外源添加剂对黄贮小麦秸秆产甲烷潜力及微生物群落的影响
闫 晶,陆冰圆,席华悦,孟星尧,袁旭峰,朱万斌,崔宗均
外源添加剂对黄贮小麦秸秆产甲烷潜力及微生物群落的影响
闫 晶1,陆冰圆1,席华悦1,孟星尧2,袁旭峰1,朱万斌1,崔宗均1※
(1. 中国农业大学农学院,北京 100193; 2. 北京工商大学环境科学与工程学院,北京 100048)
为探讨添加不同外源添加剂对黄贮小麦秸秆产甲烷潜力和微生物群落结构的影响,该研究以小麦秸秆为黄贮原料,添加乙酸(3‰ ACE组)和异型发酵乳酸菌复合系(3‰ MI1组,6‰ MI2组),调整含水率至50%,黄贮65 d后,以黄贮小麦秸秆为厌氧发酵原料,探究发酵过程中的指标性质和微生物群落结构。研究发现黄贮预处理后,发酵系统中的初始挥发性有机酸中的乳酸和乙酸增加、甲烷含量增加、可溶性化学需氧量(sCOD)增加。添加了外源添加剂的ACE组、MI1组、MI2组的累积甲烷产量较干黄小麦秸秆(WS组)提高了4.7%~10.6%,而未添加添加剂的CK组的累积甲烷产量较WS组降低了9%。发酵系统中的主要优势细菌为和,优势古菌为甲烷鬃菌属,黄贮预处理改变了发酵系统微生物的群落结构,促进了小麦秸秆的甲烷转化,为木质纤维素的沼气转化提供参考。
秸秆;添加剂;黄贮;产甲烷潜力;微生物群落
0 引 言
中国农作物秸秆资源丰富,每年产生各类秸秆约10亿t,因其富含有机质,具有很好的产沼气潜力,秸秆沼气工程受到了越来越多的关注[1-2],是目前秸秆资源化利用的有效途径。但秸秆中复杂、坚固的木质纤维素结构和高碳氮比的特性,使得秸秆沼气工程存在发酵启动缓慢、发酵周期长、秸秆降解不充分、沼气产量低、品质差等问题[3-5],另一方面作物秸秆的季节性、时效性和易腐性不仅限制了沼气工程全年连续运行和原料持续供给的可行性。因此,秸秆长期保质贮存成为秸秆沼气工程所面临的重要问题。
目前常用的秸秆贮存方式有青贮、黄贮。青贮是把新鲜作物秸秆(含水率60%~75%)密封保存,秸秆上附着的乳酸菌在厌氧条件下利用可溶性碳水化合物生成乳酸等物质,降低秸秆pH值,抑制其他微生物的活动,从而保存秸秆营养成分、减少物质损失,实现新鲜秸秆的长期贮存,但此方法具有季节性[6]。黄贮与青贮原理一样,不同的是以风干黄化的干秸秆为原料,可全年制作,但由于干秸秆纤维化、木质化程度高,组成结构复杂坚韧,可溶性碳水化合物含量低,自然附着的乳酸菌少,因此需要在制作过程中添加适宜水分和少量添加剂[7]。黄贮添加剂有3种类型,一类是发酵促进剂,通过促进乳酸发酵来改善黄贮进程,如乳酸菌、纤维素酶、葡萄糖、蔗糖等[8-12];一类是发酵抑制剂,通过降低原料pH值,直接形成适宜乳酸菌生活的环境来改善黄贮进程,如甲酸、乙酸、甲醛等[13-14];还有一类是营养剂,提高原料营养价值,如糖蜜、尿素、磷酸二铵等[15-17]。崔宪等[18]研究了协同添加葡萄糖与同型乳酸菌、异型乳酸菌、乙酸对干黄玉米秸秆品质和甲烷产量的影响,发现添加添加剂后的黄贮玉米秸秆,干物质损失少,产甲烷潜力高,黄贮过程更加稳定。
目前关于青贮、黄贮秸秆的研究大部分集中于畜禽饲草的跨季节贮存,将其应用于沼气工程原料贮存方面的研究较少。因此本文以自然风干黄化的小麦秸秆为原料,通过添加乙酸、异型发酵乳酸菌复合系来调控黄贮发酵过程,分析不同外源添加剂对黄贮小麦秸秆产甲烷潜力和微生物群落结构的影响,为秸秆沼气工程原料高效贮存提供技术支撑。
1 材料与方法
1.1 试验材料
小麦秸秆为江苏省徐州市沛县干黄小麦秸秆,其基本性质为:总固体93.3%、挥发性固体75.8%、总碳47.2%、总氮0.75%(均为质量分数,下同)。
乳酸菌复合系为中国农业大学生物质工程研究中心构建的SLP-1异型发酵乳酸菌复合系,其组成菌主要为副布氏乳杆菌,植物乳杆菌,短乳杆菌,还有少量的副干酪乳杆菌,戊糖乳杆菌,粘膜乳杆菌。将SLP-1乳酸菌复合系活化后,30 ℃培养48 h,该样品作为接种菌剂。
接种污泥取自中国农业大学生物质工程研究中心实验室运行良好的水稻秸秆与牛粪共发酵的连续发酵罐。接种污泥基本性质为:pH值7.4、总固体12.0%、挥发性固体4.5%、总碳18.2%、总氮1.66%。
