GOECP/SEOR clinical recommendations for lung cancer radiotherapy during the COVID-19 pandemic
Felipe Couñago,Department of Radiation Oncology,Hospital Universitario Quirónsalud Madrid,Pozuelo de Alarcón,Madrid 28223,Spain
Felipe Couñago,Clinical Department,Hospital La Luz,Madrid,Faculty of Biomedicine,Universidad Europea,Madrid 28223,Spain
Arturo Navarro-Martin,Department of Radiation Oncology,Institut Catalá d’Oncologia,L’Hospitalet de Llobregat,Barcelona 08908,Spain
Javier Luna,Department of Radiation Oncology,Hospital Fundación Jiménez Díaz,Madrid 28040,Spain
Nuria Rodríguez de Dios,Department of Radiation Oncology,Hospital del Mar,Barcelona 08003,Spain
Aurora Rodríguez,Department of Radiation Oncology,Hospital Ruber Internacional,Madrid 28034,Spain
Francesc Casas,Department of Radiation Oncology,Thoracic Unit,Hospital Clínic,Barcelona 08036,Spain
Rafael García,Department of Radiaiton Oncology,Hospital Ruber Internacional,Madrid 28034,Spain
Antonio Gómez-Caamaño,Department of Radiation Oncology,Hospital Clínico Universitario Santiago de Compostela,A Coruña 15706,Spain
Jorge Contreras,Department of Radiation Oncology,Hospital Regional Universitario de Málaga,29010,Spain
Javier Serrano,Department of Radiation Oncology,Clínica Universidad de Navarra,Madrid 28027,Spain
Abstract
Key words:Lung cancer;COVID-19;Pandemic;Radiotherapy;Recommendations
INTRODUCTION
As of this writing,the global pandemic caused by the novel betacoronavirus,known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),has affected more than 3 million people worldwide,with nearly 250000 deaths related directly or indirectly to the disease caused by this virus [coronavirus disease 2019 (COVID-19)],mainly the elderly and/or individuals with pre-existing conditions[1].The COVID-19 pandemic has overwhelmed health care systems around the world,which has had a major impact on human lives as well as healthcare resources.This pandemic has affected not only the medical specialties directly involved in the fight against the coronavirus,but also other specialities,most notably those-such as radiation oncologythat treat cancer patients.One of the immediate consequences of the pandemic on the organization of health care systems is that most available hospital resources are dedicated to helping to control the disease,which necessarily imposes limitations on other areas of medicine and other important medical conditions.This situation is particularly serious for cancer patients,especially those with lung cancer.
Lung cancer is an aggressive biological entity,with a high proliferative and invasive capacity.Consequently,any delay in the initiation of oncological treatment can directly influence the clinical course of the disease,negatively impacting both disease control and mortality[2-4].In the present health crisis,patients with lung cancer are especially vulnerable due to the convergence of a series of factors.First,the clinical manifestations of lung cancer often coincide with those of COVID-19 infection (cough,dyspnea,etc.),which could hinder an appropriate early diagnosis and alarm other cancer patients due to the fear of infection.In addition,the clinical characteristics of these patients-such as advanced age,the presence of an underlying lung disease,and other comorbidities (hypertension,heart disease,diabetes,among others)-place them at a higher risk of developing complications from COVID-19[2-4].In this context,the diagnostic and therapeutic procedures necessary to manage lung cancer generally require multiple visits to the hospital and/or other health care centres,thereby increasing the risk of exposure to the virus.Moreover,lung cancer patients infected with the virus are much more likely to present a clinical course that is more serious than in the general population[2-4].
Given the exceptional circumstances caused by this pandemic,which may limit our ability to offer standard radiotherapy treatment in patients with lung cancer,radiation oncologists must seriously consider alternative therapeutic strategies aimed at minimising the risk of infection in these patients while simultaneously ensuring that the alternative approach does not imply a delay in cancer treatment or a lower quality,less efficacious approach.
Due to the novelty of COVID-19,there is a lack of high-quality scientific evidence to guide clinical decision-making.Consequently,consensus-based expert recommendations are needed.Several prestigious international oncological scientific societies (ASTRO,ASCO,ESTRO) have recently published recommendations to guide the management of these patients during the pandemic[2-4].The aim of the present document,developed jointly by the Spanish Society of Radiation Oncology (SEOR)and the Oncologic Group for the Study of Lung Cancer (GOECP),is to provide an updated review of the current scientific evidence to establish clinical recommendations regarding the therapeutic options for the optimal treatment of lung cancer during the COVID-19 pandemic.
