Effectiveness and Safety of Jinye Baidu Granules(金叶败毒颗粒) for Acute Upper Respiratory Tract Infection:A Systematic Review and Meta-analysis
DAI Wen-kang (戴文康), LV Jian(吕 健), SUN Meng-hua (孙梦华),XIE Yan-ming (谢雁鸣), JIANG Jun-jie (姜俊杰)
1. Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China 2. Wang Jing Hospital, China Academy of Chinese Medical Sciences, Beijing 100102, China
ABSTRACT To evaluate the effectiveness and safety of Jinye Baidu Granules (金叶败毒颗粒) for acute upper respiratory tract infection(AURTI). Database such as CNKI, Wan-fang, VIP, SinoMed, Web of science, Clinical Trials gov, Medline、EMBASE, CENTRAL, Cochrane Library were retrieved to collect randomized controlled trials (RCTs) on Jinye Baidu Granules (金叶败毒颗粒) in treating AURTI from the estabslishment of the database to March 2019. A total of 2 reviewers independently screened the literature according to the inclusion and exclusion criteria, and extracted material and the quality evaluation of the included studies. Quality evaluation adopted Cochrane Handbook 5.1 evaluation standards and tools. RevMan5.3 was used to perform Meta-analysis for the adopted study. Finally a total of 4 RCTs involving 636 patients were included. Meta-analysis results showed that: compared with conventional Western medicine alone, Jinye Baidu Granules (金叶败毒颗粒) combined with Western medicine in the treatment of acute upper respiratory tract infection can improve the total effective rate of clinical ef ficacy [RR=0.13, 95% CI (0.06, 0.29),P<0.00001], shorten the time of antipyretic time for acute upper respiratory tract infection [MD=–1.22,95% CI (–1.43,–1.00),P<0.00001], shorten the time of pharyngeal pain [MD=–1.97, 95% CI (–2.97,–0.96),P<0.0001] and shorten the cough disappear time[MD=–1.97, 95% CI (–2.97,–0.96),P<0.0001]. There were 2 papers reporting adverse reactions during the study period, and one of them speci fically reported diarrhea, nausea, vomiting and stomachache in the experimental group. In the control group: diarrhea, nausea and adverse reactions disappeared after drug withdrawal; the incidence of adverse reactions was 3.92% in the control group and 5.88% in the observation group. There was no signi ficant difference between the 2 groups (P>0.05). Based on existing data and methods, the systematic evaluation showed that, compared with Western medicine alone, Jinye Baidu Granules (金叶败毒颗粒) combined with Western medicine alone could improve the total effective rate of clinical ef ficacy, reduce the time of fever, sore throat, and the disappearance of cough with less adverse reactions. However, due to the low quality of the included study, large samples, multicenter,randomized, double-blind trials and trials are still needed to randomized controlled trials with reference to the CONSORT standard and the STRICTA statement.
KEYWORDS Jinye Baidu Granules(金叶败毒颗粒); Acute upper respiratory tract infection; Randomized controlled trial; System evaluation; Meta-analysis
Acute upper respiratory tract infection[1]is referred to as "upper sensation", which is caused by various viruses and bacteria. It is clinically manifested by symptoms such as fever, cough, and sore throat etc. It can occur throughout the year,but it occurs frequently in winter and spring. It often occurs in the elderly, child patients and people with chronic respiratory diseases. If not treated in time,tracheitis, pneumonia, and acute myocarditis etc.can occur, which seriously affect the patient's health.
For the treatment of acute upper respiratory tract infections, Western medicine mainly provides symptomatic supportive treatments such as antivirus and anti-infection etc. However, many studies have reported[3-6]that drug resistance has increased significantly due to the extensive abuse of antibiotics. The course of treatment is longer. The symptom relief of the patients is slower, and the effects are not good[6]. The disease belongs to the category of wind-warmth lung heat disease (heat in lung-wei pattern of traditional Chinese medicine.The principle of treatment is to to clear heat through the surface.
The treatment results of previous clinical studies have shown that: Jinye Baidu Granules (金叶败毒颗粒) has a clear clinical effects in treating wind-warmth lung heat disease (heat in lung-wei pattern ), having a good advantage especially in terms of improving pharyngeal symptoms, with comparatively better safety[10].
