Analysis of Rhizosphere Microbial Community Structure in Different Pathogenesis Stages of Watermelon Fusarium Wilt
HE Ying, ZHANG Yi, ZHU Fei-ying, XIAO Ji-ling, WEI Lin, TANG Yan-ying, LIANG Zhi-huai*
1. College of Longping, Graduate School of Hunan University, Changsha 410125, PRC;
2. Hunan Agricultural Biotechnology Research Institute, Changsha 410125, PRC;
3. Hunan Institute of Plant Protection, Changsha 410125, PRC
Abstract High-throughput sequencing technique was applied to analyze the microbial community structure of rhizosphere soil at different stages of watermelon fusarium wilt to find out the difference of dominant microbial community in rhizosphere during the occurrence of watermelon fusarium wilt. Illumina-Hiseq high-throughput sequencing platform was used to sequence 16S and ITS rDNA in rhizosphere soil. The soil was named CK1 before planting, CK2 at peak stage and CK3 at stable stage. The results showed that the soil bacterial diversity was in the order of CK1> CK3> CK2, indicating no significant difference between CK1 and CK3 and a significant difference between CK2 and CK1, CK3. At the genus level, the dominant bacteria were Mizugakiibacter (21.929 9%), Rhodanobacter (5.093 3%), and Lactobacillus (3.192 1%). The diversity of soil fungi was in the order of CK1> CK3> CK2, all showing significant differences. At the genus level, the dominant fungus was Lysurus (54.460 1%), Papulaspora (12.425 2%), Acrophialophora (3.172 9%). The results showed that the diversity and abundance of bacteria and fungi in rhizosphere soil decreased during the peak period of watermelon fusarium wilt. With the gradual stabilization of the disease, the diversity and abundance of bacteria and fungi in rhizosphere soil recovered to a certain extent.
Key words Watermelon fusarium wilt; Soil bacterial diversity; Soil fungal diversity; High-throughput sequencing
1. Introduction
Watermelon fusarium wilt is a major soil-borne fungal disease infected by watermelon Fusarium oxysporum which would cause a reduction in the quality and production of watermelon. It is of great significance to study the diversity of microbial community of rhizosphere soil at different stages of watermelon fusarium wilt, analyze the species and abundance variation of dominant bacteria of watermelon plants in rhizosphere soil at the different stages, identify the major microbial species during the peak period of watermelon fusarium wilt, and explore the rhizosphere effect so as to prevent watermelon fusarium wilt and promote the sustainable development of watermelon industry. Recent findings showed that rhizosphere microorganisms have close relationship with the resistance of plants to soil-borne diseases[1]. The disease resistance of crops may come from specific microbial communities in their rhizosphere soil, especially microbial communities with antagonistic effect[2]. Therefore, the health level of watermelon plants might be connected with the microbial community structure, diversity and dominant bacterial community of the rhizosphere soil[3]. For instance, the diversity of microorganisms in rhizosphere soil at different growing stages of watermelon plants received different impacts of continuous cropping[4]. However, there have been no systematic and specific researches on the microbial community of rhizosphere soil at different stages of watermelon fusarium wilt. Based on previous experiments, this study used the watermelon greenhouse soil for planting watermelon Zaojia (8424) with 5 years continuous cropping as the material, adopted the high-throughput sequencing technique to measure the diversity of microorganisms in rhizosphere soil, and analyzed the species and functions of dominant bacteria of sick plants in rhizosphere soil in an attempt to discuss the relationship between watermelon fusarium wilt and microbial community structure of rhizosphere soil and provide scientific and reliable theoretical basis for the development of biological approach to overcome continuous cropping obstacles of watermelon.
2. Materials and Methods
2.1. Test materials
Watermelon Zaojia (8424) was used as the test variety; the test soil was obtained from the 5 years continuous cropping watermelon greenhouse of Gaoqiao Test Base of Hunan Academy of Agricultural Sciences.
2.2. Experimental design
Mix the test soil fully and put them separately into 40 cm×30 cm×30 cm pots, each pot with 15 kg soil, repeat five times. Adopt conventional ways for pregermination and seeding, sow 30 watermelon seeds in each pot. Conduct routine administration after sowing.