RADIATION ONCOLOGY DURING THE COVID-19 PANDEMIC
Although lung cancer patients are assumed to be at increased risk of death from SARSCoV-2 infection,the evidence to support this claim is limited and largely based on retrospective,single-centre studies with small sample sizes and significant methodological deficiencies[5-9].To overcome these limitations,several professional organizations,including the European Society for Medical Oncology,the International Association for the Study of Lung Cancer,and the European Platform for Thoracic Oncology,have developed a global registry called TERAVOLT,whose purpose is to better understand the effect of COVID-19 on patients with thoracic cancers to help guide oncologists in the optimal management of these patients.An analysis of preliminary data from this registry indicates that patients with comorbidities present higher hospitalization and mortality rates,but the available data do not indicate that cancer treatment has negatively affected these patients[5].
Radiotherapy plays an essential role in the treatment of all stages of lung cancer,and it is also a safe and effective alternative to surgery in certain cases.Accordingly,any limitations in patient access to radiotherapy,or delays in the start of treatment,would negatively affect survival and quality of life outcomes in these patients.Due to the pandemic,radiation oncology departments face important challenges as they attempt to strike a balance between ensuring that patients receive the appropriate oncological care and minimizing the risk of COVID-19 infection in patients and health care professionals.In this context,there is a clear need for a multi-level communication policy based on rigorous,up-to-date,and convincing data to raise awareness among patients,their families,and health care professionals,of the importance of implementing appropriate preventive measures.
Since SARS-CoV-2 first began to spread widely,various official bodies,organizations,and experts have all urged radiation oncologists to implement standards and protocols designed to ensure the continued safety of oncological treatments[10-13].These recommendations and protocols include posters and brochures,screening measures to detect infected individuals upon arrival at the department (e.g.,respiratory symptoms,fever,or contact with a possible or confirmed positive COVID case),limitations in the number of people allowed to accompany the patient,strict compliance with scheduled appointment times,as well as the routine use of masks and gloves,hand hygiene,and social distancing.In addition to these basic safety measures,the department must also develop and implement a comprehensive plan for cleaning and disinfecting physical spaces (controls,waiting rooms,booths,bunkers),contact surfaces (counters,screens,keyboards,telephones,handles),linear accelerators,the simulation computed tomography (CT) scanner,treatment tables,and immobilization devices.
The implementation of these measures in the radiotherapy department—aimed at guaranteeing the appropriate sanitary conditions-can potentially limit the department’s capacity to offer treatment due to the risk of an outbreak among staff,reassignment of personnel other departments involved in the front-line fight against COVID-19,and/or reuse of physical and material resources.For this reason,a rational approach to planning cancer care is essential,with decision-making based on high scientific and ethical standards in which the indications for treatment-priority,delay,interruption,or even refusal-are clearly defined.To achieve this in a clinical scenario with limited capacity,we must balance the risk of exposure to the coronavirus (and the associated morbidity) with the risk of failing to treat a potentially fatal cancer according to the usual standards.Multiple factors must be considered,including the patient’s general condition,life expectancy,potential for cure,existence of alternative treatments with a similar therapeutic efficacy,and the presence of an active infection.The decision to delay or interrupt radiotherapy treatment in a COVID-19-positive lung cancer patient must be carefully evaluated to avoid infecting other patients and healthcare professionals.The best approach to managing this complexity is to include other specialists involved in the treatment of this pathology in the decision-making process,which implies that thoracic tumour boards should continue to meet,albeit in a virtual setting,to ensure quality of care.
From a practical point of view,it is essential to develop a contingency plan that considers all of the following:(1) The division of departmental staff into independent operating groups (i.e.,shift-based health care activity);(2) The possibility of working from home;(3) The reorganization of physical resources (waiting rooms,consultations,linear accelerator treatment room) to minimize the number of people in the same place,for example by converting a waiting room for a clinical consultation or CT scan into a waiting room for the linear accelerator;(4) The rational use of hypofractionated regimens;a simplified approach to treatment verification and administration;and (5)Measures to minimize follow-up consultations for toxicity.Whenever possible,followup consultations should be performed by telephone or videoconference.
If a patient undergoing radiotherapy develops COVID-19,an individualized assessment must be performed to select the appropriate therapeutic approach.In all cases,the main priority is the patient’s health,which may involve treatment continuation or a temporary interruption until the infection resolves.Various factors must be considered,including those related to COVID-19 infection-severity,presence or absence of pneumonia,and the patient’s respiratory status and general conditionwhich will determine whether the patient is able to receive radiotherapy.Other factors related to the neoplasm include the extent of the oncological disease,the presence or absence of tumour-related symptoms (e.g.,obstruction,hemoptysis,or pain),and the risk of tumour progression.