Jinye Baidu Granules (金叶败毒颗粒) is composed ofLonicera japonica Thumb(Jin Yin Hua),Folium Isatidis(Da Qing Ye),Herba Taraxaci(Pu Gong Ying) andHerba Houttuyniae(Yu Xing Cao), and has the function of cooling and dispersing, clearing heat and removing toxicity[16].Modern pharmacological studies have shown that:Herba Taraxaci(Pu Gong Ying) andHerba Houttuyniaeboth(Yu Xing Cao) have effects such as antibacterial, antiviral, anti-inflammatory,and immune-enhancing etc[17];Lonicera japonica Thumb(Jin Yin Hua) has effets such as antiviral,antipyretic, anti-inflammatory, antibacterial, and pharmacological that inhibit endotoxin.Folium Isatidis(Da Qing Ye) can be antibacterial and antiviral to improve immunity[22]. Although Jinye Baidu Granules (金叶败毒颗粒) has been used in clinical practice, no related research in the field of evidence-based evaluation has been reported, and there is a lack of systematic evaluation of related efficacy. Therefore, the purpose of this study is to systematically evaluate the clinical research results of Jinye Baidu Granules (金叶败毒颗粒) for acute upper respiratory tract infection, with a view to providing more reliable evidence for the clinical application of Jinye Baidu Granules(金叶败毒颗粒).
MATERIALS AND METHODS
Screening Criteria
Study type
Randomized controlled trial, without restrictions on publication language.
Research subjects
In accordance with the diagnostic criteria for acute upper respiratory infections/wind-warmth lung heat disease (heat in lung-wei pattern)[20], regardless of age, gender, ethnicity, and no other allergic diseases.
Intervention test group
Experimental group: Jinye Baidu Granules(金叶败毒颗粒) or control group intervention +Jinye Baidu Granules (金叶败毒颗粒). The control group: conventional Western medicine (antiviral,anti-infective drugs such as amoxicillin Capsules,clarithromycin, cephalosporin antibiotics, ribavirin),and the control group did not contain Jinye Badudu Granules (金叶败毒颗粒).
Outcome indicators
Main outcome indicators: total effectiveness;secondary outcome indicators: antipyretic time, sore throat time, cough disappearance time, and adverse reactions. In the case of consistent ef ficacy standards,the total effective index is used, the total effective rate = (the number of signi ficant cases + the number of effective cases) / the total number of cases×100%.
Exclusion criteria
①Research reports that did not provide the basic information of the subjects or on relevant information on the intervention measures;②Repetitive publications or data duplication studies(the former retained 1 paper, and the latter retained 1 paper of the most complete data); ③The research data was seriously wrong.
Retrieval Strategy
Chinese databases searched included:SinoMed, CNKI, VIP, Wanfang Database; Web of science, Clinical Trials, Medline searching through PubMed, clinical trial registry of EMBASE,CENTRAL, and Cochrane Library searched by Ovid.The retrieval time range of each database was from the earliest index date of the database to March 2019. Chinese search terms were "金叶败毒颗粒", "急性上呼吸道感染", "风温肺热病 (热在肺卫证)", and English search terms were "acute upper respiratory tract infection", "Jinye Baidu". Based on the characteristics of the database, a comprehensive search of the subject words combined with free words is performed. In addition, gray papers such as dissertation and conference papers were searched in the Chinese database mentioned above, by using Google, Baidu, other search engines and manual search as supplements, and the references included in the literature were traced.
Evaluation Method
Literature screening
In this study, a total of 2 researchers independently screened the literature based on the inclusion and exclusion criteria. For those literature inconsistent with the screening results and dif ficult to determine whether to be included, a third party was requested to evaluate them. The screening process was to use Note Express software to check the literature, to remove duplicate literature, to read the titles and abstracts of the literature, and to exclude the documents that obviously did not meet the inclusion criteria. The documents that may meet the criteria were downloaded and the full text was read to rescreen, to determine whether to include.
Data extraction
Data extraction was performed for the included literature, and the speci fic contents mainly included:first author, study year, sample size, number of men and women, patient age, intervention measures,course of treatment, outcome indicators, and ef ficacy standards. When the study had 1 or more common intervention groups, the method recommended by the Cochrane manual was selected, by grouping and combining. And the multi-arm trial was converted to a two-arm trial[23].