1.2 试验设计
1.2.1 小麦秸秆黄贮试验设计
干黄小麦秸秆切成2~5 cm,喷洒自来水,调节水分至50%。CK组不添加添加剂,作为黄贮对照组,ACE组添加3‰乙酸,MI1组添加3‰乳酸菌复合系,MI2组添加6‰乳酸菌复合系,混合均匀后逐层装入1 L蓝盖瓶中,压实密封贮存65 d。
1.2.2 批次厌氧发酵试验设计
黄贮秸秆(CK组、ACE组、MI1组、MI2组)和干黄秸秆(WS组)作为原料进行批次厌氧发酵试验,采用500 mL蓝盖瓶作为反应器,发酵体积为300 mL。原料总固体1%(3 g),接种污泥总固体4%(12 g),体积不足300 mL时用自来水补足,以只加接种污泥和水作为发酵空白,每个处理3次重复。装瓶后,通3~5 min氮气,橡胶塞封口后置于(36±2)℃的恒温培养室内进行厌氧发酵,发酵周期20 d。发酵过程中测定日产气量和甲烷含量,并于发酵的0、2、4、6、8、10、15、20 d测定反应体系的pH值、挥发性脂肪酸、可溶性化学需氧量等指标,取厌氧发酵20 d样品测定微生物群落结构。
1.3 测定指标
1.3.1 理化性质测定
总固体(Total Solid,TS)采用烘干法,105℃烘干至恒质量;挥发性固体含量(Volatile Solid,VS)采用马弗炉550 ℃灼烧4 h;pH值测定采用便携式笔式pH计(上海三信,SX610);总碳(Total Carbon,TC)采用重铬酸钾-硫酸氧化法;总氮(Total Nitrogen,TN)采用凯氏定氮法;纤维素、半纤维素、木质素采用范氏(Van Soest)洗涤法[19]。
1.3.2 沼气及其成分测定
日产沼气量测定采用压力法;甲烷含量采用气相色谱仪(GC-2014, Shimadzu, Japan);可溶性化学需氧量(soluble Chemical Oxygen Demand,sCOD)采用COD快速检测仪(Lovibond E799718, 德国)消解,分光光度法测定;挥发性脂肪酸(Volatile Fatty Acids,VFA)采用岛津LC-20A 高效液相色谱仪,色谱柱为BIO-RAD HPX-87H Lon Exclusion Column。
1.3.3 微生物高通量测序
对厌氧发酵20 d样品进行细菌和古菌群落结构测定。高通量(Illumina Miseq)测序由上海美吉生物医药科技有限公司进行。使用E.Z.N.A.® soil试剂盒 (Omega Bio-tek, Norcross, GA, U.S.) 进行总DNA抽提,细菌使用通用引物338F(ACTCCTACGGGAGGCAGCAG)和806R (GGACTACHVGGGTWTCTAAT)、古菌使用通用引物524F10ext(TGYCAGCCGCCGCGGTAA)和Arch958Rmod(YCCGGCGTTGAVTCCAATT)对V3-V4可变区进行PCR扩增,利用Illumina公司的Miseq PE300平台对扩增子文库进行测序。并在上海美吉生物云平台对微生物进行菌群结构分析。
1.4 数据分析
数据处理和统计分析使用 Microsoft Excel 2010、SPSS 20、Origin 9.1和Canoco for Windows 4.5。方差分析使用 Duncan的多范围检验,其显著性<0.05。
2 结果与讨论
2.1 外源添加剂对黄贮小麦秸秆性质的影响
表1为黄贮小麦秸秆的基本性质,所有处理的pH值都较低,在4.2~4.7之间,酸香味醇厚,说明黄贮发酵良好。
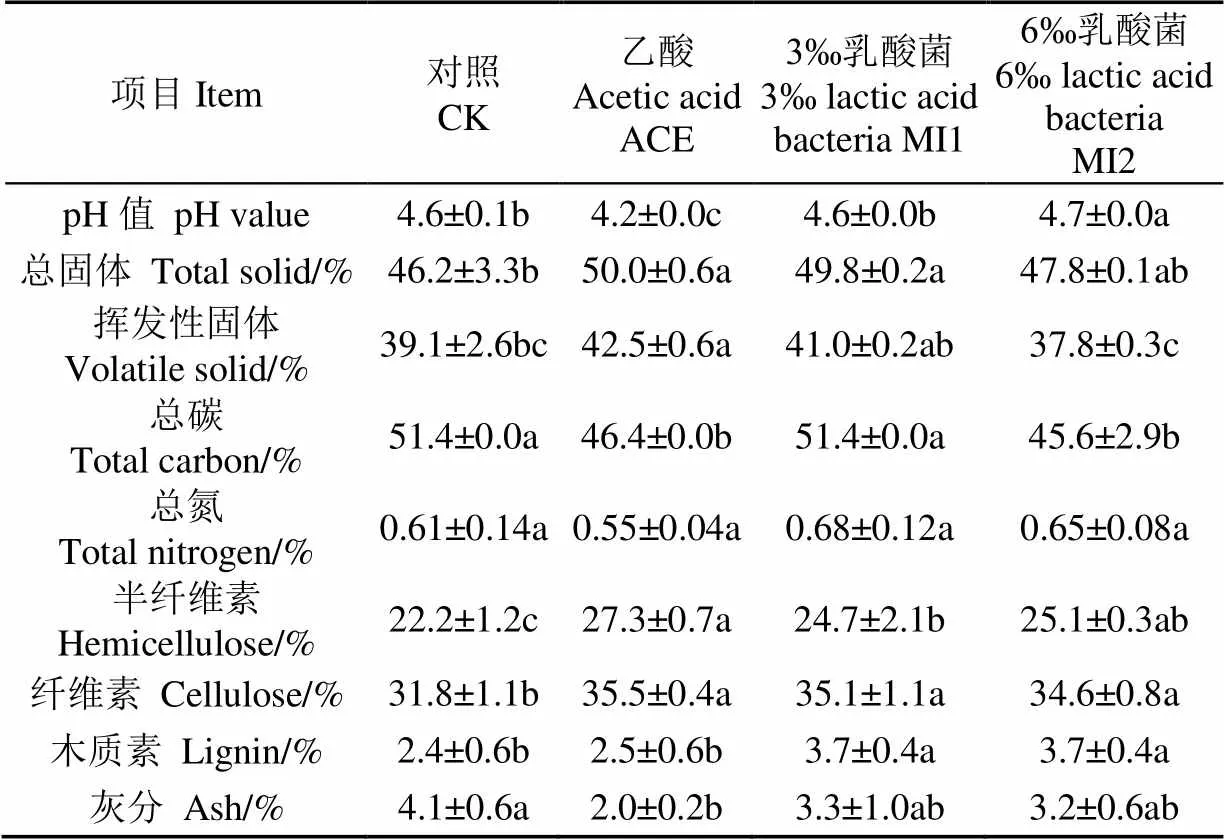
表1 外源添加剂对黄贮小麦秸秆性质的影响
注:所有数值均表示为平均值±标准偏差(=3),同一行不同字母表示差异显著(<0.05)。
Note: All values were expressed as means ± standard deviation (=3); Different letters showed significant difference (<0.5).
