Antifungal Secondary Metabolites Isolated from Mangrove Rhizosphere Soil-Derived Penicillium Fungi
SHEN Nanxing, LIANG Zhaoyang, 2), LIU Qing, TU Chuandeng, DONG Kunming,WANG Changyun, and CHEN Min,
Antifungal Secondary Metabolites Isolated from Mangrove Rhizosphere Soil-DerivedFungi
SHEN Nanxing1), LIANG Zhaoyang1), 2), LIU Qing1), TU Chuandeng1), DONG Kunming1),WANG Changyun2), 3), and CHEN Min1),*
1),,,225127,2),,,,266003,3),,266237,
Fungi isolated from mangrove rhizosphere soil ofthe South China Sea were investigated for the production of potential antifungal metabolites. With 28 fungal isolates, the strainsHK1-23 andHK1-6 showed significant antifungal activities. A bioassay-guided investigation ofthe two fungal strains led to the isolation of two secondary metabolites, brefeldin A and penicillic acid, with high yields of 143 and 423mgL−1, respectively. Penicillic acid showed potent antifungal activities towardand, with 67.5% and 76% growth inhibition, respectively, at 50μgmL−1. Brefeldin A showed strong activity toward, with 56.4% growth inhibition at 50μgmL−1. The research highlights the importance of exploring microbes from mangrove rhizosphere soil for the identification of bioactive metabolites for future fungicide development.
mangrove rhizosphere soil-derived fungus;;brefeldin A; penicillic acid; antifungal
1 Introduction
Marine-derived fungi and other microorganisms represent an incredibly rich reservoir of natural products, which often show potent bioactivity and are exploited in various contexts, ranging from human medicine to crop protection (Bugni and Ireland, 2004; Blunt., 2018). Mangrove-derivedfungi, growing in the saline coastal habitats in the tropics and subtropics, are considered to be a pro- mising source of structurally unique and biologically active natural products considering the uniqueness of the in- tertidal zone will give rise to equally unprecedented na- tural products (Zhou., 2013). Indeed, to date, an increasing number of new, bioactive, and structurally unique metabolites have been identified from mangrove-derived fungi (Wu., 2008; Xu, 2014), demonstrating that this special ecosystem could be utilized in the exploration of potential new drugs, such as anticancer, antiviral, antibac- terial, and anti-inflammatory agents (Yasuhara-Bell and Lu, 2010; Deshmukh., 2017; Ancheeva., 2018). How- ever, there are relatively few reports about the agricultural fungicidal effects of these marine natural compounds.
The prevention and control of phytopathogens is particularly important to avoid major crop failure and financial loss. For example, rice sheath blight, caused by, is one of the most important rice fungal diseases, leading to great economic losses in all temperate and tropical rice-growing regions throughout the globe (Lee, 1983). Fungicide is one of the important measures used to control rice sheath blight (Boukaew., 2013). In China, validamycin A (jinggangmycin) has been widely used for controlling sheath blight disease for over 40 years (Mew., 2004). However, the intensive use of fungicides has adverse effects, including fungicide resistance and residual toxicity (Aguín., 2006). Therefore, it is necessary to develop new environmentally safe fungicides. Nowadays the screening of microbial metabolites for agricultural fungicide has become a hot topic (Strange and Scott, 2005; Suprapta, 2012; Boukaew and Prasertsan, 2014). Natural products from marine-derived fungi as sources for the development of fungicidal agents may be one of the most plausible solutions.
Therefore, the main objective of this study was to systemically investigate the antifungal potential of fungi isolated from mangrove rhizosphere soil collected from Dong- zhaigang mangrove natural reserve in the South China Sea and isolate and evaluate potential exploitable antifungal secondary metabolites brefeldin Aand penicillic acid (Fig.1) fromtwo active strains,HK1-23 andHK1-6.
