Effectiveness of a novel,fixed dose combination of netupitant and palonosetron in prevention of chemotherapy induced nausea and vomiting:A real-life study from India
Bharat Vaswani,Medical Oncology,Yashoda Cancer Institute,Secunderabad 500003,India
Sagar Bhagat,Saiprasad Patil,Hanmant Barkate,Medical Services,Glenmark Pharmaceutical limited,Mumbai 400099,India
Abstract
Key words:Chemotherapy induced nausea vomiting;Netupitant;Palonosetron;Cancer;Chemotherapy
INTRODUCTION
Chemotherapy induced nausea and vomiting (CINV) is one of the most feared adverse events in various cancer chemotherapy regimens[1].Evidence suggests that the incidence of acute CINV varies from 30% to 90% and that of delayed CINV is reported to be 28%-50%[2-4].Rates of nausea (28.8% to 53.5%) and vomiting (9.4% to 19.2%) in the overall phase reported from Asia Paicifc region after first cycle of chemotherapy were varied[5].A study from North India observed the CINV prevalence of 25.5%[6].These data suggest that CINV may affect upto half of all the patients receiving highlyemetogenic or moderately-emetogenic chemotherapies (HEC/MEC).
Pathomechanistically,serotonin and substance P are major neurotransmitters involved in acute and delayed CINV.Serotonin binds to 5HT3 receptor present mainly in the gastrointestinal tract and Substance P binds with neurokinin-1 (NK1) receptors in the nucleus tractus solitarius and induces vomiting.Therefore,targeting serotonergic and neurokinin pathways are helpful in prevention of CINV[7].The European Society of Medical Oncology and the Multinational Association of Supportive Care in Cancer guidelines recommend 5HT3 receptor antagonist,dexamethasone and NK1 receptor antagonist in acute CINV,whereas later two are advised in delayed CINV[8].Recently,a new,oral fixed dose combination (FDC) of netupitant (highly selective NK1 receptor antagonist,300 mg) with Netupitant and palonosetron (5HT3 receptor antagonist,0.5 mg) (NEPA) was approved in India[9].NEPA + DEX has been found to be clinically superior to monotherapy of palonosetron+ DEX in preventing both acute and delayed CINV[10,11].Being a recent and novel FDC antiemetic with limited evidence in Indian setting,there is need to further understand its efficacy and safety.Hence,we planned this observational study to determine efficacy of NEPA in prevention of CINV.
MATERIALS AND METHODS
Study design
This single-centre,retrospective study was conducted in patients treated with HEC/MEC.
Ethics
Study was initiated after the approval from independent ethics committee and was conducted according to good clinical practice and applicable regulatory guidelines.
Setting
This study was conducted in tertiary care centre in Hyderabad,India.This centre provides super-specialty services in management of various malignancies.It caters to the urban,semi urban and rural population.
Participants
Adults aged >18 years of either sex who were treated with HEC/MEC and prescribed NEPA irrespective of the number of chemotherapy cycles from June 2019 to December 2019 were identified from the patient database at our centre.Any patient treated with low-emetogenic chemotherapy or those who received chemotherapy with minimal emetogenic potential were excluded.
Treatment schedule in participants
After identifying the patients from the database,their demographic and baseline data mentioned in medical records was captured in structured case record form.Demographic data included age,gender,and clinical data on type of chemotherapy,current number of cycles,etc.were noted.As a standard practice,the given treatment schedule was followed in all patients for prevention of CINV.
Before initiating chemotherapy,all patients were treated with a single oral capsule of netupitant 300 mg and palonosetron hydrochloride 0.5 mg.After 60 min,chemotherapy was initiated.Dexamethasone (12 mg intravenous once) was concomitantly administered intravenously in all patients.Data on nausea and vomiting was captured by patients in patient diaries which were available with their medical records.From these diaries,events of nausea and vomiting were identified during first 24 h and over day 1 to day 5.Events that occurred within first 24 h were considered as acute CINV and those between day 2 and day 5 were considered as delayed CINV ( Figure 1).
Outcome measurement
The main outcome assessed was complete repose (CR) to NEPA.CR was defined as no emesis or no requirement of rescue medication.CR was determined in acute phase (0-24 h),delayed phase (24-120 h) and in overall phase (0-120 h).Overall CR was primary outcome measure.Effect of study drug was also evaluated by emetogenicity of chemotherapy as high and moderate as well as in by the cycle of chemotherapy.