不同黄贮处理后秸秆的粗纤维素含量不同,其中ACE组的半纤维素、纤维素含量最高,分别为27.3%、35.5%,CK组则显著低于其他处理,分别为22.2%、31.8%,MI1组、MI2组间的则无显著性差异。可见黄贮添加剂的添加有利于纤维素等可利用成分的保存。这是因为ACE组加入乙酸后,原料pH值迅速降低,直接形成适合乳酸菌但抑制其他微生物生长的环境,减少了黄贮过程中对木质纤维素的降解[20-21]。MI1组、MI2组添加乳酸菌复合系后,体系中乳酸菌迅速生长繁殖,加快了其形成优势菌属的时间,并产生大量乳酸、乙酸(乙醇)和CO2,使pH值下降,从而抑制体系中其他微生物的活动,亦减少了半纤维素和纤维素的降解[22]。CK组在黄贮过程中先是附着在原料上的酵母菌、腐败菌、霉菌和醋酸菌等好气性微生物利用原料的可溶性糖类生长繁殖,形成厌氧环境后原料上的肠细菌、梭菌、霉菌、酵母菌和乳酸菌等专性和兼性厌氧菌开始生长繁殖[23-24],这一过程中,部分分解菌会将原料的木质纤维素降解为可溶性糖类和有机酸来提供体系内微生物生长繁殖所需的营养,随着黄贮过程的推进,体系内pH值下降,从而抑制了其他微生物的活动。体系中的有机酸亦能破坏秸秆的木质纤维素结构,对秸秆起到了一定的前处理作用[25]。
2.2 外源添加剂对黄贮小麦秸秆产甲烷的影响
2.2.1 外源添加剂对黄贮小麦秸秆厌氧发酵甲烷含量的影响
将黄贮小麦秸秆(CK组、ACE组、MI1组、MI2组)和干黄秸秆(WS组)作为厌氧发酵的原料,在含固率1%条件下进行批次厌氧发酵试验。图1为批次厌氧发酵中甲烷含量的箱形图,箱形图是一种统计数据分散情况的方法,能够直观显示小麦秸秆在厌氧发酵产沼气过程中甲烷含量的数据分布以及不同处理之间的差异,从而确定有利于甲烷生产的处理[26]。从图中可以看出,黄贮组的上四分位数、中位数、下四分位数、平均值均高于WS组,说明黄贮组的甲烷含量整体水平高于WS组,即黄贮预处理提高了甲烷发酵中的甲烷含量。结合发酵数据可知,最小值为发酵第1 d的甲烷含量,WS组的初始甲烷质量分数最低,为9%,而黄贮组的初始甲烷质量分数在11%~12%,可见黄贮预处理亦能提高小麦秸秆厌氧发酵的起始甲烷含量。其原因是黄贮预处理能够为产甲烷菌提供更充足的营养物质,增强产甲烷菌的活性和代谢强度,增加甲烷含量。

图1 厌氧发酵过程中的甲烷含量
2.2.2 外源添加剂对黄贮小麦秸秆日产甲烷量和累积产甲烷量的影响
图2为批次厌氧发酵中的日产甲烷量及累积产甲烷量,从图中可以看出,WS组在整个发酵过程中仅在第4 d出现了1个产甲烷高峰,为15.3 mL/g (以TS计)。而黄贮组的小麦秸秆均出现了2个产甲烷高峰,其中CK组出现在第3、8 d,分别为16.56、14.40 mL/g;ACE组和MI1组均出现在第4、8 d,分别为19.45、17.2和18.34、16.68 mL/g;MI2组则是在第4、9 d出现2个产甲烷高峰,分别为17.15和16.24 mL/g。可见黄贮组不仅增加了发酵过程中产甲烷高峰出现的次数也提高了小麦秸秆的最大日产甲烷量,较WS组分别提高了8.2%、27.1%、19.9%、12.1%。CK组、ACE组、MI1组、MI2组、WS组的累积产甲烷量分别为175.8、213.7、202.2、207.9、193.2 mL/g,其中CK组的累积产甲烷量在前4 d高于WS组,之后一直低于WS组,最终比WS组降低了9.0%,其原因是黄贮过程中微生物生长代谢形成的小分子物质,如乳酸,乙酸,甲醇,乙醇,H+,CO2等,在发酵前期提高了小麦秸秆的产甲烷量或产甲烷速率,之后小分子物质被消耗后,再加上秸秆中可被厌氧发酵微生物利用的营养物质,如半纤维素、纤维素,在黄贮过程中已被利用一部分,使得之后发酵过程的产甲烷量或产甲烷速率低于WS组。ACE组、MI1组、MI2组的累积产甲烷量始终高于WS组,最终比WS组提高了10.6%、4.7%、7.6%,比CK组提高了21.5%、15.0%、18.3%。黄贮组的小麦秸秆均在第13 d时甲烷产量达到累积甲烷产量的80%,而WS组是在第14 d时达到的,可见黄贮预处理能够缩短甲烷产量达到累积产甲烷量的80%所用的时间,提高小麦秸秆的产甲烷效率。
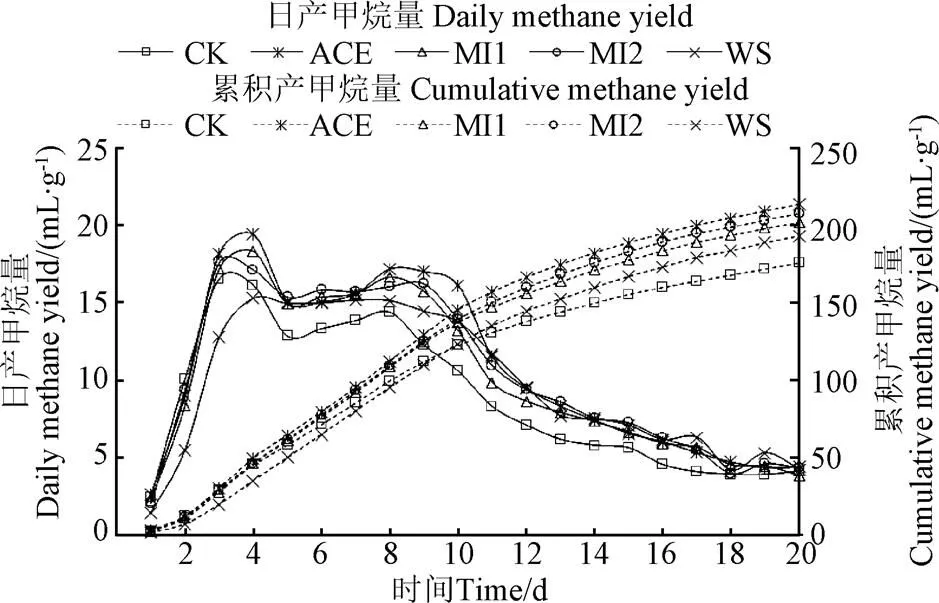
图2 日产甲烷量及累积产甲烷量
2.3 外源添加剂对黄贮小麦秸秆发酵环境的影响