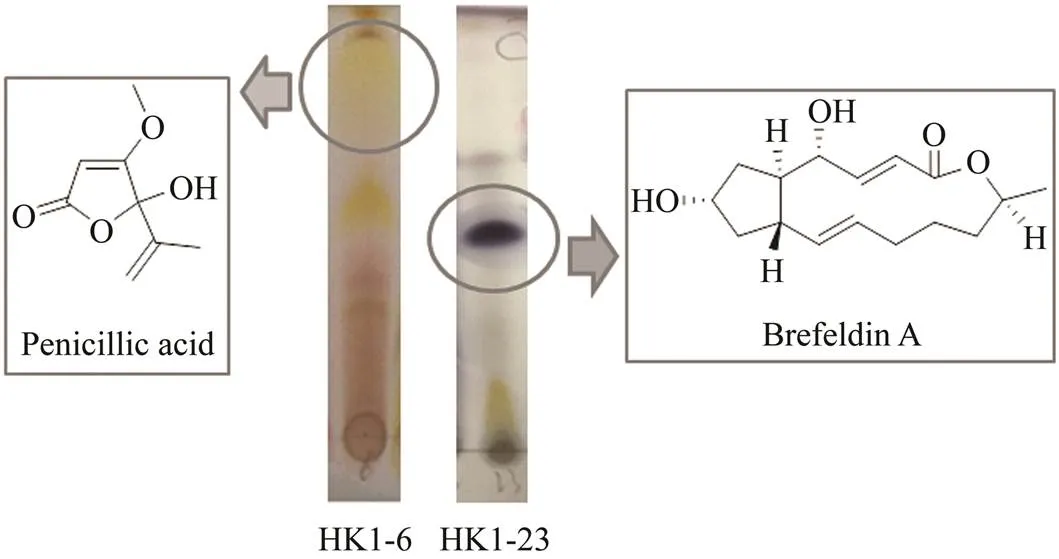
Fig.1 Thin layer chromatography (TLC) analyses and the chemical structures of penicillic acid and brefeldin A.
2 Materials and Methods
2.1 Sampling and Isolation of Fungi
A mangrove rhizosphere soil sample was collected from the Dongzhaigang mangrove natural reserve in the South China Sea in September 2015. The sample was stored in sterilized polythene bags, transported to the laboratory, and processed immediately for isolation and cultivation of fungi.
The fungi were isolated by the serial dilution method (1:10, 1:100, and 1:1000) using Potato Dextrose Agar (PDA) medium supplemented with sea salt (30gL−1), ampicillin (25mgL−1), and streptomycin sulfate (25mgL−1). The inoculated plates were cultured at 25℃ for 1–3 weeks and observed the growth of fungi intermittently. Fungal isolates were chosen and transferred onto new corresponding agar plates on the basis of their morphological traits. The strains were deposited at the Marine Science & Technology Institute, College of Environmental Science & Engineering, Yangzhou University, Yangzhou, China.
2.2 Screening of Fungi
2.2.1 Preparation of extracts
The analysis-scale fermentations of 28 fungal isolates (HK1-1–HK1-28) were carried out, respectively, in 1-L Erlenmeyer flask containing 400mL of potato dextrose broth medium (20g of dextrose and 30g of natural sea salt in 1L of potato infusion) at 25℃ without shaking for 4 weeks. The fermented broth was separated through cheese cloth to obtain the filtrate and the mycelia. Then the broth was extracted three times with an equal volume of EtOAc, and the mycelia were extracted three times with MeOH. The organic extracts were combined and concentrated under vacuum as a total crude extract for further antifungal assay.
2.2.2 Antifungal assay
All 28 fungal extracts were screened for antifungal activity against the rice sheath blight pathogen,, by the mycelium linear growth rate method as Yang.(2015). Each fungal extract was dissolved in dimethyl sul- foxide (DMSO) and then was added to the assay flasks containing heat-sterilized PDA media (50℃) with a final concentration of 100μgmL−1. After a thorough mixing, the treated medium was poured into Petri dishes (90mm×15mm). The control plates were treated with corresponding DMSO only. When the medium in the plate was partially solidified, a 5-mm thick and 5-mm diameter disc of fungus which had been cut from subcultured Petri dish, was placed at the center of the semisolid medium. The inoculated plates were kept in an incubator at 28℃ for 48h. Three replicates were analyzed simultaneously. The radial growth of mycelia (colony diameter in mm) in all plates was measured 48h after inoculation with a caliper in three different directions, and the growth inhibition rates were calculated according to the following formula and ex- pressed as means±S.D.