Interrupting or delaying treatment should only be considered in patients with COVID-19-related symptoms in whom a temporary interruption or delay in starting treatment is unlikely to lead to significant tumour progression (for example,prophylactic cranial irradiation,postoperative radiotherapy,etc.).Once the COVID-19 symptoms have resolved and the patient tests negative for the disease (PCR),treatment should be restarted or initiated as soon as possible.In the event that the decision is made to continue radiotherapy in a COVID-19-positive patient,this should be done according to recommendations of national and international organizations,which include the implementation of strict safety measures to protect health care personnel.The main safety measures include avoiding physical contact between infected and non-infected patients in the department (i.e.,different rooms for COVID-19 patients) and/or by treating these patients at different times,and strict cleaning and disinfection protocols[14,15].
STEREOTACTIC BODY RADIOTHERAPY FOR STAGE T1-T2 NON-SMALL CELL LUNG CANCER
The recommendations described here are based on an appropriate balance between the risk and benefits of the proposed radiotherapy regimens.The main risk associated with stereotactic body radiotherapy (SBRT) in patients with lung cancer is the potential for treatment-related toxicity,which is mainly a function of the location of the target lesion,the total dose and dose per fraction,and lesion size.Timmermanet al[16]found that the risk of treatment-related toxicity is higher in central versus peripheral lesions for the same dose fraction.For this reason,several groups[17,18]have proposed basing the fraction size on the tumour location (applicable to tumours with a maximum diameter <5 cm).In addition to the established indication for SBRT in inoperable patients,in the context of this pandemic,if the surgery department is at full capacity,then SBRT should also be considered in operable patients in accordance with recommendations of both American and European experts[3].
Fractionation in central tumours
The appropriate treatment approach to central tumours is based mainly on the phase 1/2 RTOG 0813 trial (19)[19],a dose escalation trial performed to assess a five-fraction SBRT schedule ranging from 10 to 12 Gy/fraction (total dose,50 to 60 Gy) delivered every other day.In that trial,the maximum tolerated dose was 12.0 Gy/fraction,with≥ grade (G) 3 toxicity rate of 7.2%.These regimens can be compared to more conservative regimens,such as that described by Haasbeeket al[20],in which patients received 60 Gy delivered in 8 fractions,with a local control rate at 3 years of 92.6% and≥ G3 toxicity rate of 7.9%.
Based on the available evidence for the risk of toxicity versus the probability of achieving local control in central tumours,a reasonable recommendation would be a total dose of 50-60 Gy delivered in 5 fractions,with dose adjustment (10 to 12 Gy/fraction) as appropriate to comply with dose limits to the organs at risk (OAR)[21].Table 1 summarizes the recommended treatment regimens according to tumour location.
Lesions adjacent to the chest wall
In patients with lesions adjacent to (<2 cm) or in contact with the chest wall,the European recommendations[22]allow for a dose of up to 48 Gy in 4 fractions.Another proposed fractionation schedule in this location is 60 Gy in 5 fractions[23].Based on the published results of these two schedules in terms of local control and toxicity,we recommend 48 Gy in 4 fractions.
Peripheral lesions in the "safe" zone
Tumours located in a “safe” zone are understood as those located in an area that cannot be considered central,and at least 2 cm from the chest wall.In these cases,extreme hypofractionation consisting of a single fraction of 30-34 Gy-based on the findings of two prospective phase 2 trials[24,25]may be considered in well-selected patients.The classic fractionation regimen (60 Gy in 3 fractions) proposed by Timmermanet al[26]offers excellent 3-year local control rates (90.6%-94%) in peripheral tumours,with an acceptable toxicity rate (≥ G3),ranging from 10%-16.3%[27].In short,administration of a single fraction of 30-34 Gy can be considered in lesions located in the safe zone provided that dosimetric restrictions to the OARs are met[28-32];otherwise,the Timmerman scheme (54 Gy in 3 fractions),corrected for heterogeneity,should be administered.
RADIOTHERAPY IN LOCALLY-ADVANCED NON-SMALL CELL LUNG CANCER
Concomitant chemoradiotherapy (CRT) is considered the standard treatment for unresectable,stage III non-small cell lung cancer (NSCLC)[33].However,the impact of the SARS-CoV-2 pandemic on surgery departments,including thoracic surgery,will likely increase the number of patients with potentially-resectable stage III NSCLC who receive non-surgical treatment-that is,some combination of chemotherapy and radiation therapy.Nevertheless,in patients in reasonably good physical condition[performance status (PS) 0-1,weight loss <5 kg,good lung function,no significant comorbidities],if the available human and material resources allow,the treatment of choice is the standard external radiotherapy regimen:60-66 Gy (30-33 fractions)administered concomitantly with platinum-based chemotherapy[34].However,during a pandemic,the greater toxicity associated with concomitant CRT in these patients,especially lymphopenia,deserves special consideration as it could significantly increase the risk of complications in patients with COVID-19 (in whom lymphopenia is a common symptom),potentially leading to a worse prognosis[35-37].