2.3.1发酵过程中pH值的变化
发酵过程中pH值能影响体系中微生物活性而改变厌氧发酵的产甲烷效率[27]。厌氧发酵最适pH值在6.8~7.4之间,体系中可溶性有机物和木质纤维素等被水解酸化,转化为VFA并逐渐积累,pH值迅速下降,之后VFA被产甲烷菌利用生成甲烷,pH值开始上升。本试验所有处理pH值变化差异不大(图3),初始pH值在7.3~7.4之间,第4天左右下降至6.6,之后缓慢上升,发酵结束时所有处理pH值均稳定在7.0。

图3 厌氧发酵过程中的pH值变化
2.3.2 发酵过程中VFA的变化
VFA是厌氧发酵过程重要的中间产物,是影响厌氧发酵过程稳定性的关键指标。图4为发酵过程中VFA的变化,从图中可以看出所有处理均只在发酵前8 d检测到了VFA,可能是因为此后体系中有机物水解酸化转化为VFA的速率与产甲烷菌将VFA转化为甲烷的速率相一致的原因。此外本发酵体系中丙酸一直存在,这在纯秸秆的厌氧发酵中是常见的,过多的丙酸会造成厌氧发酵酸败,一般来说,丙酸质量浓度达到1~2 g/L时会抑制体系中的产甲烷菌,本试验中的丙酸含量较低未对发酵过程产生抑制作用[26,28]。在WS组中,初始VFA由乙酸和丙酸组成,浓度为0.51 g/L,在第4天是达到峰值0.63 g/L后降低。而黄贮组的初始VFA由乳酸、乙酸和丙酸组成,其初始值即为峰值,随着发酵的进行,体系中的VFA含量逐渐减少。CK组乳酸的初始质量浓度为1.1 g/L,高于ACE组的0.7 g/L,MI1组的0.6 g/L、MI2的0.4 g/L,乙酸的初始质量浓度为0.16 g/L,低于ACE组的0.23 g/L,MI1组的0.38 g/L、MI2的0.42 g/L。这一结果说明了CK组在黄贮过程中以同型乳酸菌发酵为主,故其乳酸含量最高、乙酸最低,由于乳酸并不是有效的抗真菌剂,因此小麦秸秆中的可利用营养物质可能会被酵母菌等所利用,物质损失较大,累积产甲烷量反而降低。MI1组、MI2组黄贮过程中添加的是异型乳酸菌复合系,其发酵过程中除了生成乳酸外,还会生成乙酸(乙醇)和CO2,乙酸是有效的抗真菌剂,既能抑制微生物活性,减少物质损失,又易被产甲烷菌利用,提高甲烷产量。
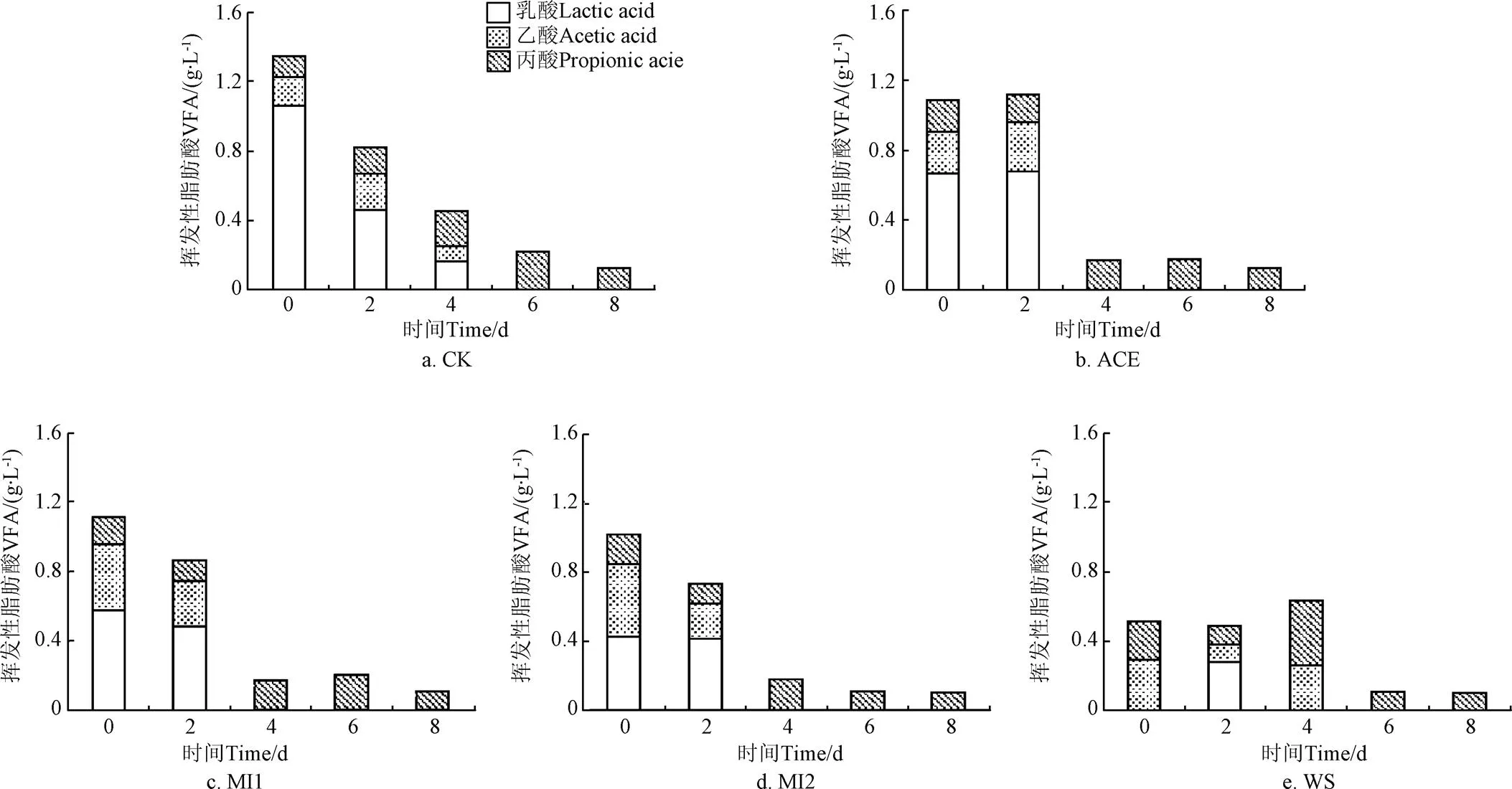
图4 厌氧发酵过程中VFA变化
2.3.3 发酵过程中sCOD的变化
在厌氧发酵过程中,发酵液sCOD能够反应发酵液中VFA、可溶性糖以及亚硝酸盐、亚铁盐、硫化物等还原性物质可被氧化的数量,从而掌握发酵进程[29]。发酵过程见图5。
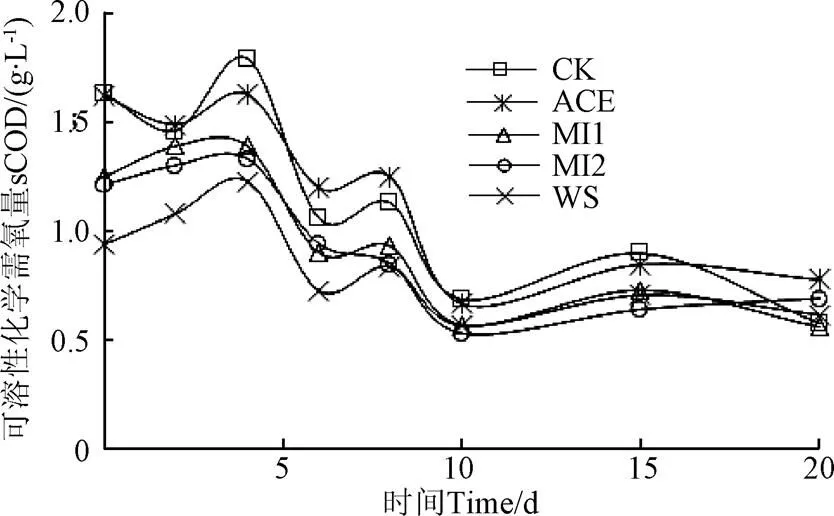
图5 厌氧发酵过程中sCOD变化
在发酵初始时(图5),WS组的sCOD最低,为0.94 g/L,而黄贮组在1.21~1.63 g/L,高于WS组,原因是黄贮过程中乳酸菌发酵产生大量的乳酸、乙酸、乙醇等可溶性有机物,再加上其对木质纤维素的分解,使得秸秆的sCOD 升高。在发酵过程中,所有处理均在第4、8天出现2个峰值,这与日产甲烷变化是相一致的。之后随着发酵天数的增加,sCOD降低。
2.4 外源添加剂对黄贮小麦秸秆厌氧发酵系统中微生物群落结构变化规律的影响
2.4.1 外源添加剂对黄贮小麦秸秆微生物群落多样性的影响
Alpha多样性即为样品物种多样性,其中Chao、Ace指数反映微生物群落的丰富度,即群落中物种总数。Shannon、Simpson指数反映微生物群落多样性,Shannon指数值越大群落多样性越高,而Simpson指数值越大群落多样性越低。Coverage反映微生物群落的覆盖度。从表2可以看出,发酵体系中细菌的物种多样性指数均远远高于古菌,说明古菌微生物多样性远远低于细菌。接种污泥细菌和古菌的Chao、Ace、Shannon指数均为最低、Simpson指数最高,说明小麦秸秆厌氧发酵后的微生物丰富度和多样性均高于接种污泥。黄贮组的细菌丰富度和多样性均高于WS组,即黄贮预处理能够提高体系中的细菌多样性,使体系更加稳定。相反古菌中WS组Shannon指数最高、Simpson指数最低,说明其古菌多样性高于黄贮组,但其甲烷含量和产量却比较低。可见黄贮预处理有利于发酵体系中的细菌多样性增加和古菌多样性减少,故而有利于甲烷产量的提高。
注:相似性水平97%。
Note: Similarity level 97%.