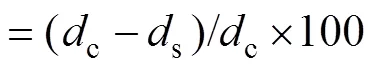
wherecis the diameter of a fungal colony in the blank test, andsis the diameter of a fungal colony in the fungal extract-treated test.
2.2.3 Thin-layer chromatography analysis
All 28 fungal extracts were analyzed by TLC. Precoat- ed silica gel plates (Yan Tai Zi Fu Chemical Group Co.; G60, F-254) were used for TLC analyses. A mixture of CH2Cl2:MeOH (v:v, 15:1) was used as the developing sol-vent for TLC. Saturated vanillin sulfuric acid solution (17%) was used as the chromogenic agent.
2.3 Identification of Fungi
The genomic DNA of the fungal strains ofHK1-6 andHK1-23 were isolated by using the protocol described previously (Zheng., 2012). The DNA quality was checked and amplified using ITS1- (5’-TCC GTA GGT GAA CCT GCG G-3’) and ITS4- (5’-TCC TCC GCT TAT TGA TAT GC-3’) primers. The ITS sequences were submitted to the NCBI GenBank database.
2.4 Isolation and Structure Characterization of Brefeldin A and Penicillic Acid
The fungal strainsHK1-23 andHK1-6 were cultivated, respectively, in 30L and 10L of potato dextrose brothmedia at 25℃ without shak- ing for 4 weeks. The extraction of broth and mycelia were carried out as described above to supply 40g and 28g of total extract, respectively. Brefeldin A (4.3g) and penicillic acid (4.23g) were separated as described above. Silica gel (Qing Dao Hai Yang Chemical Group Co.; 200–300 mesh) was used in CC.1H and13C NMR spectra was obtained from AVANCE 600 NMR spectrometer (600MHz for1H and 150MHz for13C), using TMS as internal stan- dard. ESIMS data were obtained from Bruker Daltonics Apex-Ultra 7.0 T mass spectrometer. Both of the results were employed to confirm the structures.
2.5 Antifungal Activities ofBrefeldin A and Penicillic Acid
Brefeldin A and penicillic acid were screened for antifungal activities against crop pathogens including,,var.,,,, andby using the mycelial linear growth rate method (Yang., 2015) as described above. Brefeldin A and penicillic acid were test-ed at three concentrations, including 50, 10, and 2μgmL−1. Control plates were treated with corresponding amounts of DMSO only. Each test was performed in triplicate. Car- bendazim was used as the positive control and showed antifungal activities towardand, with 57% and 73% growth inhibitions, respectively, at 10μgmL−1.
3 Results and Discussion
3.1 Antifungal Activity of Fungi Derived from Mangrove Rhizosphere Soil
Cultivation of fungi from the mangrove rhizosphere soil sample yielded a total of 42 isolates. Reduplicate isolates were excluded under the guidance of observation on morphological differences, especially the visible examination of growth characteristics, mycelia, and diffusible pigment. As the results, 28 independent strains (HK1- 1−HK1-28) were selected for the screening of antifungal activity against the rice sheath blight pathogen,. As shown in Fig.2, 18 isolates (64.3%) displayed differ- ent growth inhibition levels againstat 100μgmL−1. It is worth noting that five isolates viz. HK1-6, HK1-12, HK1-14, HK1-20, and HK1-23, exhibited significant an- tifungal activities with growth inhibition of 54.2%, 64.4%, 53.3%, 51.6%, and 61.3%, respectively. Additionally, 28 fungal extracts were examined by thin-layer chromatog- raphy (TLC) for the diversity of secondary metabolites. The bioactive isolates HK1-23 and HK1-6 were proposed to produce characteristic metabolites indicated by the darkblue or yellow-green spots, respectively, from the TLC plates. Therefore, HK1-23 and HK1-6 were selected as the bioactive target fungi to identify the responsible bio- active constituents.