In this context,sequential administration of systemic therapy followed by radiotherapy could be an option,for three main reasons.First,sequential treatment would reduce the potential immunosuppressive effects of concurrent CRT,which would,in turn,minimize the risk of complications caused by COVID-19.Second,a sequential therapeutic regimen would minimize exposure of the patient to the hospital environment,where the risk of infection is high.Third,it would optimize the available human and material resources in the radiation oncology department,which have been greatly depleted during the peak of the pandemic.
Therefore,it is reasonable to propose the use of hypofractionated thoracic radiotherapy in selected cases and in situations of severe shortage of radiotherapyresources.Some schemes,such as 15-20 fractions administered at doses ranging from 2.75 to 4 Gy per fraction to the target volume,with or without integrated boost have been tested.These regimens would maintain an appropriate biologically-equivalent dose,which previous studies have shown to be both safe and effective[38,39].

Table1 Stereotactic body radiotherapy schemes for stage T1-T2 non-small cell lung cancer
The use of these hypofractionated regimens has long been routine in the United Kingdom[40].In lung cancer patients,the use of hypofractionated radiotherapy administered sequentially after chemotherapy was widely used before the current pandemic,mainly due to its efficiency and favourable toxicity profile,and this approach should be considered as a possible strategy of choice in the current context[41].Table 2 summarizes the most commonly used hypofractionated schedules.References in Table 2[41-43].
However,the current evidence to support hypofractionated radiotherapy combined with chemotherapy remains limited.Although a systematic review found that some studies reported high toxicity[44],many of those studies used older radiotherapy techniques,rather than the more modern-and more precise-techniques available today.Nevertheless,the findings of several studies suggest that concomitant CRT [with a risk-adjusted chemotherapy (cisplatin + vinorelbine) dose] can be considered in wellselected patients,although a greater risk of toxicity must be assumed.
In terms of quality standards for radiotherapy,it is advisable-regardless of whether a conventional (30-33 fractions) or hypofractionated regimen is prescribed-to perform a simulation CT (preferably 4D-CT) to assess respiratory motion.In addition,whenever possible,highly conformal techniques with dynamic modulation such as intensity-modulated radiotherapy or volumetric modulated arc therapy should be used.Appropriate image guidance and positioning verification (image-guided radiotherapy) using cone beam CT is essential-particularly for hypofractionated regimens-in order to continuously monitor the treatment volume to minimize the radiation dose to the OARs[45-47].
Once sequential CRT has been completed,depending on the patient’s PD-L1 status,maintenance treatment with durvalumab for one year should be considered[48].However,because the patients in the PACIFIC trial were treated with concomitant CRT,there is only limited evidence to support maintenance therapy with durvalumab in sequentially-treated patients.Moreover,the costs of the adjuvant treatment with durvalumab in sequentially treated patients may not be covered by the Spanish public health care system.Consequently,in patients with PD-L1 >1%,the application of sequential schemes could negatively influence overall survival;nevertheless,consolidation treatment after sequential CRT is currently being investigated in the PACIFIC 6 trial (ClinicalTrials.gov Identifier:NCT03693300).An unplanned subgroup analysis of the PACIFIC trial data found that patients who started treatment with durvalumab within 14 d from the end of consolidation treatment had better outcomes;nonetheless,in the full patient cohort,durvalumab was initiated from 1 to 42 d post-CRT with good outcomes,findings that should be taken into account in the current situation.
To conclude,our recommendation is to prescribe,whenever possible,the standard external radiotherapy regimen-60-66Gy (30-33 fractions)-administered concomitantly with platinum-based chemotherapy followed by consolidation with Durvalumab accordingly with the patient´s PD-L1 status.However,if concomitant treatment is not possible,a valuable strategy could be the use of hypofractionated schemes in a sequential manner to chemotherapy.