2.4.2 外源添加剂对黄贮小麦秸秆厌氧发酵系统中细菌和古菌群落结构的影响
图6a为细菌的微生物群落结构,从图中可以看出,在门水平上,不同处理发酵系统中细菌种类是相似的,只有相对丰度是不同的,其中主要菌群为拟杆菌门()、厚壁菌门()、变形菌门()、绿弯菌门()、互养菌门()、、螺旋体门()等。其中和为优势菌,占细菌总丰度的44.6%~69.6%,研究表明和是厌氧发酵水解酸化阶段发挥主要作用的细菌群[30],能够将复杂的大分子有机物如蛋白质、脂肪、碳水化合物等水解酸化为小分子有机酸[26]。ACE组、MI1组、WS组细菌和古菌丰度之和较接种污泥分别提高了22.4%、5.5%、5.5%,而MI2组、CK组却降低了21.6%、2.1%,结合产甲烷量发现,在本试验中两者丰度与甲烷产量并无直接关系。和这3类菌群多见于厌氧发酵体系中,对有机大分子物质的水解起到一定的作用[31]。是一类能够利用纤维素和半纤维素等碳水化合物的菌群[32],厌氧发酵后各处理中的相对丰度较接种污泥提高了300~1 500倍,其中WS组中相对丰度最高,说明该处理中纤维素和半纤维素较多,水解酸化受到限制,这也是WS组产气量不高的原因。
从古菌菌群结构中可以看出,本试验厌氧发酵系统中的古菌在门水平上(图6b)有2种优势菌门,广古菌门()约占56.6%~75.9%,泉古菌门()约占23.7%~42.1%。目前已知的产甲烷菌主要属于,能将厌氧发酵水解酸化过程中有机大分子物质分解产生的H2和CO2、乙酸、甲基类等小分子物质转变成甲烷[33]。
在属水平上(图6c)主要有甲烷鬃菌属()、深古菌()、甲烷杆菌属()、、甲烷马赛球属()、甲烷八叠球菌属()等。是一类专营性嗜乙酸型古菌,其占优势说明本试验中各处理均是以嗜乙酸产甲烷途径为主要的产甲烷途径。该菌属在黄贮组的相对丰度为49.0%~70.1%,而WS组仅41.1%,是因为黄贮后的秸秆,特别是CK组,含有大量以乳酸和乙酸为主的有机酸,乳酸在发酵过程中亦可以转化为乙酸,因此有利于其生长。为中一类具有多样代谢方式的古菌属,既能降解蛋白质、碳水化合物、脂肪酸、芳香族化合物和甲基化合物等有机质进行异养代谢,也能通过利用H2和CO2产乙酸途径进行自养代谢,同时还能异化还原亚硝酸盐和硫酸盐、参与甲烷代谢循环,发生甲烷厌氧化作用等[34-36]。厌氧发酵后各处理中其相对丰度增加了0.37~1.43倍,推测该菌在纤维素分解中起到了积极作用。WS组中的相对丰度均高于黄贮组,分别为6.1%、5.0%、3.2%,是一种嗜氢营养型产甲烷菌,能将H2和CO2代谢生成CH4;是一类专营性嗜甲基型产甲烷菌,通过H2还原甲硫醇产甲烷[37-38];是一类只有在H2存在的条件下,才能还原甲胺、甲醇生成CH4的甲基营养型产甲烷菌[39-40],说明WS组利用H2产甲烷途径较黄贮组多。可见黄贮预处理影响了厌氧发酵系统中产甲烷古菌的分布,但由于决定古菌主导种类的因素是发酵底物类型,所以并未使主导型古菌发生变化。
2.4.3 微生物结构与发酵环境因子之间的相关性分析
图7为微生物与发酵初始指标及累积产甲烷量之间的相关性分析。图7a为细菌门水平上的相关性分析,从图中可以看出,RDA1对细菌群落的影响占88.5%,其中WS组、MI1组、MI2组物种组成相近,而ACE组、MI2组分列两侧,物种差异较大,其中在ACE组丰度最高,且与sCOD和pH值成正相关,说明这些细菌有利于sCOD的转化,同时适宜的pH值促进了纤维素分解速率,提高酸化水解速率[41],这也是ACE组甲烷产量最高的原因。而MI2组中、、的相对丰度较高,与乙酸成正相关,说明这些细菌有利于乙酸的形成,其甲烷含量仅次于ACE组。整个发酵体系中影响水解酸化阶段细菌群落结构变化的主要环境因子为sCOD和VFA,有利于后续产甲烷阶段甲烷的生成。
图7b为古菌属水平上的相关关系,从图中可以看出,甲烷产量与乙酸、丙酸呈正相关,与乳酸、VFA、sCOD呈负相关,这是因为在复杂的厌氧环境中,微生物能够利用乙酸、丙酸等生成甲烷,但过高的乳酸则会抑制甲烷的产生,这与Zhao等人[24]的发现一致。甲烷产量与呈负相关,即其相对丰度越高对应的甲烷产量越低;与其他古菌属呈正相关,即等古菌的相对丰度越高对应的甲烷产量越高。和是唯一可以利用乙酸产甲烷的产甲烷古菌,研究表明对乙酸的亲和性更高,乙酸浓度较低时,甲烷鬃菌属更易成为优势菌属,而则在乙酸浓度较高时更易成为优势菌属[42-44],因此与乙酸呈正相关,与乙酸呈负相关。整个发酵体系中影响产甲烷阶段古菌群落结构变化和甲烷产量的主要环境因子为乙酸,即乙酸浓度影响了菌群中的相对丰度,从而决定了甲烷产量。
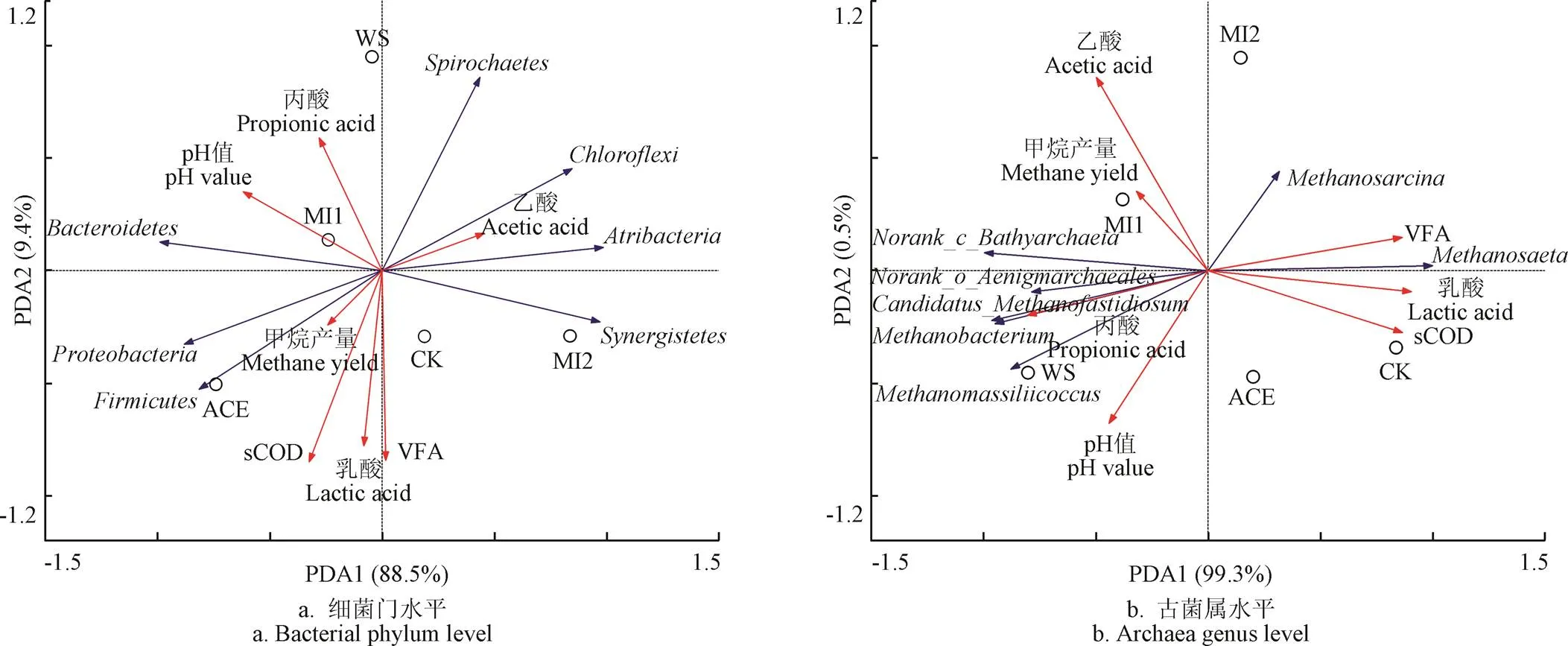
图7 细菌门水平、古菌属水平与环境间的相关性分析
3 结 论
1)添加外源添加剂减少了黄贮过程中小麦秸秆的半纤维素、纤维素等营养成分的损失,有利于后续厌氧发酵过程甲烷的生成,其中CK组的累积产甲烷量为175.8 mL/g,较WS组的193. 2 mL/g降低了9%,而ACE组、MI2组、MI1组的黄贮小麦秸秆的累积产甲烷量分为213.7、207.9、202.2 mL/g,较WS组提高了4.7%~10.6%,较CK组提高了15.0%~21.5%。
2)在厌氧发酵过程中,黄贮组起始VFA浓度增加,主要为乳酸和乙酸的增加,随着发酵的进行,丙酸成为VFA的主要组成酸;黄贮处理能提高沼气中的甲烷含量,对小麦秸秆沼气化利用有积极的作用。
3)厌氧发酵体系中优势细菌均为,优势古菌为,添加外源添加剂虽未改变厌氧发酵体系中细菌和古菌的优势菌群,却改变了各自的群落结构,细菌多样性增加,古菌多样性减少,更有利于甲烷的生成。其中,对细菌群落结构影响最大的环境因子为VFA和sCOD;对古菌群落结构和甲烷产量的影响最大的环境因子为乙酸。
综上,以干黄秸秆为沼气工程原料时,添加乙酸、异型乳酸菌复合系的黄贮处理,不仅有利于秸秆的长期保存,还有效提高其产甲烷潜力。
[1] 石祖梁,贾涛,王亚静,等. 我国农作物秸秆综合利用现状及焚烧碳排放估算[J]. 中国农业资源与区划,2017,38(9):32-37.
Shi Zuliang, Jia Tao, Wang Yajing, et al. Comprehensive utilization status of crop straw and estimation of carbon from burning in China[J]. Chinese Journal of Agricultural Resources and Regional Planning, 2017, 38(9): 32-37. (in Chinese with English abstract)