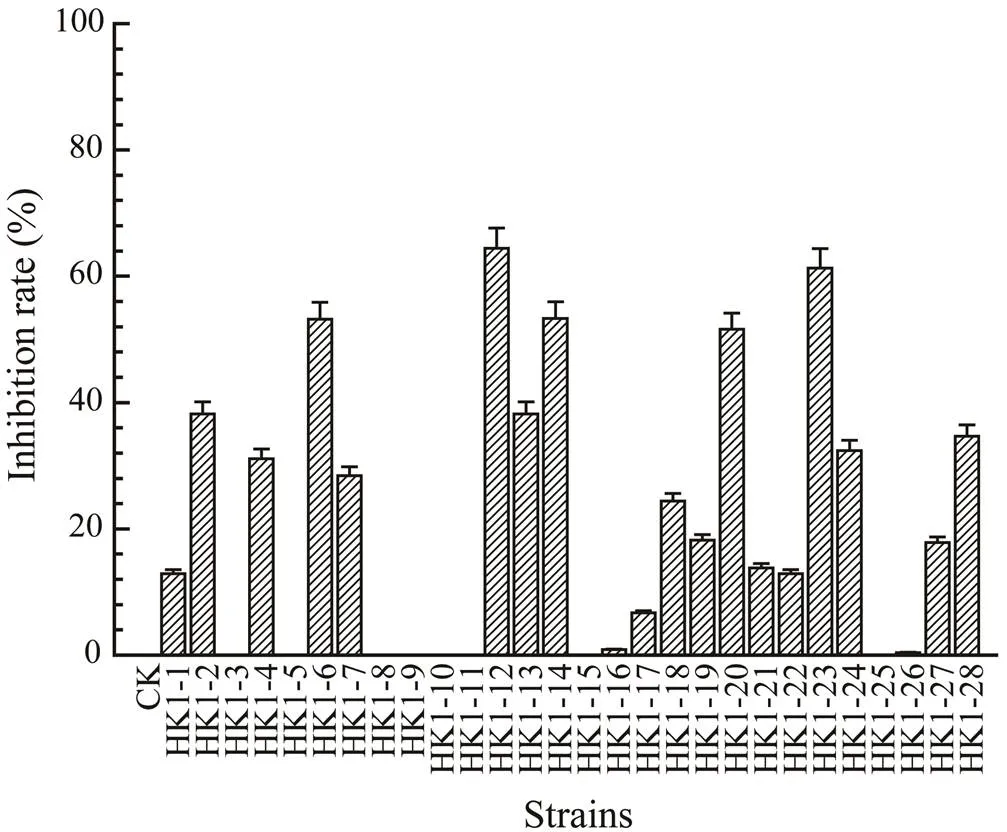
Fig.2 Antifungal activities of crude extracts from 28 mangrove rhizosphere soil-derived fungal strains (HK1-1–HK1- 28) against Rhizoctonia solani at 100μgmL−1.
3.2 Identification of the Bioactive Strains HK1-23 and HK1-6
HK1-23 was identified on the basis of its morphological traits and a molecular protocol by amplification and sequencing of the DNA sequences of the ITS region of the rRNA gene. A phylogenetic tree based on the ITS rRNA gene sequences of the closely related species was constructed by using the neighbor joining tree method and is illustrated in Fig.3. HK1-23 was closely related to(MH877078.1), and its sequence has been submitted to NCBI GenBank with accession number of MH 628211. The identification ofHK1-6 has been described previously (GenBank KY412802) (Chen., 2017).