Methodological quality evaluation of included literature
A total of 2 researchers independently evaluated the methodological quality of the literature back-to-back, and in case of disagreement,discussed or decided by a third researcher. The quality of the included literature was assessed according to the risk bias assessment tool in the evaluation manual developed by the Cochrane Working Group, including ①random sequence generation; ②allocation concealment; ③ blind method for patients and testers; ④ blind method for outcome assessors; ⑤ incomplete result data;⑥ selective reporting; ⑦ other biases (such as potential biases or statements of fraud related to research and special research design etc.). Finally,the judgments of "low risk", "high risk", and "unclear risk" were made on the included research literature.After the paper was completed, a self-assessment was performed according to the PRISMA statement and the AMSTER standard.
Statistical analysis
This study used RevMan 5.3 software provided by Cochrane Collaboration Network for Meta-analysis.Relative data (RR) was used as the statistical analysis of efficacy, and the mean difference (MD)used by measurement data was as the efficacy analysis statistics. Both were expressed by the effect size and its 95% confidence interval (CI).I2test was used to evaluate the statistical heterogeneity: ifI250%, it indicated that the statistical homogeneity was good, and a fixed effect model was used for Meta-analysis; ifI2> 50%, it indicated that the statistical heterogeneity was greater. Random effect model was used for Meta-analysis. If the results of the heterogeneity were too large and the study was not suitable for Meta-analysis, a descriptive analysis was performed. If papers included in an outcome indicator10, a funnel chart was used to analyze whether there was publication bias.
RESULTS
Literature Search Results
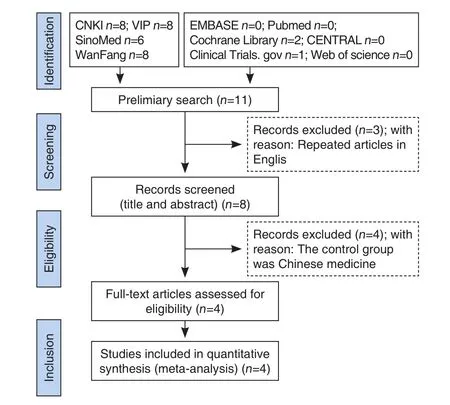
Figure 1. Literature Screening Process
A total of 33 related literature were initially collected. There were 3 English literature and 30 Chinese literature. Bibliographic titles were imported into the Note Express software. A total of 22 duplicate papers duplicated were excluded, and 11 papers were obtained after excluding. A total of 2 papers in the Cochrane Library were translated from Chinese and were recorded as as duplicate literature. After reading the title and abstract, a total of 8 papers were included and initially screened, and the remaining 8 papers were downloaded and read for the full text. Finally,4 papers were included. The literature screening process and results were shown in Figure 1.basic data of each included study, including: author,year of publication, number of included cases(experimental group, control group, total number of cases), specific interventions, course of treatment,outcome indicators and efficacy criteria (cured:clinical symptoms, body temperature; laboratory tests returned to normal; markedly effective: clinical symptoms markedly improved; body temperature returned to normal; and laboratory tests were all close to normal; invalid: clinical symptoms improved,body temperature lowered, and laboratory tests have not improved compared with the previous ones, and even worsened).[26]The study mentioned that the experimental group and the control group were similar in baseline and comparable, with treatment courses ranging from 1 to 7 days. The basic characteristics of the included studies were shown in Table 1.
Basic Characteristics of Included Studies
The 4 RCTs studied were all located in China,with a total of 636 patients. The interventions included in the literature were: Jinye Baidu Granules(金叶败毒颗粒) + conventional Western medicine vs conventional Western medicine. We extracted the
Risk Bias Evaluation of Included Studies
Random grouping was mentioned in 4 studies,and 1 study[12]reported speci fic random methods. All studies did not mention the allocation concealment of the random allocation scheme. Nor mentioned the report on the implementation of blind methods.Other bias sources were unclear. The results were shown in Figure 2 and Table 2.