[2] Sawatdeenarunat C, Nguyen D, Surendra C, et al. Anaerobic biorefinery: Current status, challenges, and opportunities[J]. Bioresource Technology, 2016, 215: 304-313.
[3] 毛春兰. 小麦秸秆与猪粪混合物料厌氧发酵特征及微生物调控机制研究[D]. 杨凌:西北农林科技大学,2018.
Mao Chunlan. Anaerobic Co-digestion Characteristics and Microbial Regulatory Mechanism of Wheat Straw and Swine Manure[D]. Yangling: Northwest A&F University, 2018. (in Chinese with English abstract)
[4] 苏小红,范超,王欣,等. 黄贮时间对东北地区玉米秸秆的产甲烷性能的影响[J]. 黑龙江科学,2017,8(8):27-29. Su Xiaohong, Fan Chao, Wang Xin, et al. The effect of maize straw yellow storage time on the methane producing properties in the northeast region[J]. Heilongjiang Science, 2017, 8(8): 27-29. (in Chinese with English abstract)
[5] 袁玲莉,刘研萍,袁彧,等. 存储时间对玉米秸秆理化性状及产甲烷潜力的影响[J]. 农业工程学报,2019,35(13):210-217. Yuan Lingli, Liu Yanping, Yuan Yu. Effect of storage time on physiochemical properties and methanogenesis potential of maize straw[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(13): 210-217. (in Chinese with English abstract)
[6] 霍立娇,杜静,叶小梅,等. CO2气调贮存秸秆及对厌氧发酵产沼气性能的影响研究[J]. 中国沼气,2019,37(2):12-18. Huo Lijiao, Du Jing, Ye Xiaomei, et al. CO2gas controlled storage of straw and the effect on its biogas production[J]. China Biogas, 2019, 37(2): 12-18. (in Chinese with English abstract)
[7] 任海伟,王聪,窦俊伟,等. 玉米秸秆与废弃白菜的混合青贮品质及产沼气能力分析[J]. 农业工程学报,2016,32(12):187-194. Ren Haiwei, Wang Cong, Dou Junwei, et al. Mixed ensiling quality of maize straw with waste cabbage and biogas production potential analysis[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(12): 187-194. (in Chinese with English abstract)
[8] Comino L, Tabacco E, Righi F, et al. Effects of an inoculant containing a Lactobacillus buchneri that produces ferulate-esterase on fermentation products, aerobic stability, and fibre digestibility of maize silage harvested at different stages of maturity[J]. Animal Feed Science and Technology. 2014, 198: 94-106.
[9] Acosta A Y, Jatkauskas J, Vrotniakiene V. The effect of a silage inoculant on silage quality, aerobic stability, and meat production on farm scale[J]. ISRN Veterinary Science, 2012, 2012: 345927.
[10] 焉石. 碳水化合物添加剂和不同收获期对青贮玉米青贮品质的影响[D]. 哈尔滨:东北农业大学,2010. Yan shi. The Effect of Carbohydrate Additives and Different Harvest Stages on Corn Silage Q uality[D]. Harbin:Northeast Agricultural University, 2010. (in Chinese with English abstract)
[11] Addah W, Baah J, Groenewegen P, et al. Comparison of the fermentation characteristics, aerobic stability and nutritive value of barley and corn silages ensiled with or without a mixed bacterial inoculant[J]. Canadian Journal of Animal. 2011, 91(1): 133-146.