2.3. Sample collection
Use multipoint sampling method to collect rhizosphere soil sample at the depth of 5~15 cm, including the soil of CK1 before planting, CK2 at peak stage and CK3 at stable stage, according to different development stages of watermelon fusarium wilt. Then sieve the soil samples through 20 meshes and preserve them under -70℃ for further tests of their physicochemical properties and soil microbial community structure and diversity. The physicochemical properties were tested by Hunan Agrochemical Center.
2.4. Data processing and analysis
The microbial diversity of rhizosphere soil was tested by Novogene Co., Ltd. Illumina-Hiseq high-throughput sequencing technique was used to sequence and analyze 16S and ITS rDNA in CK1, CK2 and CK3 totally 15 soil samples.
The experimental process is as follows: DNA extraction, sampling and testing, PCR amplification, product purification, library preparation and inspection, and Hiseq sequencing. The primers for bacteria 16S rDNA amplification are 5'-GTGCCAGCMGCC GCGG-3' for 515F and 5'-CCGTCAATTCMTTTRAG TTT-3' for 907R, respectively; the primers for fungi ITS1-ITS2 amplification are 5'-GGAAGTAAAAGT CGTAACAAGG-3' for 1737F and 5'-GCTGCGTTC TTCATCGATGC-3' for 2043R, respectively.
Clean data was acquired after splicing and filtering the raw data obtained from sequencing. Then OTU (Operational Taxonomic Units) clustering and taxonomic analysis was conducted based on the clean data. After sequence optimization analysis, the data obtained from Illumina-Hiseq sequencing was read and spliced. The samples, after quality inspection, were differentiated according to the barcode labels at two ends of the sequences and their primers sequences to obtain the effective sequences. The direction of the sequences was rectified, and the optimized sequences of different samples were classified under the similarity level of 97% for bioinformatics statistical analysis. The diversity of bacteria was then analyzed according to the Alpha Diversity indexes (Shannon, Simpson, Chao, ACE, Coverage) of different samples under the consistency threshold of 97%.
3. Results and Analysis
3.1. Physicochemical properties of soil for testing
The test soil was 5 years watermelon continuous cropping acidic sandy soil; soil organic matter: 27.3 g/kg; total nitrogen: 1.98 g/kg; total potassium: 22.7 g/kg; total phosphorus: 0.83 g/kg; available nitrogen: 0.16 g/kg; available potassium: 0.16 g/kg; available phosphorus: 0.12 g/kg; pH value: 5.3.
3.2. Analysis of soil bacterial diversity at different stages of watermelon fusarium wilt
3.2.1. Analysis of soil bacterial diversity and abundance indexes
Table 1 showed the statistics of the Alpha Diversity indexes of soil bacterial diversity and abundance. As shown, all the Coverage indexes were above 99%. Specifically, the soil bacterial diversity throughout the watermelon fusarium wilt was CK1> CK3>CK2, and the soil bacterial abundance was CK3>CK1>CK2. There was no significant difference between CK1 and CK3, and there was a significant difference between CK2 and CK1, CK3. The results showed that the diversity and abundance of bacteria and fungi in rhizosphere soil decreased during the peak period of watermelon fusarium wilt. With the gradual stabilization of the disease, the bacteria diversity and abundance in rhizosphere soil recoveredto a certain extent.