Table2 Hypofractionated radiotherapy for locally-advanced non-small cell lung cancer
NEOADJUVANT AND POSTOPERATIVE RADIOTHERAPY IN NON-SMALL CELL LUNG CANCER
The neoadjuvant approach (radiotherapy +/- chemotherapy followed by surgery) in stage III NSCLC (stage IIIA and,in some cases,potentially-resectable stage IIIB) is highly complex and should only be performed in centres with multidisciplinary experience.Although some phase 2 trials conducted at centres of excellence have reported excellent 5-year survival rates (up to 50%) with low morbidity and mortality,a recent meta-analysis found no significant survival benefit for neoadjuvant CRT plus surgery versus CRT alone[49].For this reason,most international guidelines that have explored the optimization of radiotherapy resources during the COVID-19 pandemic have largely omitted any discussion of the neoadjuvant approach.Even the recommendations of the Memorial Sloan-Kettering Cancer Center,a well-known supporter of neoadjuvant chemotherapy in selected stage III patients,do not include this indication[50].The Fox Chase Cancer Center guidelines comprehensively address the treatment of NSCLC during the COVID-19 pandemic stating “Patients with resectable disease can be treated with definitive non-operative management if surgical resources are limited or the risks of perioperative care are high...”[51].Nevertheless,it is essential that the treatment approach be individualised within the parameters set by an interdisciplinary tumour board.For example,after induction therapy,the patient should undergo surgery within 4-12 wk,keeping in mind the high risks and high resource utilization in surgery departments.The final decision to perform surgery will ultimately depend on the phase of the pandemic and the impact of COVID-19 on surgery departments.Clearly,surgery is more feasible in the initial phases of the pandemic,whereas in advanced phases hospital resources are likely to be limited.If thoracic surgery is considered,all patients (even those who are asymptomatic) should undergo a CT scan and be tested for COVID-19 to check for the presence of bilateral pulmonary infiltrates.
In patients in whom neoadjuvant CRT is indicated,the GOECP/SEOR recommends,if possible,the use of radical CRT alone,without surgery.The GOECP/SEOR also recommends against performing induction chemotherapy as this is a suboptimal cancer treatment unless subsequent radical local treatment (surgery or radiotherapy)can be guaranteed.
Postoperative radiotherapy (PORT) has several evidence-based clinical indications[52].PORT is recommended in patients with NSCLC with involvement of multiple nodal stations (pN2) and/or capsular rupture,according to the explicit indication of the ESTRO-ASTRO guidelines[3].These same guidelines note there is a strong consensus (82% of experts) that PORT could be delayed for 4-6 wk,with a recommended dose of 54 Gy at standard fractionation (2 Gy per fraction).Those guidelines do not recommend hypofractionated regimens in these patients due to the increased risk of morbidity and mortality.
Various international guidelines,such as those published by the MSKCC,emphasize the clinical utility of PORT in patients with involved surgical margins[53,54],recommending a total dose of 54-60 Gy (1.8-2 Gy/fraction) to the high risk surgical bed.Other guidelines advise a shorter fractionation schedule (50 Gy in 25 fractions) to limit exposure to the hospital setting.However,some guidelines do not even specifically include PORT among the recommendations[55].
The GOECP/SEOR believes that PORT,as an adjuvant treatment,should be deferred,whenever possible,until the current pandemic is under control.In our view,PORT is indicated in patients with involved margins (including massive capsular rupture) and in patients with multi-station mediastinal node involvement,with a recommended dose of 60 Gy in the former group and 50-54 Gy in the latter,in both cases using standard fractionation schedules (2 Gy/fraction),assuming that patients have good PS (0-1) and appropriate functional tests.Indications for other clinical scenarios,such as involvement of the parietal pleura and chest wall without clearly affected margins,are more ambiguous due to the scant published data;these cases should be presented to the weekly tumour board and discussed with pathologists and thoracic surgeons.As a general rule,we do not recommend hypofractionation in these patients due to the risk of toxicity.In any case,as with any adjuvant therapy,each case must be evaluated individually to assess potential benefits and risks.If the likely benefit in local control and/or survival is modest,then treatment should be delayed.Finally,in patients who test positive for COVID-19 during treatment,radiotherapy(both neoadjuvant and PORT) should be interrupted.
STEREOTACTIC BODY RADIOTHERAPY IN PATIENTS WITH OLIGOMETASTIC NON-SMALL CELL LUNG CANCER
It is estimated that 60%-70% of patients with NSCLC are diagnosed with stage IV disease and,of these,approximately 20% meet criteria for oligometastatic disease[56],although this percentage may be even higher,as staging techniques such as positronemission tomography become more widely used.Oligometastatic disease has two main forms of presentation:“de novo” oligometastasis (≤ 3-5 lesions at diagnosis) or“induced” oligometastasis (persistence of ≤ 3 lesions after treatment).
The concept of induced oligometastatic disease was recently described by Guckenbergeret al[57],who subclassified this into three clinical presentations:(1)Oligopersistence:<5 lesions persisting after systemic treatment;(2) Oligoprogression:Progression in ≤ 3 sites after systemic treatment;and (3) Oligoresistance:Response to systemic treatment,with evidence of disease in ≤ 3 sites.