[12] 崔宪,张乐平,孙辉,等. 碳氮比对干黄秸秆贮存及后续甲烷产量的影响[J]. 农业工程学报,2019,35(23):250-257. Cui Xian, Zhang Leping, Sun Hui. Effects of C/N ratio of wilted maize straw on wet storage process and subsequent methane production[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(23): 250-257. (in Chinese with English abstract)
[13] 马旭光,刘晶晶,郑泽慧,等. 乙酸和乳酸对玉米秸秆青贮料有氧稳定性和甲烷产率的影响[J]. 中国农业大学学报,2015,20(1):44-52. Ma Xuguang, Liu Jingjing, Zheng Zehui. Effects of acetic and lactic acid in corn stover silage on aerobic stability and methane yield rate[J]. Journal of China Agricultural University, 2015, 20(1): 44-52. (in Chinese with English abstract)
[14] Yuan X, Wen A, Dong Z, et al. Effects of formic acid and potassium diformate on the fermentation quality, chemical composition and aerobic stability of alfalfa silage[J]. Grass and Forage Science. 2017, 72(4): 833-839.
[15] 孙启忠,于艳冬,王美容,等. 添加尿素和乳酸菌制剂对玉米秸秆青贮料品质的影响[J]. 中国畜牧杂志,2009,45(3):37-40. Sun Qizhong, Yu yandong, Wang Meirong. Effects of urea and lactic acid bacteria on the quality of corn stalk silages[J]. Chinese Journal of Animal Science, 2009, 45(3): 37-40. (in Chinese with English abstract)
[16] Ball C, Mctaggart I P, Scott A. Mitigation of greenhouse gas emissions from soil under silage production by use of organic manures or slow-release fertilizer[J]. Soil Use and Management, 2004, 20(3): 287-295.
[17] Aksu T, Baytok E, Karsli M, et al. Effects of formic acid, molasses and inoculant additives on corn silage composition, organic matter digestibility and microbial protein synthesis in sheep[J]. Small Ruminant Researc, 2004, 61(1): 29-33.
[18] 崔宪,郭建斌,徐艳,等. 秸秆湿贮存过程添加剂协同调控对甲烷产量的影响[J]. 农业机械学报,2018,49(9):302-310. Cui Xian, Guo Jianbin, Xu Yan. Effect of wet-storage additives on fermentation performance and biomethane potential of corn stover[J]. Transactions of the Chinese Society for Agricultural Machinery, 2018, 49(9): 302-310. (in Chinese with English abstract)
[19] Guo P, Mochidzuki K, Cheng W, et al. Effects of different pretreatment strategies on corn stalk acidogenic fermentation using a microbial consortium[J]. Bioresource Technology. 2011, 102(16): 7526-7531.
[20] 时建青,徐红蕊,艾尼瓦尔·艾山. 青贮饲料添加剂及其研究应用现状[J]. 现代畜牧兽医,2005(12):22-23.
[21] Zhao X L, Liu J H, Liu J J. Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass[J]. Bioresource Technology, 2017, 241: 349-359.
[22] Zhang H, Wu J W, Gao L J, et al. Aerobic deterioration of corn stalk silage and its effect on methane production and microbial community dynamics in anaerobic digestion[J]. Bioresource Technology. 2018, 250: 828-837.
[23] Mceniry J, Allen E, Murphy J D, et al. Grass for biogas production: The impact of silage fermentation characteristics on methane yield in two contrasting biomethane potential test systems[J]. Renewable Energy, 2014, 63: 524-530.
[24] Zhao Y B, Yu J D, Liu J J, et al. Material and microbial changes during corn stalk silage and their effects on methane fermentation[J]. Bioresource Technology. 2016, 222: 89-99.
[25] Tanjore D, Richard T L, Marshall M N. Experimental methods for laboratory-scale ensilage of lignocellulosic biomass[J]. Biomass & Bioenergy, 2012, 47: 125-133.
[26] 赵肖玲. 产纤维素酶菌株筛选优化及对秸秆厌氧发酵的促进机制[D]. 北京:中国农业大学,2018. Zhao Xiaoling. Screening and Optimization of Cellulase-producing Strains and Promotion Mechanism During Anaerobic Digestion of Straw[D]. Beijing: China Agricultural University, 2018. (in Chinese with English abstract)
[27] 罗晓莎. 蘑菇菌糠与牛粪、餐厨垃圾厌氧共发酵产甲烷潜力及熟化特性[D]. 北京:中国农业大学,2018. Luo Xiaosha. Biogas Production and Characteristics of Maturity During Anaerobic Co-digestion of Spent Mushroom Substrate and Dairy Manure or Kitchen Waste[D]. Beijing: China Agricultural University, 2018. (in Chinese with English abstract)
[28] Cavinato C, Cinzia D R, Pavan P, et al. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage[J]. Bioresource Technology, 2017, 223: 59-64.
[29] 蔡亚凡. 微量元素对水稻秸秆厌氧发酵产甲烷的影响及微量元素生物利用度调控[D]. 北京:中国农业大学,2019. Cai Yafan. Effect of Trace Elements on Methane Production by Anaerobic Digestion of Straw and Regulating the Bioavailability of Trace Elements[D]. Beijing: China Agricultural University, 2019. (in Chinese with English abstract)
[30] Ziganshin A M, Liebetrau J, Proter J, et al. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials[J]. Applied Microbiology and Biotechnology, 2013, 97(11): 5161-5174.
[31] 刘晋欢. 刈割次数、青贮添加剂与温度对柳枝稷甲烷发酵效率的影响[D]. 北京:中国农业大学,2015. Liu Jinhuan. Effect of Harvest Time, Silage Additives and Temperature on Methane Yield of Switchgrass[D]. Beijing: China Agricultural University, 2019. (in Chinese with English abstract)
[32] Dubinina G, Grabovich M, Leshcheva N, et al. Spirochaeta perfilievii sp.nov., an oxygen-tolerant, sulfide-oxidizing, sulfur- and thiosulfate-reducing spirochaete isolated from a saline spring[J]. International Journal of Systematic and Evolutionary Microbiology, 2011, 61: 110-117.
[33] 吴麒,陈颖,邱凯瑞,等. 产甲烷条件下岩溶湿地沉积物中古菌群落的变化规律[J]. 微生物学通报,2019,46(12):3193-3204. Wu Qi, Chen Ying, Qiu Kairui. Characterization of archaeal communities in a karst wetland under methanogenic conditions[J]. Microbiology China, 2019, 46(12): 3193-3204. (in Chinese with English abstract)
[34] 陈玉连,潘杰,周之超,等. 滨海深古菌的研究进展[J]. 微生物学通报,2017,44(7):1690-1698. Chen Yulian, Pan Jie, Zhou Zhichao. Progress in studies on Bathyarchaeota in coastal ecosystems[J]. Microbiology China, 2017, 44(7): 1690-1698. (in Chinese with English abstract)
[35] 赵肖玲,郑泽慧,蔡亚凡,等. 哈茨木霉和黑曲霉粗酶液预处理改善秸秆产甲烷性能[J]. 农业工程学报,2018,34(3):219-226. Zhao Xiaoling, Zheng Zehui, Cai Yafan. Pretreatment by crude enzymatic liquid from Trichoderma harzianum and Aspergillus sp improving methane production performance during anaerobic digestion of straw[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(3): 219-226. (in Chinese with English abstract)
[36] Evans P N, Parks D H, Chadwick G L, et al. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics[J]. Science, 2015, 350(6259): 434-438.
