%,5%,7%Pd/Ce-MOF by microwave irradiation method.Interestingly,the trend of the catalytic activity was not directly proportional with increasing content of Pd(mass fraction).5%Pd/Ce-MOF showed the highest activity(T50:ca.77 ℃,T100:ca.96 ℃).They explained that this was probably due to the aggregation of the Pd nanoparticles on the surface of the Ce-MOF at high loading,which could decrease the surface area of the catalyst.
3.3 Preparation&Pre-treatment Techniques
The catalytic activity of synthesized catalysts strongly depends on the preparation and pre-treatment methods as they directly affect the quality and quantity of the active sites as well as the dispersion of the nanostructured catalysts.
Ye et al.[45]employed a dielectric-barrier discharge(DBD)plasma to treat Cu3(BTC)2and got amorphized Cu3(BTC)2.They found that crystalline and amorphized Cu3(BTC)2were both proved to be excellent catalysts for CO oxidation.Moreover,the amorphized Cu3(BTC)2had a better activity than the crystalline one.
In order to obtain Cu/CuOx/C nanocomposites,Chen et al.[28]annealed Cu-BTC at 350,400,500,600 and 700℃in N2atmosphere and designated the obtained samples as S-350,S-400,S-500,S-600 and S-700,respectively(Table 1,Entries 18—22).Sample S-500 exhibited the T100at 155℃,which was the best for oxidation of CO among the catalysts reported in their work.This work revealed that different preparation temperatures to deal with the same MOFs resulted in various performances(Fig.3).
Before the proceeding of the catalytic reaction,the as-prepared catalysts are always treated with a pretreatment process,which is also frequently called the activation of the catalysts.Herein,we talk simply from two perspectives,activation temperature and activation atmosphere,to discuss the impact of the pretreatments on the catalysts’performance.
With respect to activation temperature,Qiu et al.[24]made a detailed report about CuBTC and how this factor affected the catalytic performance for CO oxidation.They activated the samples in a flow of nitrogen at 443,473,503,523 and 553 K,to give CuBTC-443,CuBTC-473,CuBTC-503,CuBTC-523 and CuBTC-553,respectively.The catalytic activity of CuBTC samples increased in the order CuBTC-553<CuBTC-443≈CuBTC-473<CuBTC-503<CuBTC-523[Fig.4(A)].It was demonstrated that too high activation temperatures of CuBTC would decrease its activity owing to the destruction of the frameworks.
Recently,Xu et al.[40]reported the fabrication of metal/quasi-MOF composites by partial deligandation of metal/MOF composites by calcinations at different temperatures(Fig.5).In this article,the researchers found that at 373 K,the guest H2O molecules were free from MIL-101.At 473 K,accessible Cr—O sites were created upon removal of the coordinated H2O molecules and OH/F groups.More accessible Cr—O sites on“quasi-MIL-101”can be fabricated at 573 K due to the release of CO2on the terephthalate ligand.However,a harsh temperature above 673 K resulted in the collapse of the framework.Thus,the obtained Au/quasi-MIL-101(573)catalyst maintained 100%CO conversion at-120℃for up to 4000 min.
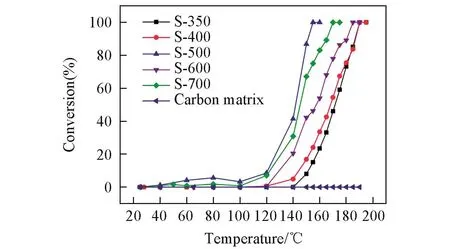
Fig.3 Metal(oxide)/carbon nanocomposites derived from MOFs[28]

Fig.4 Effects of activation temperature and atmosphere on CO oxidation
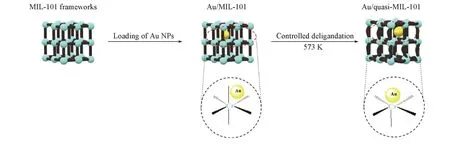
Fig.5 Impacts of calcination temperature on the formed crystal structure
Activation atmosphere remains equally important in the pre-treatment process.Zhanget al.[29]proved that different activation atmospheres had distinct impact on the properties of CuBTC MOFs for CO oxidation.In their work,thermal activation by reductive H2severely destroyed the structure of CuBTC and Ar had a weak influence,while O2was effective for CuBTC catalysts since active CuO species were obtained from the slight collapsing of CuBTC structure[Fig.4(B)].Moreover,the highest activity could be obtained when activated in CO reaction gas,since many pores and more effective Cu2O were formed during the process(Entries 23—26 in Table 1).
Considering the injecting sequence of reactant gas,Wanget al.[59]used UHV-FTIRS data to demonstrate how the factor affected the reactions on Cu-MOFs(HKUST-1 and MOF-14)at 105 K.HKUST-1 and MOF-14 samples were first exposed to O2and then to CO at 105 K,the corresponding UHV-FTIR spectra showed no indication for the formation of CO2,revealing that in this case the oxidation reaction did not take place.However,the opposite sequence gave different results.The authors proposed that O2molecule was activated in the presence of pre-adsorbed CO and interacted simultaneously with two isocarbonyl species at neighboring Cu2+CUS to yield two CO2molecules.
3.4 Reaction Temperature
In catalytic reaction,temperature is one of the most important parameters which should be emphasized.Kimet al.[30]reported that Co-MOF-74 achieved 87%CO conversion at 130 ℃,while they could not further study the catalytic activity of Co-MOF-74 after 130 ℃ due to the catalyst’s instability against the catalytic reaction conditions.The author revealed that the PXRD of the degassed Co-MOF-74 after heating above 150℃for 5 h in the air showed the deformation of the original structure,which was eventually transformed to Co3O4at 230℃.It has been generally accepted that at high temperature,the frameworks of MOFs are fragile and may transform into other material,such as metal oxides.Accordingly,taking the reaction temperature into consideration in evaluating the MOFs catalysts’properties comes into being important.
4 Conclusions and Perspectives
In conclusion,MOFs have been proven to be suitable for catalytic CO transformation not only due to their permanent porosity,high surface area,structural diversity and tunability,but also because they can be utilized as platforms or sacrificial precursors to create various MOFs-based catalysts.Researchers in this field have spared no effort to synthesize high-activity catalysts based on MOFs and made great progress in understanding of the catalytic mechanism.Some important factors must be taken into consideration.A trivial change on these factors may cause distinct activities.There are still many challenges to fully take advantage of the unique MOF merits,such as the periodic network,precise structural information,permanent porosity,and high surface area.Particularly,it is essentially important to gain deep insights about the relationship between the structure and their catalytic activity,which may greatly help us to inspect and reconsider the conventional mechanism.Despite the intensive studies and progress in this filed,efficient MOFs or MOF-based catalysts are still highly desired,which need further experimental and theoretical investigations.With constant efforts devoted to this field,we believe more efficient MOF catalysts and profound understanding for CO oxidation will be obtained in the near future.
This paper is supported by the National Key R&D Program of China(No.2017YFA0206801).
——李振声