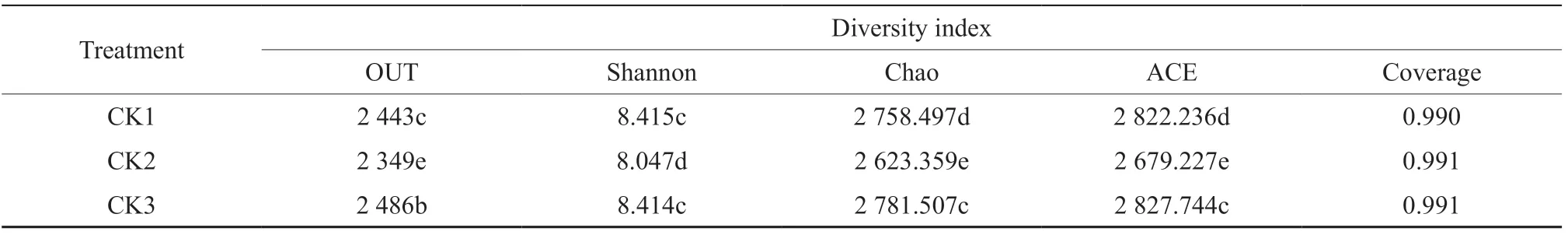
Table 1 Comparative analysis of soil bacterial diversity and abundance
3.2.2. Principal component analysis of soil bacterial community structure
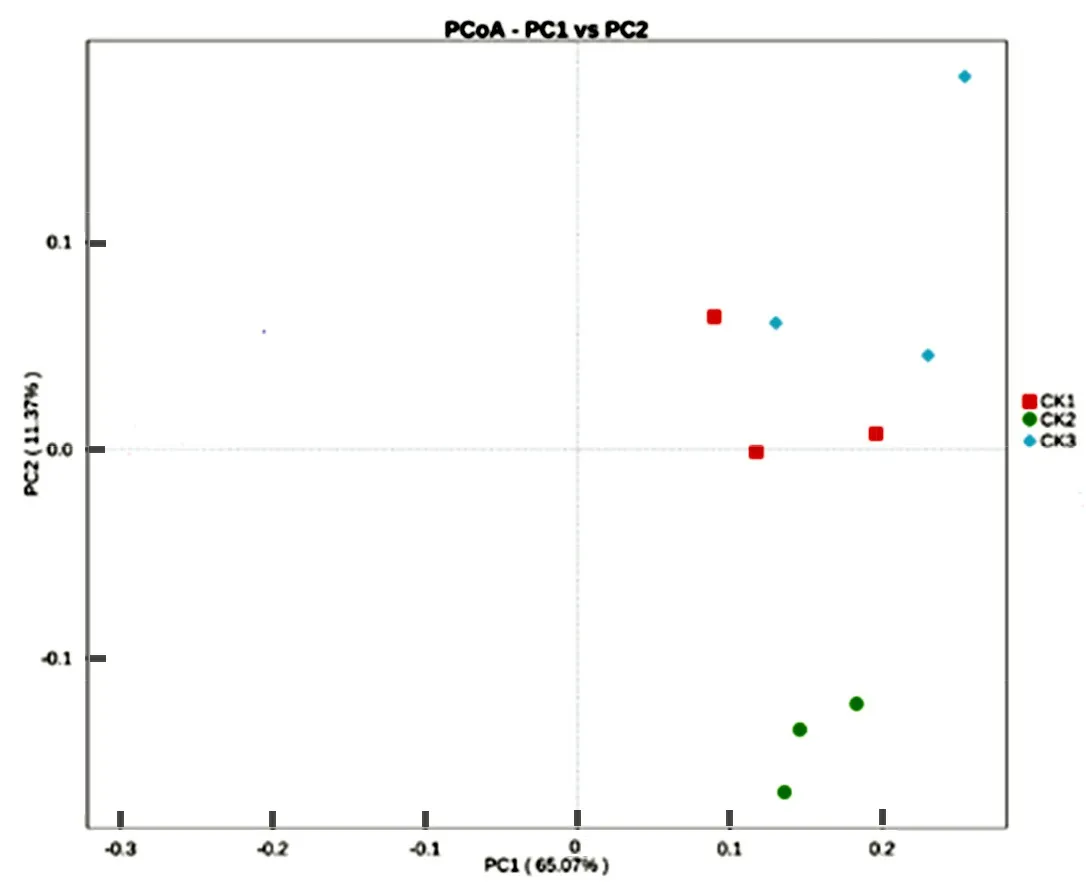
Fig.1 Principal component analysis of soil bacterial community
Fig.1 showed that the cumulative variance contribution rate of the first two principal components of the soil bacterial community was 76.44%, which explains 65.07% and 11.37% of the variable variance, respectively. This indicated that these two principal componentd (PC1, PC2) were principle factors to explain the difference of the bacterial community. Specifically, there was a small distribution distance between CK1 and CK3, and a large distribution distance between CK2 and CK1, CK3, showing a good differentiation between CK2 and CK1, CK3 on PC2 axle. Therefore, PC2 was regarded as the principle component causing the difference of soil bacterial community between CK1, CK2 and CK3.