在技术方面,我们常见的花鸟题材主要有三种画法,“工笔”“写意”还有“兼工带写”。这三种不同的画法是的花鸟题材以不同的表现形式绽放出其独特的美感。粉彩瓷绘画与中国国画有着密不可分的联系。当花鸟画的技术进入到粉彩瓷的领域中时,使得粉彩瓷的表现形式更加多样,再加上花鸟题材的点睛作用,是的粉彩瓷焕发了别样的生机。
RESULTS
Baseline characteristics
In total,403 patients were identified and analysed.Baseline characteristics of the study patients are shown in (Table 1).Mean age of the participants was 56.24 ± 11.11 years with majority being in age group of 51 to 65 years (51.36%).Proportion of females was slightly higher than males (55.09%vs44.91% respectively).Among study participants,most common malignancy was of breast (25.06%) followed by colon (15.63%),oral cavity (10.66%) and others as shown in (Table 1).54.6% patients had received HEC whereas remaining were treated with MEC.Also,patients were in different cycles of chemotherapy regimens as shown in (Table 1).
Outcome assessment
CR in overall population:For overall phase,the CR in our study was 93.79%.CR in acute and delayed phase CINV was 98.01% and 93.79% respectively (Table 2).
CR as per emetogenic potential of chemotherapy:We further analysed the CR according the chemotherapy regimen.In participants who received HEC (n= 220),overall CR was observed in 93.63% whereas 97.27% had CR in acute phase,and 93.63%had CR in delayed phase.Similarly,in patients receiving MEC (n= 183),overall response was seen in 93.98% whereas CR in acute and delayed CINV was 98.90% and 93.98% respectively.
CR as per number of chemotherapy cycles:All the enrolled participants were onvarious cycles of chemotherapy (Tables 1 and 3).Overall CR was 90% or more in all groups of chemotherapy cycles except in the group of patients with 4 cycles in whom overall CR was 83%.Similarly,the CR in acute CINV was over 90% in all chemotherapy cycle groups except patients who had 4 chemotherapy cycles in whom CR in acute CINV was 88.88%.Acute CINV CR was 100% in patients who had 5 chemotherapy cycles.CR in the delayed CINV phase was similar to overall CR in all chemotherapy cycle groups.

Table1 Baseline characteristics of enrolled patients
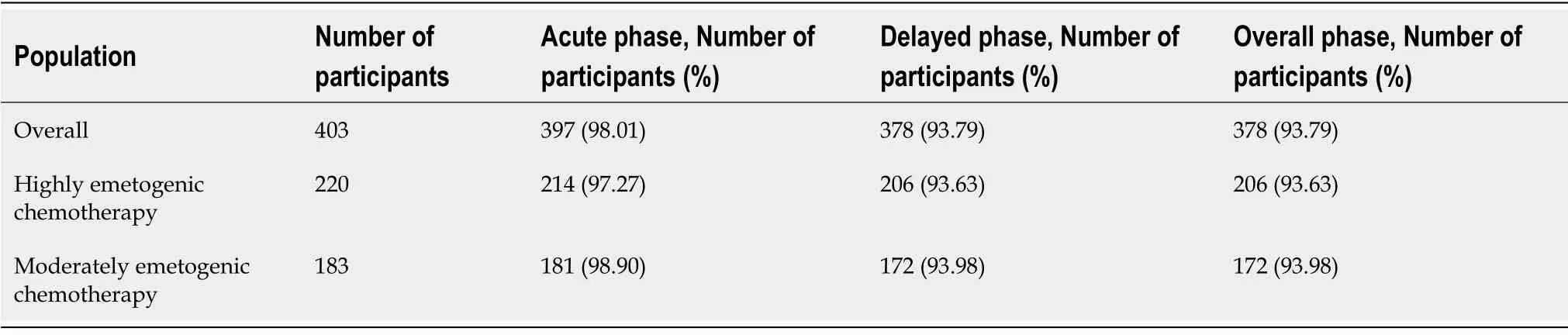
Table2 Outcome assessments

Table3 Complete response rate among enroled patients

Figure1 Study flow chart.
DISCUSSION
Combination of netupitant and palonosetron is first of its own kind FDC for prevention of CINV.In this study,we demonstrated that NEPA was effective in preventing CINV as shown by CR of 93.79% in overall and delayed phase with CR of 98.01% in acute phase.Compared to the finding of Heskethet al[10]who observed CR in 89.6% patients,CR in our study was substantially higher.This is probably attributable to the differences in participants in two studies as Heskethet al[10]included patients receiving HEC only.Badalamentiet al[12](2019) also reported overall CR in first chemotherapy cycle to be 88.9%[12].This indicates overall excellent efficacy of NEPA in preventing acute and delayed phase CINV.The combination has also been found to be more effective than monotherapy with palonosetron.In randomized,double-blind,study involving patients on MEC,Aaproet al[13]demonstrated that the CR in overall phase,acute phase and delayed phase was 74.3%,88.4% and 76.9% in NEPA group and 66.6%,85.0% and 69.5% with palonosetron monotherapy.Dexamethasone was coadministered in both treatment groups[13].Heskethet al[10]also reported NEPA was superior to palonosetron in preventing CINV in patients receiving HEC[10].This suggest that NEPA is highly effective in preventing CINV in any level of emetogenic chemotherapy.Further,CINV due to chemotherapy can lead to reduced quality of life,impairment in home and occupational activities,may add to increased cost and cause organ damage in the long run,preventing CINV is one of the primary goals of therapy[14-17].Therefore,single oral dose of NEPA can contribute the improved quality of life of patients receiving chemotherapeutic regimens.