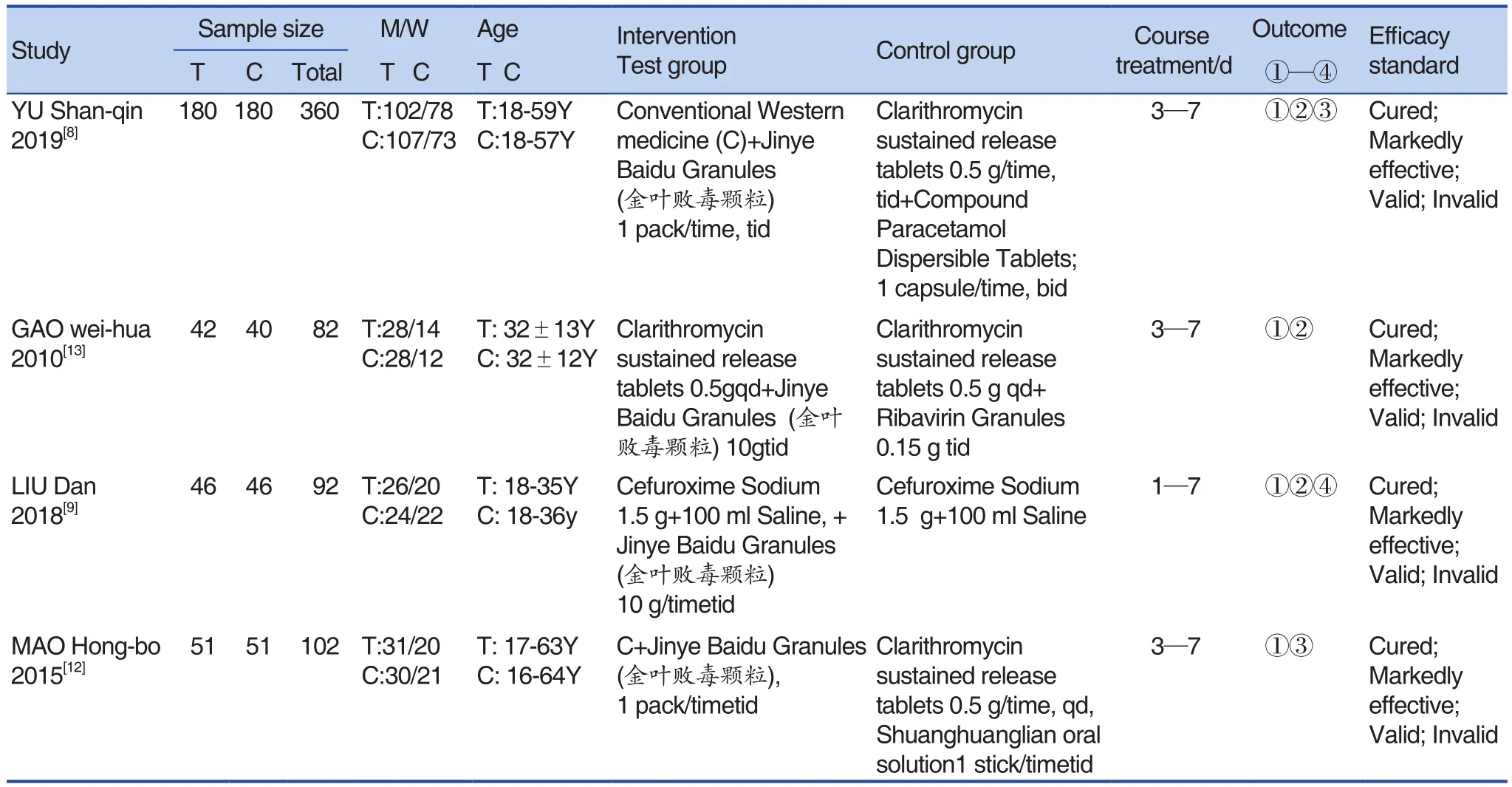
Table 1. Basic Information Included in the Study
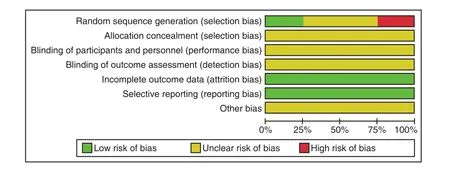
Figure 2. Percentage of Projects Included in the Study That Produced a Risk of Bias
Meta Analysis Results
Total effective rate of clinical ef ficacy
A total of 4 studies[8,9,12,13]with 636 patients were included. The heterogeneity among the results was small (P=0.92,I2=0%), so a fixed effect model was used for Meta-analysis. The results showed that:Jinye Baidu Granules (金叶败毒颗粒) + conventional Western medicine was superior to conventional Western medicine in the total effective rate of clinical ef ficacy [RR=0.13, 95% CI (0.06, 0.29),P<0.00001].See Figure 3.