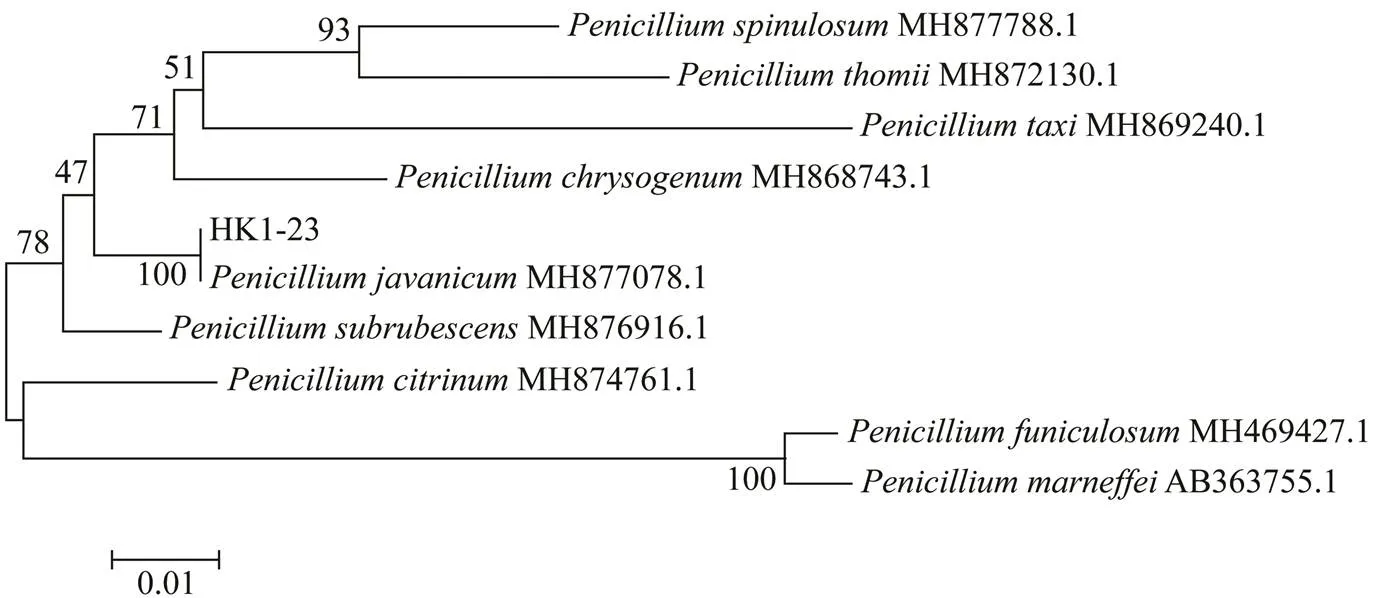
Fig.3 Phylogenetic tree of ITS rRNA sequences of closely related P. javanicum HK1-23. Reference sequences were downloaded from NCBI with the accession numbers indicated in parentheses. Distances and clustering was performed by MEGA 4.1. Bootstrap values based on 1000 replications are listed at the branching points.
3.3 Production of Bioactive Metabolites
To investigate the bioactive constituents ofHK1-23, a scale-up fermentation was performed in 30L of potato dextrose broth medium. After harvest, its organic extract was isolated by silica gel column chromatography (CC) using a step gradient elution with EtOAc- petroleum ether (0–100%) to fractionate it into eight fractions (Frs.1–8). Frs.6–8 displayed remarkable antifungal activitieswith percentages of growth inhibition greater than 50% at 100μgmL−1. Subsequently Frs.6–8 were further purified by repeated silica gel CC and recrystallization with MeOH−EtOAc (v:v, 3:1) to yield brefeldin A (4.3g), a 13-member lactone. Its structure was determined on the basis of its1H and13C NMR spectra and ESIMS data, and by comparison with data previously reported in the literature (Fukushima, 1993). Brefeldin A was displayed as dark blue spots on TLC plates.
The fungal strainHK1-6 was cultivated in 10L of potato dextrose broth medium. An extraction similar to the one described above produced an extract, which was separated by silica gel CC to provide eight fractions (Frs.1–8). Frs.4–5 showed antifungal activities and were purified by repeated silica gel CC and recrystallization from EtOAc-petroleum ether (v:v, 1:5) to obtain penicillic acid (4.23g), whose structure was confirmed by the1H and13C NMR spectra and ESIMS data (Namikoshi., 2003). Penicillic acid was indicated as yellow-green spots on TLC plates.
3.4 Antifungal Activities of Brefeldin A and Penicillic Acid
Brefeldin A has been reported to exhibit various biolo- gical actions, including antibacterial, antitumor, antiviral, antimitotic, and cytostatic activities (Harri., 1963). How- ever, its antifungal activities against crop pathogens are present in relatively few reports. This is the first report on the antifungal activity of brefeldin A against the rice sheath blight pathogen,. The results (Fig.4) displayed that brefeldin A exhibited potent antifungal activity againstwith growth inhibition of 62.2%, 49%, and 35% at 50, 10, and 2μgmL−1, respectively. Additionally, to dis- cover broader antifungal functions, brefeldin A was screen- ed for antifungal activities against a series of crop pathogens including,var.,,,, andThe results(Fig.4) demonstrated that brefeldin A showed different levels of inhibitory activities against these tested crop pathogens. Especially, brefeldin A showed strong anti- fungal activity toward, with growth inhibition of 56.4% at 50μgmL−1. Not coincidentally, a 14-member lactone, LL-Z1640-2, showed excellent agricultural fungicidal activity againstandin the whole-plant assay (Liu., 2014).