We observed persistent CR in patients from different number of chemotherapy cycles suggesting that effectiveness of NEPA is not affected in repeated administration or initiating at any chemotherapy cycle.The overall CR in first cycle was similar to those who had more than five chemotherapy cycles.Similar finding was observed by Grallaet al[18]in evaluation of patients receiving HEC or MEC.They found consistent overall CR which was 81%,86%,91%,90%,92% and 91% in cycles 1,2,3,4,5,and 6 of chemotherapy[18].Combined with our observation,the evidence is clear that NEPA is highly effective in preventing CINV over multiple cycles of HEC/MEC.This has important clinical implications as single dose is effective and there is no need of repeat administration or rescue medications.With improved patient education,compliance to chemotherapy regimens can be improved substantially with appropriate intake of antiemetics[19].
We observe certain strengths and limitations in our study.Study has inherent limitations of retrospective design.We assessed the response acute and delayed phase but its efficacy in anticipatory,breakthrough,and refractory CINV in Indian population require further assessment.Although efficacy in low emetogenic chemotherapy was not assessed,NEPA is expected to be efficacious in these group of patients as it had proved its efficacy in HEC/MEC.Further,age and gender difference in efficacy as well as efficacy in different tumours can be assessed to identify population that can get most benefited with use of NEPA.Also,we did not compare the efficacy with existing therapies which would have provided more insights in understanding the benefits with NEPA.Nonetheless,our initial experience with NEPA suggests its effective utility in preventing CINV in HEC/MEC.
A novel FDC of netupitant and palonosetron has been approved for prevention of CINV.We observed that this FDC is effective in preventing CINV in patients receiving HEC/MEC with complete response rate of 93.79% with near complete response in acute phase of CINV.Also,the response was maintained irrespective of HEC or MEC administration as well as repose was consistent across number of chemotherapy cycles.Thus,in real-world setting,we find that NEPA is effective for preventing CINV over multiple cycles of highly or moderately emetogenic potential chemotherapy regimens.These finding need to be further confirmed in larger,randomized,comparative studies.
ARTICLE HIGHLIGHTS
ARTICLE HIGHLIGHTS
Research background Chemotherapy induced nausea and vomiting (CINV) is one of the most feared adverse events with patient receving chemotherapy regimens.Pathomechanistically,serotonin and substance P are major neurotransmitters involved in acute and delayed CINV,targeting both optimizes CINV control.NEPA,an oral fixed dose combination Netupitant (300 mg) and Palonosetron (0.50 mg),was recently approved in India for the management of CINV.He nce th ere was a need to evaluate the effectiveness of NEPA in Indian setting in real worl d scenario.
Research motivation
To analyse the effectiveness of NEPA in prevention of CINV among Indian patients who have received highly an d moderately emetogenic chemotherapy regimen.
Research objectives
To elucidate the clinical effectiveness of NEPA,in terms of the complete response in acute-delayed and overall phase of nausea-vomiting irrespective of the chemotherapy cycle.Thereby,we hope to generate the real world evidnce on the usefulness of NEPA in the management of C INV patients in India.
Research methods
Medical records and patient diaries of adults cancer patients who were treated with highly emetogenic or moderately emetogenic chemotherapy and received NEPA irrespective of the number of chemotherapy cycles from June 2019 to December 2019 were retrieved.Relevant clinical variables such as presence or absence of nauseavomiting and if present,the severity of nausea on visual analog scale and cycle wise distribution of the data were captured.
Research results
The study demonstrated that complete response in overall phase was 93.79% whereas it was 98.01% in acute CINV and 93.79% in delayed CINV.Overall complete response in highly emetogenic chemotherapy group of patients was 93.63% and in moderately emetogenic group of patients was 93.98%.
Research conclusions
We found that the oral fixed dose combination of netupitant 300 mg and palonosetron hydrochloride 0.5 mg is effective in preventing CINV in patients receiving highly or moderately emetogenic chemotherapy regimen in the real world setting.Also,the response was consistent across number of chemotherapy cycles.
Research perspectives
This study demonstrated the clinical effectiveness of NEPA among Indian patients in managing CINV,and serves as an impetus for future research.
 World Journal of Clinical Oncology2020年8期
World Journal of Clinical Oncology2020年8期
- World Journal of Clinical Oncology的其它文章
- Intravascular lymphoma with hypopituitarism:A case report
- Proton beam therapy of periorbital sinonasal squamous cell carcinoma:Two case reports and review of literature
- Concurrent renal cell carcinoma and hematologic malignancies:Nine case reports
- Management of neuroblastoma in limited-resource settings
- Mutational analysis of Ras hotspots in patients with urothelial carcinoma of the bladder
- Novel molecular targets in hepatocellular carcinoma