Antipyretic time
A total of 3 studies[8,9,13]were included with 534 patients. The heterogeneity between the results of each study was large (P<0.0001,I2=90%), so a random effect model was used for Meta-analysis. The results showed that Jinye Baidu Granules (金叶败毒颗粒) combined with conventional Western medicine was superior to conventional Western medicine in shortening the time of fever reduction [MD=–1.22,95% CI (P1.43,P1.00),P<0.00001]. When the LIU Dan's study was removed,P=0.19,I2=42%, and the results of the Meta-analysis showed: MD=–1.12, 95%CI (–1.26, –0.99),P<0.00001. The analysis of the reasons for the large heterogeneity may be related to the different course of treatment included in the LIU Dan's study. See Figure 4.
Sore throat resolution time
A total of 3 studies were included with 534 patients. There was statistical heterogeneity between the results of the studies (P<0.00001,I2=95%), so a random effect model was used for Meta-analysis. The results showed that Jinye Baidu Granules (金叶败毒颗粒) + conventional Western medicine treatment was superior to conventional Western medicine treatment in shortening the sore throat time [MD=–1.97, 95% CI (–2.97, –0.96),P<0.0001]. When LIU Dan's study was removed,P=0.66,I2=0%, and the Meta-analysis results showed that MD=–1.47, 95% CI (–1.72, –1.21), andP<0.00001. The major reasons analyzed resulting in heterogeneity may be related to the different treatment courses. See Figure 5.
Cough disappearance time
A total of 3 studies[8,9,13]were included with 534 patients. There was statistical heterogeneity between the results of the studies (P<0.0001,I2=89%), and a random effect model was used for Meta-analysis.
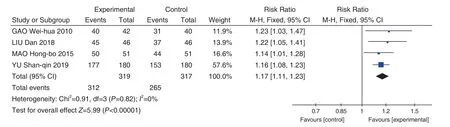
Figure 3. Forest Map of Total Effective Rate
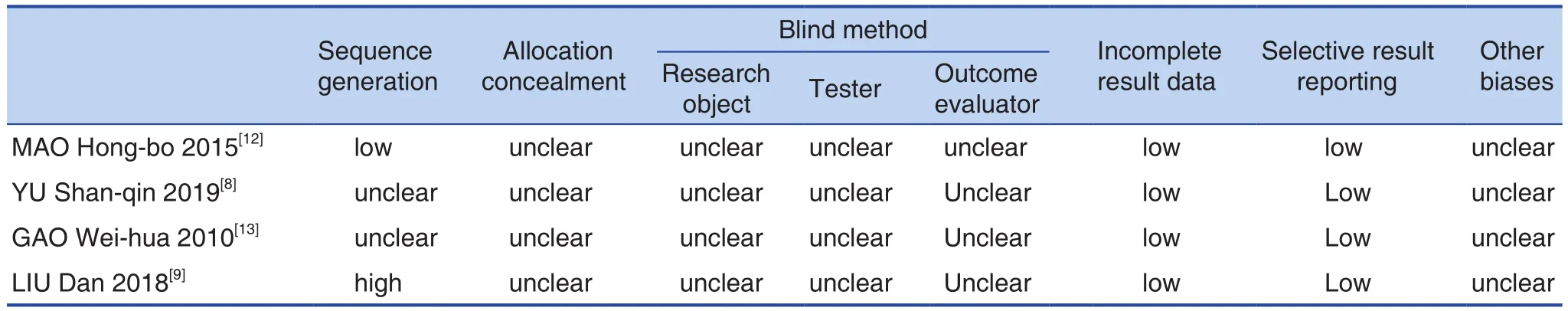
Table 2. Risk Assessment of Bias in the Included Studies
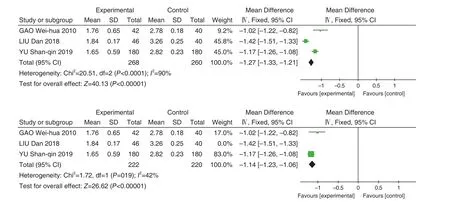
Figure 4. Forest Map of Antipyretic Time
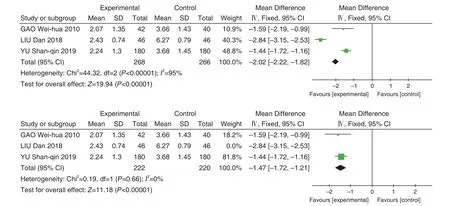
Figure 5. Sore Throat Regression Time Forest Map
The results showed that Jinye Baidu Granules (金叶败毒颗粒) + conventional Western medicine treatment was better than conventional Western medicine in shortening the cough disappearing time [MD=–1.97, 95% CI (–2.97, –0.96),P<0.0001].When the LIU Dan's study was removed,P=0.2,I2=40%. The results of the Meta-analysis showed that MD=–1.65, 95% CI (–1.95, –1.35),P<0.00001,and the reasons for heterogeneity were the same as above. See Figure 6.