3.2.3. Diversity analysis of soil bacterial community structure
As shown in Fig.2, of the top 30 rhizosphere soil bacteria species in relative abundance at the genus level, the abundance of Mizugakiibacter, Lactobacillus, Gemmatimonas, Gemmatirosa, Pseudomonas, Pseudoxanthomonas, [Polyangium]-brachysporum_group, and Turicibacter at the peak stage of watermelon fusarium wilt all increased by more than 0.1%, whereas the abundance of Rhodanobacter, Flavisolibacter, Sphingomonas, Frateuria, unidentified Chitinophagaceae, Dyella, Bryobacter, Alistipes, Alicyclobacillus, and Isosphaera at the peak stage of the disease all decreased by more than 0.1%. Specifically, Mizugakiibacter was the most dominant bacteria in CK1, CK2 and CK3 soils, the abundance of which increased by more than 6%, occupying 15.899 1%, 21.929 9% and 13.885 6% in CK1, CK2 and CK3, respectively; secondly, the abundance of Lactobacillus at the peak stage increased by more than 2%, occupying 0.759 6%, 3.192 1% and 0.665 2% respectively in CK1, CK2 and CK3; the abundance of Pseudoxanthomonas at the peak stage of the disease increased by more than 1%, occupying 0.827 8%, 2.216 3% and 0.395 4% in CK1, CK2 and CK3, respectively. Due to different cardinal numbers of rhizosphere soil bacteria at different stages of watermelon fusarium wilt, the relative abundance variations may not reflect the real changes of the bacteria species but may provide certain reference for identifying the relationship between the microbial community structure of rhizosphere soil and the occurrence of watermelon fusarium wilt.
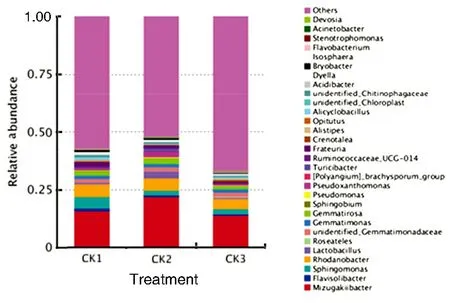
Fig.2 The relative abundance of top 30 species of bacteria community at the genus level
3.3. Analysis of soil fungal diversity at different stages of watermelon fusarium wilt
3.3.1. Analysis of soil fungal diversity and abundance indexes
Table 2 showed the statistics of the Alpha Diversity indexes of soil fungal diversity and abundance. As shown, the soil fungal diversity throughout the watermelon fusarium wilt was CK1>CK3>CK2, and the soil fungal abundance was CK1>CK3>CK2, all presented significant difference. The results showed that the diversity and abundance of bacteria and fungi in rhizosphere soil decreased during the peak period of watermelon fusarium wilt. With the gradual stabilization of the disease, the fungal diversity in rhizosphere soil recovered to a certain extent.
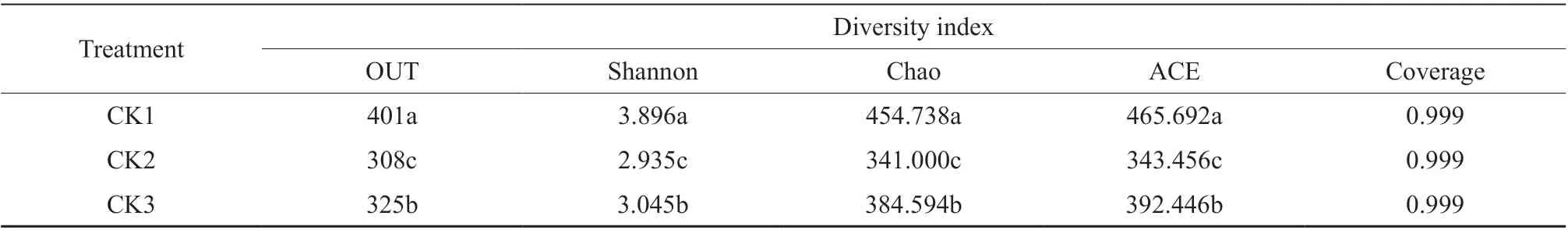
Table 2 Soil fungal diversity and abundance
3.3.2. Principal component analysis of soil fungal community structure
Fig.3 showed that the cumulative variance contribution rate of the first two principal components of the soil fungal community was 33.4%, which explains 20.64% and 12.76% of the variable variance, respectively. This indicated that these two principal components (PC1, PC2) were the principle factors to explain the difference of the fungal community. Specifically, there was a large distribution distance between CK1, CK2 and CK3 with good differentiation on PC1 axle, showing that PC1 was the principle component causing the difference of soil fungal community between CK1, CK2 and CK3; the distribution distance between CK1 and CK3 was small; the distribution distance between CK2 and CK1, CK3 was large with good differentiation on PC1 axle, indicating that PC1 was the principle component causing the difference of soil bacterial community at different stages of watermelon fusarium wilt.