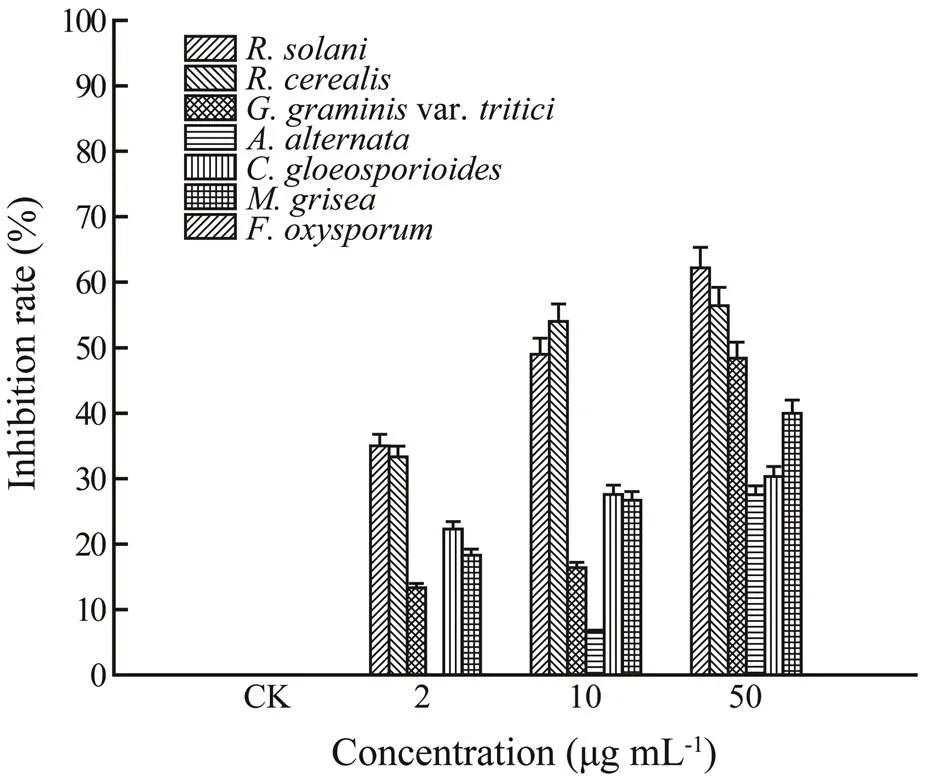
Fig.4 Antifungal activities of brefeldin A against 7 strains of crop pathogens.
A literature survey revealed that penicillic acid showed a variety of bioactivities, including but not limited to antimicrobial (Kang and Kim, 2004) and cytotoxic (Vansteelandt., 2013) functions. The antifungal activities of penicillic acid were also evaluated against seven strains of crop pathogens (Fig.5). Encouragingly, penicillic acid showed potent antifungal activities towardandwith 67.5% and 76% growth inhibition, respectively, at 50μgmL−1. Therefore, penicillic acid and brefeldin A are expected to be two promising compounds for further development of novel fungicides.