Adverse reactions
Of the 4 studies, 2 studies reported that no adverse reactions of the related drugs occurred during the treatment period, and 2 other studies reported adverse reactions occurred during the study period, of which 1 study[8]had adverse reactions in the experimental group: diarrhea, nausea, vomiting,stomach pain; adverse reaction rate was 3.33%;adverse reactions in the control group were: diarrhea,nausea; adverse reaction rate was 1.67%; the incidence of adverse reactions in the control group of 1 study[12]was 3.92% (2/51), and the observation group was 5.88% (3/51). There was no significant difference between the 2 groups of data, and it was not statistically significant (P>0.05). Symptoms disappeared after discontinuation of drugs.
Evaluation report bias
In view of the fact that the funnel chart was applicable to the case where the number of studies were greater than 10. The largest number of studies included in this study was the total effective rate, the number of which was 4, so no funnel chart analysis publication bias was performed
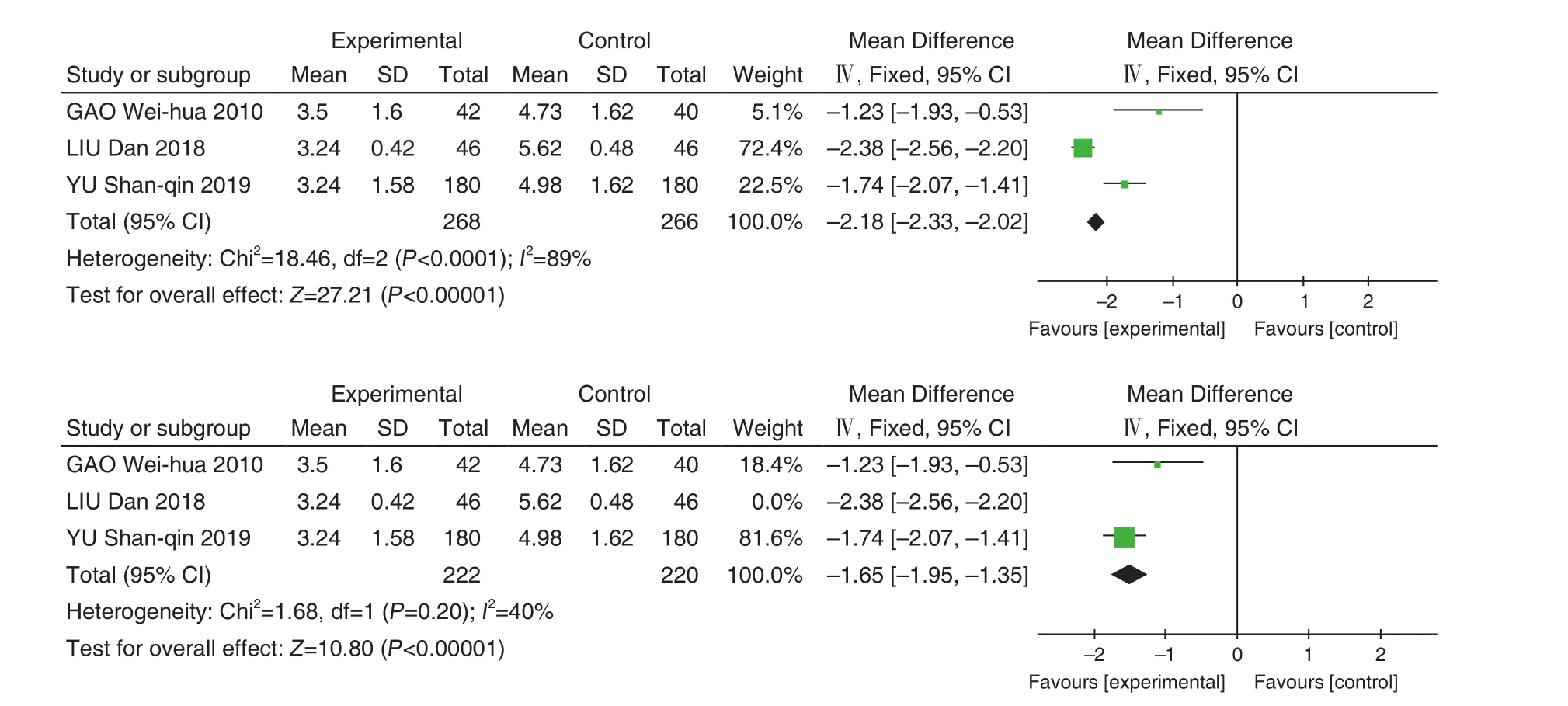
Figure 6. Forest Map of Cough Disappearing Time
DISCUSSION
Effectiveness of Jinye Baidu Granules (金叶败毒颗粒) in the Treatment of Acute Upper Respiratory Tract Infection
This study mainly systematically evaluated the effectiveness of Jinye Baidu Granules (金叶败毒颗粒) in the treatment of acute upper respiratory tract infections from the total effective rate, antipyretic time, sore throat disappearance time, cough disappearance time, and adverse reaction. Jinye Baidu Granules (金叶败毒颗粒) +conventional Western medicine was superior to conventional Western medicine in terms of the overall effectiveness of clinical ef ficacy. Jinye Baidu Granules (金叶败毒颗粒) as an adjuvant drug combined with conventional Western medicine in the study to improve the overall effectiveness was analyzed; in terms of shortening the antipyretic