Fig.3 Principal component analysis of soil fungal community
3.3.3. Diversity analysis of soil fungal community structure
As shown in Fig.4, of the top 30 rhizosphere soil fungal species in relative abundance at the genus level, the relative abundance of Papulaspora, Acrophialo- phora, Emericellopsis, Fusarium, Penicillium, Aspergillus, Cephalotheca, Chaetomium, Engyodontium, Thielavia, Trichoderma, Spizellomyces, Hortaea, and Rhizopus at the peak stage of watermelon fusarium wilt all increased by more than 0.1%, while the abundance of Tomentella, Monosporascus, Lophodermium, Rhizopycnis, Kohlmeyeriopsis, Populocrescentia and Corynespora at the peak stage of the disease was zero. Specifically, the abundance of Lysurus increased significantly, which occupied 0.555 9%, 54.460 1% and 0.169 8% in CK1, CK2 and CK3, respectively; the abundance of Papulaspora at the peak stage decreased significantly, which occupied 38.503 2%, 12.425 2% and 42.858 3% in CK1, CK2 and CK3, respectively. Therefore, due to different cardinal numbers of rhizosphere soil fungi at different stages of watermelon fusarium wilt, the relative abundance variations may not reflect the real changes of the fungal species but may provide certain reference for identifying the relationship between the microbial community structure of rhizosphere soil and the occurrence of watermelon fusarium wilt.
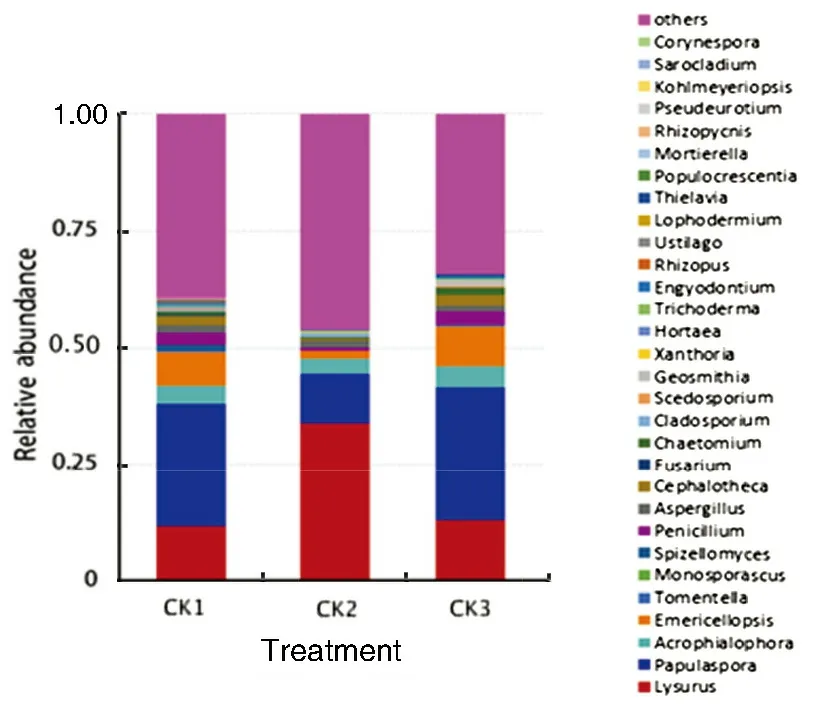
Fig.4 The relative abundance of top 30 species of fungal community at the genus level
4. Discussion
The analysis of the relative abundance of top 30 species of bacteria community at the genus level (Fig.2) showed that the bacteria with a significant increase in abundance during the peak period of watermelon fusarium wilt are sensitive to changes in soil properties, the mechanism of which are mainly applied for wastewater treatment[5], soil remediation agent and soil improvement agent[6]. The bacteria with a decrease in abundance during the peak period of watermelon fusarium wilt can degrade toxic substances in the soil to improve the soil environment[7], or secret a variety of carbohydrates[8]and promote the use of glycogen in plants[9]. Some bacteria can activate the plant immune system or directly inhibit the growth of plant pathogens[10]. For example, Sphingomonas can improve the rhizosphere environment of watermelon, reduce the continuous cropping obstacles of watermelon, and promote the growth of watermelon plants[11]. Hence, this bacterial genus has certain ability to degrade harmful substances in soil, directly promote the growth of plants, and it can be used to achieve a good prevention and control effect. However, few cases were reported in dealing with watermelon fusarium wilt through this bacterial genus, the prevention and control mechanism of which needs further discussions.