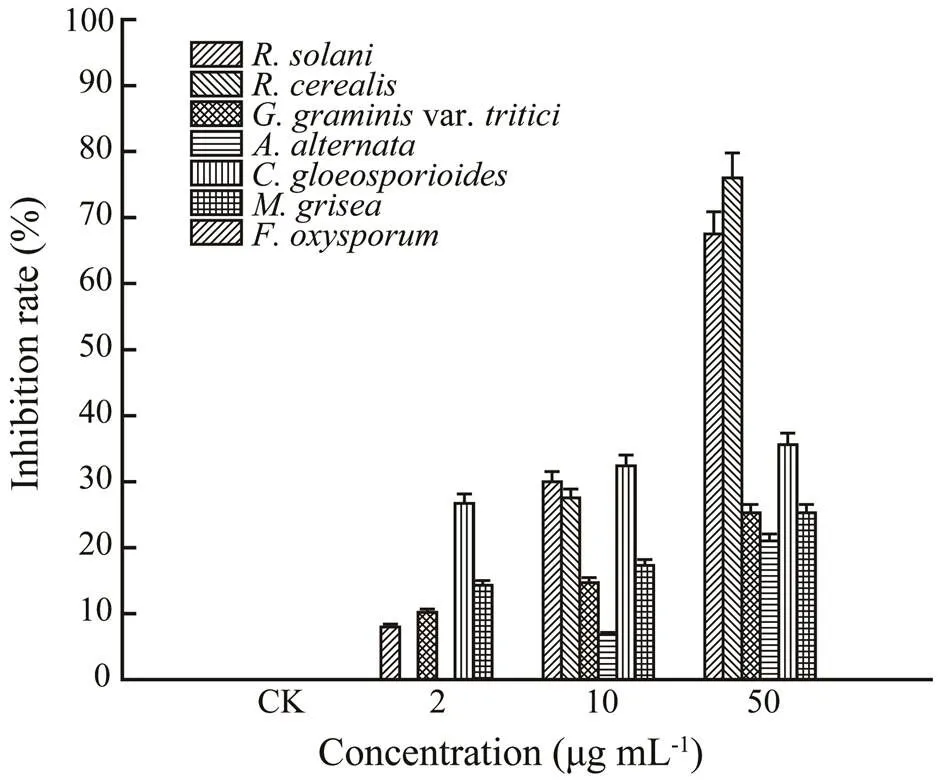
Fig.5 Antifungal activities of penicillic acid against 7 strains of crop pathogens.
3.5 High Yield of Brefeldin A and Penicillic Acid
Cost minimization is particularly important in the development of fungicide for crop protection. Natural pro- ducts are often found to have a variety of biological activities; however, their further exploitation is frequently hampered because of their limited yields and/or low purity, resulting in a high cost of application. In the present study, the fungal strainsHK1-23 andHK1-6 could produce the antifungal compounds brefeldin A and penicillic acid in potato dextrose broth medium with high yields of 143 and 423mgL−1, respectively, under common laboratory conditions. Furthermore, both brefeldin A and penicillic acid could be purified by using comprehensive separation methods including silica gel CCand recrystallization. These fermentation and purification methods are convenient approaches to yield brefeldin A and penicillic acid with high yields and purities.
4 Conclusions
This study demonstrated that the fungal strainsHK1-23andHK1-6 isolated frommangrove rhizosphere soil were efficient producers of bre- feldin A and penicillic acid, respectively. Penicillic acid and brefeldin A displayed strong antifungal activities against the crop pathogensand, and also have different degrees of inhibition towardvar.,,,, and. Based on the present results, microorganisms from mangrove ecosystems may be a large reservoir of bioactive natural products for future agrochemical devel- opment, and brefeldin A and penicillic acid could be in- teresting lead compounds for further development of no- vel fungicides.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81703411), the Natural Science Foundation of Jiangsu Province (Nos. BK20180940, BK20180937), the Open Fund of CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences (No. KLME ES201802), and the Taishan Scholars Program, China.
Aguín, O., Mansilla, J. P., and Sainz, M. J., 2006.selection of an effective fungicide againstand control of white root rot of grapevine in the field., 62: 223-228.
Ancheeva, E., Daletos, G., and Proksch, P., 2018. Lead compounds from mangrove-associated microorganisms., 16: 319-350.
Blunt, J. W., Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R., 2018. Marine natural products., 35: 8-53.
Boukaew, S., and Prasertsan, P., 2014. Suppression of rice sheath blight disease using a heat stable culture filtrate fromRM-1-138., 61: 1-10.
Boukaew, S., Klinmanee, C., and Prasertsan, P., 2013. Potential for the integration of biological and chemical control of sheath blight disease caused byon rice., 29: 1885- 1893.
Bugni, T. S., and Ireland, C. M., 2004. Marine-derived fungi: A chemically and biologically diverse group of microorganisms., 21: 143-163.
Chen, M., Shen, N. X., Chen, Z. Q., Zhang, F. M., and Chen, Y., 2017. Penicilones A−D, anti-MRSA azaphilones from the marine-derived fungusHK1-6.,80: 1081-1086.
Deshmukh, S. K., Prakash, V., and Ranjan, N., 2017. Marine fungi: A source of potential anticancer compounds., 8: 2536.