Studies have shown that the use of local therapy to treat patients with oligometastatic disease can improve overall survival[58].Three prospective trials evaluated subgroups of patients with oligometastatic disease at diagnosis[59-61],finding that metastatic patients who respond to-or who do not develop disease progressionafter systemic treatment are more likely to benefit from local therapies such as radiotherapy.Although immunotherapy was not considered within the treatment arms in those trials,data from more recent trials that did assess the role of immunotherapy have changed the treatment paradigm for patients with metastatic disease[62],with numerous clinical trials currently underway to assess the immunotherapy combined with local radiation therapy.
In the current pandemic,our recommendation is to identify the “true”oligometastatic patient,defined as the patient who,after systemic treatment,shows a response or at least no evidence of progression.If the event that the conditions related to the pandemic did not allow for systemic treatment,then patients with the best prognosis should be selected for locally ablative therapy.In retrospective series of patients with oligometastatic disease,the factors most consistently associated with better prognosis were as follows:Gender (malevsfemale);histology (adenocarcinomavsother histologies);presentation (metachronousvssynchronous);performance status(PS 0-1vsthe rest);the number of metastatic lesions (1vs2-3vsthe rest);size (<3 cmvsthe rest);and location (lung and bonevsadrenal glands and lymph nodesvsother sites)[63-69].
The most appropriate SABR/SBRT treatment regimen will depend on the characteristics of each patient,although the objective should always be to offer the treatment approach that most limits the patient’s exposure to the hospital environment and-depending on the metastatic location-the treatment with the lowest risk of toxicity.It is important to stress that,depending on the tumour localisation a more or less aggressive regimen can be selected.The most common sites for metastases in patients with stage IV NSCLC are the brain,lung,liver,bones,and adrenal glands.Table 3 shows the currently accepted doses and fractions for the treatment of these different metastatic locations[70-82].
RADIOTHERAPY IN PATIENTS WITH SMALL-CELL LUNG CANCER
Small-cell lung cancer (SCLC) accounts for 13% of all lung cancers.The standard treatment in limited stage SCLC (LS-SCLC) is concomitant radiotherapy initiated in the first or the second cycle of chemotherapy.Consolidation thoracic radiotherapy has become widely used in clinical practice in patients with extensive stage SCLC (ESSCLC) who show a good response to systemic treatment.In both of the aforementioned clinical scenarios,prophylactic cranial irradiation (PCI) is recommended in responders.New treatments such as immunotherapy are being progressively incorporated into the therapeutic armamentarium for SCLC,although more slowly than in NSCLC[83].
Limited-stage SCLC
A key lesson that has been learned in recent years with regard to the management of SCLC is that time is crucial factor in treatment outcomes due to the aggressive nature of this histological subtype,which has a rapid doubling time,a tendency towards early dissemination,and-in some cases-rapid symptom onset.For this reason,curativeintent treatment should not be delayed by more than 4-6 wk;however,in patients with COVID-19,radiotherapy should be deferred,when possible,until the patient is asymptomatic and tests negative for the disease.If the diagnosis of COVID-19 is made when the patient has already started radiotherapy,the decision to interrupt treatment should consider all relevant factors,including the presence of symptoms (due to the tumour or virus),and the phase of treatment (i.e.,closer to the start or finalisation of radiotherapy).
The standard recommendation for treatment sequencing in terms of early concomitant therapy in the first and second cycles of chemotherapy should be reviewed in these circumstances.Studies have shown that overall survival outcomes are better when radiotherapy is administered in the first eight weeks after chemotherapy[84]and in patients in which the start and completion of radiotherapy is less than 30 d[85].Recent studies suggest that radiotherapy has a similar therapeutic efficacy when administered with the third cycle of chemotherapy,a finding that implies that a slight delay is acceptable if the start of radiotherapy coincides with the onset of clinical manifestations of COVID-19[86,87].
Fractionation plays a key role in the treatment of lung cancer.In general,we do not recommend modifying the standard CRT regimens:45 Gy in 30 fractions for 3 wk (2 fractions of 1.5 Gy/day)[88]or 60-66 Gy in 30-33 fractions for 6 to 6.5 wk[89].However,the individual radiation oncology department must decide whether they prefer to extend the duration of treatment (one fraction/day) or shorten it (two fractions/day),with the latter implying an increased exposure to the hospital environment.The choice will depend on factors such as the effectiveness of prevention measures against COVID-19 or the logistical capacity of the department to administer two daily sessions.