The analysis of the relative abundance of the top 30 species of fungal community at the genus level (Fig.4) showed that a small number of fungi had a significant increase in relative abundance during the peak period of watermelon fusarium wilt. Some of these fungi have certain antifungal bioactivity (such as Lysurus)[12], some can significantly promote the growth of plants (such as Cladosporium)[13], and some can degrade the heavy metals in the soil (such as Xanthoria)[14]. Most of fungi had an obvious decrease in relative abundance during the peak period of watermelon fusarium wilt. These fungi are mainly the pathogenic fungi causing plant diseases[15-19]. For example, fusarium is the pathogenic fungi leading to watermelon fusarium wilt. However, Trichoderma is an antagonistic microbe which can decompose organic pollutants, realize biological control and promote the growth of crops[20]. As shown in Fig.4, 27 of the top 30 species of fungal community at the genus level had a significant decrease in the relative abundance during the peak period of watermelon fusarium wilt. The reason is that, according to preliminary analysis, when induced by the rhizosphere exudates of watermelon plants, some pathogenic fungi (such as fusarium) in the soil entered and settled down in the rhizosphere soil of the growing plants, which presented a trend of decrease and then a significant increase in the relevant abundance of the pathogenic fungi during the peak period of disease. But for species which have certain prevention and control effect on watermelon fusarium wilt, such as Trichoderma, there was a continuous decrease in its abundance with the development of the disease.
The Shannon index is highly consistent with the disease prevention and control of crops. The higher the Shannon index is, the better the disease prevention and control effect will be[21]. As shown in Table 2 and Table 3, during the peak period of watermelon fusarium wilt, as the Shannon index of the microbial community in rhizosphere soil decreased, the diversity and abundance of the bacteria and fungi in rhizosphere soil also decreased to a certain extent. But with the stabilization of the disease, the diversity and abundance of bacteria and fungi had certain level of recovery. This also indicates that the occurrence of watermelon fusarium wilt is related to the diversity of microbial community. LIU L L et al.[22]expressed that the rhizosphere exudates of watermelon plants may have caused the changes of the microbial community in rhizosphere soil after planting. The findings of recent studies showed that the floristic disorder of the microbial community in rhizosphere soil and the indirect effect of the ecological efficiency of rhizosphere exudates were the major cause of the continuous cropping obstacles of watermelon. It was also found that some species of microbial community, such as Acrophialophora, Monosporascus, Fusarium and other soil-borne pathogens, multiplied quickly under the mediation of specific components of rhizosphere exudates while inhibiting the growth of beneficial microorganisms such as Sphingomonas, Trichoderma and other antagonistic bacteria, which would change the composition and quantity of the rhizosphere exudates of watermelon plants and provide more carbon and energy sources for chemotaxy pathogenic microorganism, forming a vicious circle and causing adverse effects on the growth and development of watermelon plants[23]. However, the real causes to the continuous cropping obstacles and growth problems of watermelon need to be further studied.
 Agricultural Science & Technology2020年2期
Agricultural Science & Technology2020年2期
- Agricultural Science & Technology的其它文章
- Effects of γ Irradiation on Microorganisms in Edible Fungi Matrix
- Optimization of Steam Explosion Process Condition for Extracting Polysaccharides from Pseudostellaria heterophylla by Response Surface Methodology
- Investigation of Salt Stress Response Mechanisms Using Mirna Sequencing of Salt Tolerant and Salt Sensitive Jute Genotypes
- Study on Extraction Method of Carotenoids from Citrus Leaves
- A Study on Planting Adaptability of Various Soybean Varieties in Hengyang
- Analysis on Some Agronomic Characters and Pedigree in Hezihao Series Peanut Varieties