Fukushima. Y., 1993. β-Glucan biosynthesis inhibitors isolated from fungi as hyphal malformation inducer., 3: 1219-1222.
Harri, E., LoeMer, W., Singh, H. P., Stahlin, H., and Tamm, C., 1963. Die constitution von brefeldin A.,46: 1235-1243.
Kang, S. W., and Kim, S. W., 2004. New antifungal activity of penicillic acid againstspecies., 26: 695-698.
Lee, F. N., 1983. Rice sheath blight: A major rice disease., 67: 829-832.
Liu, Q. A., Shao, C. L., Gu, Y. C., Blum, M., Gan, L. S., Wang, K. L., Chen, M., and Wang, C. Y., 2014. Antifouling and fungicidal resorcylic acid lactones from the sea anemone-derived fungus., 62: 3183-3191.
Mew, T. W., Cottyn, B., Pamplona, R., Barrios, H., Li, X. M., Chen, Z. Y., Lu, F., Nil-panit, N., Arunyanart, P., Van Kim, P., and Van Du, P., 2004. Applying rice seed-associated antagonistic bacteria to manage rice sheath blight in developing countries., 88: 557-564.
Namikoshi, M., Negishi, R., Nagai, H., Dmitrenok, A., and Kobayashi, H., 2003. Three new chlorine containing antibiotics from a marine-derived funguscollected in Pohnpei., 56: 755-761.
Strange, R. N., and Scott, P. R., 2005. A threat to global food security., 43: 83-116.
Suprapta, D. N., 2012. Potential of microbial antagonists as bio- control agents against plant fungal pathogens., 18: 1-8.
Vansteelandt, M., Blanchet, E., Egorov, M., Petit, F., Toupet, L., Bondon, A., Monteau, F., Bizec, B. L., Thomas, O. P., Pouchus, Y. F., Bot, R. L., and Grovel, O., 2013. Ligerin, an antiproliferative chlorinated sesquiterpenoid from a marine-derivedstrain.,76: 297-301.
Wu, J., Xiao, Q., Xu, J., Li, M. Y., Pan, J. Y., and Yang, M., 2008. Natural products from true mangrove flora: Source, chemistry and bioactivities., 25: 955- 981.
Xu, J., 2014. Bioactive natural products derived from mangrove- associated microbes., 5: 841-892.
Yang, R., Gao, Z. F., Zhao, J. Y., Li, W. B., Zhou, L., and Miao, F., 2015. New class of 2-aryl-6-chloro-3,4-dihydroisoquino- linium salts as potential antifungal agents for plant protection: Synthesis, bioactivity and structure-activity relationships., 63: 1906-1914.
Yasuhara-Bell, J., and Lu, Y., 2010. Marine compounds and their antiviral activities., 86: 231-240.
Zheng, C. J., Shao, C. L., Guo, Z. Y., Chen, J. F., Deng, D. S., Yang, K. L., Chen, Y. Y., Fu, X. M., She, Z. G., Lin, Y. C., and Wang, C. Y., 2012. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derivedsp. fungus., 75: 189-197.
Zhou, L., Jiang, H. X., and Sun, S., 2013. Biodiversity and biotechnological potential of microorganisms from mangrove eco- systems: A review., 63: 1-19.
. Tel: 0086-514-89357891
E-mail: dieying0719@163.com
October 15, 2019;
December 17, 2019;
March 20, 2020
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年3期
Journal of Ocean University of China2020年3期
- Journal of Ocean University of China的其它文章
- Diffusion Characteristics of Swells in the North Indian Ocean
- Analysis of the Dynamic System of Wave Glider with a Towed Body
- Provenance and Tectonic Implications of Paleozoic Strata in the South Yellow Sea Basin, China–Revealed from the Borehole CSDP-2
- Ecological Risk of Heavy Metals in Sediment Around Techeng Island Special Marine Reserves in Zhanjiang Bay
- Geochemical and Grain-Sized Implications for Provenance Variations of the Central Yellow Sea Muddy Area Since the Middle Holocene
- Grain-Size Distribution of Surface Sediments in the Bohai Sea and the Northern Yellow Sea: Sediment Supply and Hydrodynamics