Although only scant evidence is available to support hypofractionated regimens in LS-SCLC,this approach could merit consideration based on the extrapolation of results obtained in patients with NSCLC using either 45 Gy or 40-42 Gy delivered in 15daily fractions,Table 4.The administration of concomitant chemotherapy should be carefully evaluated,including the possibility of administering systemic treatment sequentially to reduce the risk of significant toxicity.Given that SCLC often presents as a centrally-located bulky mass,these regimens should only be considered in wellselected patients in the context of resource restrictions related to the pandemic in which standard treatment is not possible[50,90,91].In patients with early stage LS-SCLC(T1-T2 N0M0),SBRT at doses ranging from 50-60 Gy in 5 fractions is always an option[92].
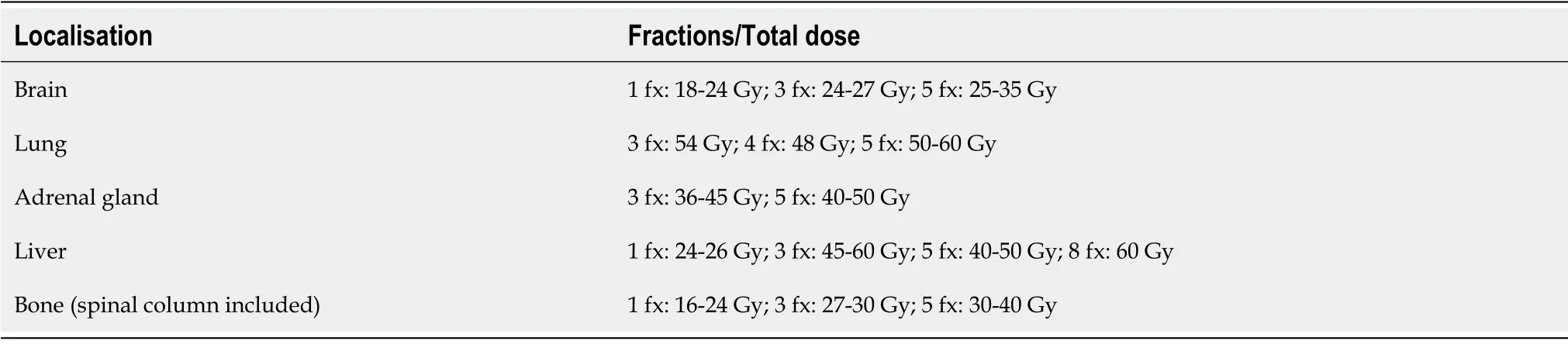
Table3 Stereotactic body radiotherapy schemes for oligometastatic non-small cell lung cancer
Prophylactic cranial irradiation at a dose of 25 Gy in 10 fractions has been shown to reduce the risk of symptomatic brain metastases and to improve survival[93,94],and therefore this fractionation schedule should not be modified,even in the present pandemic[95].Although PCI for LS-SCLC must remain the standard recommendation during the pandemic,PCI could be delayed if brain MRI is used to closely monitor the patient,an approach that has been used in patients with ES-SCLC[96].If we postpone PCI (due to the pandemic) until CRT has finalized,we would have two months to administer PCI after completing CRT.However,if instead of administering PCI,we decide to monitor the patient with brain MRI,then the minimum duration of MRIbased follow-up should be two years,performed every three months the first year and every six months the second year,as indicated in the protocol of the Japanese trial[96].In COVID-19 positive patients,PCI should be delayed or interrupted until the patient is asymptomatic and tests negative for the disease.
Extensive-stage SCLC
Consolidative thoracic radiotherapy (CTRT) has been shown to improve survival in patients with ES-SCLC who show a significant response to chemotherapy[97].PCI has also been shown to reduce symptomatic metastases and may also improve survival in this subgroup[98].Indeed,in recent years,CTRT and PCI have both been added to the therapeutic armamentarium of radiation oncology departments.However,during the present pandemic,omitting CTRT could reasonably be considered in patients with a complete pulmonary response to chemotherapy;in selected cases with tumour persistence,the traditional regimen (30 Gy in 10 fractions) could be considered.In general,our recommendation is to avoid PCI,preferring instead brain MRI to monitor brain lesions[96].
PALLIATIVE RADIOTHERAPY IN LUNG CANCER
In the context of the current pandemic,it is essential to consider the following points regarding palliative-intent radiotherapy:(1) A detailed risk/benefit analysis should be performed;(2) Consider possible repercussions of delaying radiotherapy if the patient’s clinical condition allows;(3) Consider therapeutic alternatives;(4) Palliative radiotherapy is not indicated in patients with life expectancy <3 mo;and (5) When indicated,the maximum hypofractionated dose should be applied to reduce hospital visits.