[37] 段昌海,张翠景,孙艺华,等. 新型产甲烷古菌研究进展[J]. 微生物学报,2019,59(6):981-995. Duan Changhai, Zhang Cuijing, Sun Yihua. Recent advances on the novel methanogens[J]. Microbiology China, 2019, 59(6): 981-995. (in Chinese with English abstract)
[38] Ni B J, Zeng S T, Wei W, et al. Impact of roxithromycin on waste activated sludge anaerobic digestion: Methane production, carbon transformation and antibiotic resistance genes[J]. Science of the Total Environment, 2020, 703: 134899.
[39] 韩睿. 青海农用沼气池发酵微生物群落结构与功能研究[D]. 武汉:华中师范大学,2018. Han Rui. Research on Microbial Community Structure and Their Function of Rural Household Biogas Digesters in Qinghai[D]. Wuhan: Central China Normal University, 2018. (in Chinese with English abstract)
[40] 杨斌. 低温沼气发酵系统中细菌和古菌的群落结构与多样性[D]. 昆明:云南师范大学,2017. Yang Bin. Community Structures and Diversity of Bacteria and Archaea in Low Temperature Biogas Systems[D]. Kunming: Yunnan Normal University, 2017. (in Chinese with English abstract)
[41] 王雅雅. 几种典型农业废弃物高含固率厌氧共发酵产气性能与协同机理研究[D]. 北京:中国农业大学,2018. Wang Yaya. Performance of Methane Production and Synergy Mechanism at High Solid Content Anaerobic Co-digestion Using Typical Agricultural Wastes[D]. Beijing: China Agricultural University, 2018. (in Chinese with English abstract)
[42] 承磊,郑珍珍,王聪,等. 产甲烷古菌研究进展[J]. 微生物学通报,2016,43(5):1143-1164.
Cheng Lei, Zheng Zhenzhen, Wang Cong. Recent advances in methanogens[J]. Microbiology China, 2016, 43(5): 1143-1164. (in Chinese with English abstract)
[43] 孔德望,张克强,房芳,等. 猪粪厌氧发酵消化液回流体系微生物群落结构特征与产气关系研究[J]. 农业环境科学学报,2018,37(3):559-566. Kong Dewang, Zhang Keqiang, Fang Fang. Study of microbial community and biogas production in anaerobic digestion of pig manure with digested slurry recirculation[J]. Journal of Agro-Environment Science, 2018, 37(3): 559-566. (in Chinese with English abstract)
[44] Conklin A, Stensel H D, Ferguson J. Growth kinetics and competition between Methanosarcina and Methanosaeta in mesophilic anaerobic digestion[J]. Water Environment Research. 2006, 78(5): 486-496.
Effects of yellow silage additives on methane production and microbial community dynamics during anaerobic digestion of wheat straw
Yan Jing1, Lu Bingyuan1, Xi Huayue1, Meng Xingyao2, Yuan Xufeng1, Zhu Wanbin1, Cui Zongjun1※
(1.100193;2.100048,)
Straw-biogas-fertilizer has become one of the promoted utilization modes for the agricultural waste, particularly on large amount of crop straw that produced annually in China. However, a long-term storage of straw has posed a great problem on a straw biogas plant. A commonly used method, the yellow silage, can be expected to preserve nutrient, while reduce the dry matter loss of straw during storage. This study aims to explore the effects of yellow silage additives on methane production and microbial community dynamics during anaerobic digestion of wheat straw. The experiment was divided into two parts: yellow silage and anaerobic digestion. Yellow silage treatments were inoculated additives into the dry yellow wheat straw with a moisture content of 50% for 65 days, including CK group (without additives), ACE group (acetic acid addition of 3‰), MI1 group (lactic acid bacteria community addition of 3‰), MI2 group (lactic acid bacteria community addition of 6‰). The results showed that the pH of four groups below 4.7, indicating excellent fermentation quality. Hemicellulose and cellulose decreased during yellow silage, especially CK group only 22.2% and 31.8%, respectively. In the treatment with additives, the hemicellulose and cellulose content were significantly higher than that in the CK group, indicating that the addition of additives was helpful to preserve available nutrients. In the anaerobic digestion experiment, the raw materials were the yellow silage wheat straw (CK, ACE, MI1, MI2 group) and dry yellow wheat straw (WS group). The batch tests were conducted for up to 20 days at (36±2)℃. The working volume of each reactor was 300 mL, consisting of 12 g (TS) inoculum and 3 g (TS) substrate, with a TS content of 5 % and the remaining space filled with nitrogen gas. Inoculum without any added feedstock was used as a blank. Triplicate reactors were run for each treatment. The biogas production and methane composition were measured every day, whereas, the pH value, volatile fatty acids (VFA), soluble chemical oxygen demand (s COD) were measured during anaerobic digestion. High-throughput sequencing was used to determine the microbial community structure on the twentieth day of anaerobic digestion, in order to detect the effect of yellow silage pretreatment on the bacteria and archaea community in anaerobic fermentation system. The results from the anaerobic experiment showed that the VFA concentration and s COD increased significantly in yellow silage group at the initial stage, where mainly VFA in the fermentation system were lactic acid and acetic acid. As the fermentation time increased, the VFA concentration and s COD decreased after 2 days fermentation, where the propionic acid was the main component of VFA. The cumulative methane yield of ACE group, MI1 group, MI2 group were 213.7, 202.2, 207.9 mL/g, increased by 10.6%, 4.7% and 7.6%, respectively, compared with WS group (193. 2 mL/g), while CK group were 175.8 mL/g, decreased by 9.0% compared with WS group. After anaerobic digestion, the main bacteria were, while the main archaea wereindicating that the yellow silage can affect the microbial structure in the fermentation system. This finding can provide an important theoretical and technical support for energy conversion of crop straw in large-scale biogas production.
straw; additives; yellow silage; methane yield; microbial community
闫晶,陆冰圆,席华悦,等. 外源添加剂对黄贮小麦秸秆产甲烷潜力及微生物群落的影响[J]. 农业工程学报,2020,36(15):252-260.doi:10.11975/j.issn.1002-6819.2020.15.031 http://www.tcsae.org
Yan Jing, Lu Bingyuan, Xi Huayue, et al. Effects of yellow silage additives on methane production and microbial community dynamics during anaerobic digestion of wheat straw[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(15): 252-260. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2020.15.031 http://www.tcsae.org
2020-04-06
2020-08-10
国家公益性行业(农业)科研专项(201503137)
闫晶,博士生,主要从事农业废弃物资源化利用方面的研究。Email:849449907@qq.com
崔宗均,博士,教授,主要从事生物质资源转化与利用研究。Email:acuizj@cau.edu.cn
10.11975/j.issn.1002-6819.2020.15.031
S216.4; X712
A
1002-6819(2020)-15-0252-09