For palliative thoracic radiotherapy (to treat hemoptysis,severe cough,dyspnea secondary to bronchial obstruction,or atelectasis),it is preferable to offer the most hypofractionated regimen possible,such as 20 Gy in 5 fractions ,17 Gy in 2 fractions ,or a single fraction of 10 Gy[99-102],Table 5.
In patients with vena cava syndrome,alternatives to radiotherapy (e.g.,endovascular stent,thrombolysis,etc.) should be considered.If these alternatives arenot possible,then one of the hypofractionated radiotherapy regimens described above can be administered.
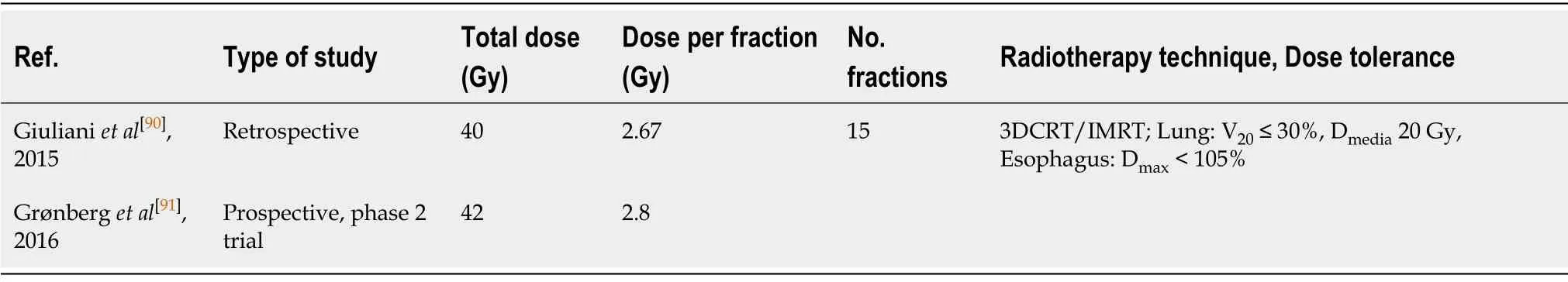
Table4 Hypofractionated radiotherapy for limited stage small-cell lung cancer

Table5 Palliative radiotherapy in lung cancer
To manage bone metastases,Table 5,the first step is to compare radiotherapy to other treatment options (e.g.,modification of analgesic therapy,bisphosphonates,etc.)to ensure that radiotherapy is the best option.If so,the available evidence indicates that a single fraction of 8 Gy is as effective as more fractionated regimens[103].Indeed,a recent trial also evaluated this regimen to treat metastatic spinal cord compression[104]and some recommendations[105]support this regimen.For pathological fractures,PORT is not recommended during the pandemic.
The use of whole brain radiotherapy to treat multiple metastases is controversial,in part because a study performed by Mulvenna and colleagues demonstrated that a similar quality of life can be achieved in many cases with corticosteroids treatment alone[106].However,those authors also showed that whole brain radiotherapy appears to improve survival in a well-defined patient subgroup (age <60 years,good PS,controlled primary tumour).Consequently,a hypofractionated regimen consisting of 20 Gy in 5 fractions would be an appropriate recommendation in this patient subgroup,Table 5.
In patients with COVID-19 scheduled to undergo palliative radiotherapy,it is better to delay or suspend treatment when possible until the patient has clinically recovered(negative PCR test).However,radiation therapy may be necessary in COVID-19+patients who present potentially life-threatening symptoms,such as spinal cord compression or grade 4 vena cava syndrome,where the risk of delay is too great.
CONCLUSION
All recommendations of the GOECP should be considered in accordance with the resources available at the treating centre.A summary of the international guidelines,expert consensus,and the recommendations of the present manuscript are shown in Table 6.

Table6 Summary of recommendations of the main clinical guidelines and of the GOECP/SEOR for lung cancer radiotherapy during the coronavirus disease 2019 pandemic
 World Journal of Clinical Oncology2020年8期
World Journal of Clinical Oncology2020年8期
- World Journal of Clinical Oncology的其它文章
- B-cell lymphoma-2 inhibition and resistance in acute myeloid leukemia
- Combination drug regimens for metastatic clear cell renal cell carcinoma
- Circular RNA and its potential as prostate cancer biomarkers
- Statins in risk-reduction and treatment of cancer
- Novel molecular targets in hepatocellular carcinoma
- Effectiveness of a novel,fixed dose combination of netupitant and palonosetron in prevention of chemotherapy induced nausea and vomiting:A real-life study from India