time and time of sore throat and cough disappearing time,wind-warmth lung heat disease being caused by wind-heat pathogen was analyzed. The oncologist YE Tianshi of the Qing Dynasty once said that"exogenous pathogen was attacked from mouth and nose and the lungs was first attacked." Exogenous pathogen invaded from the snout, skin and hair. The lung-wei was the 1stattacked. The disharmony of defensive exterior leads to fever, slight aversion to cold, and pantalgia; impaired depurative descending of lung qi leads to cough and pharyngalgia. Jinye Baidu Granules (金叶败毒颗粒) have the functions of cooling and dispersing, clearing heat and removing toxicity[16]. Jinye Baidu Granules (金叶败毒颗粒) are composed ofLonicera japonica Thumb(Jin Yin Hua),Folium Isatidis(Da Qing Ye),Herba Taraxaci(Pu Gong Ying) andHerba Houttuyniae(Yu Xing Cao). InChinese Materia Medica Volume 2 Selected Book, it is said thatLonicera japonica Thumb(Jin Yin Hua) is sweet and cold, is good at clearing the heat of the lung-wei, and is light and fragrant. It also has the function of dispersing. At the beginning of the treatment of warm disease, the pathogen is in the lung-wei, the fever is severe and the aversion to cold is light and thirsty as the main medicine.";Herba Houttuyniae(Yu Xing Cao) is pungent, slightly cold into the lung channel, having the function of clear heat and remove toxicity,detumescence and expel pus, diuretic and free strangury, as a minister drug, withLonicera japonica Thumb(Jin Yin Hua) clearing the lung heat.Folium Isatidis(Da Qing Ye), which is bitter and cold, enters the 2 channels of the stomach and the heart, having the functions of clearing heat and removing toxicity,cooling blood and removing spots.Herba Taraxaci(Pu Gong Ying), which is bitter and sweet cold,enters the liver and stomach channels, and has the functions of clearing heat and removing toxicity,detumescence and resolving masses, diuretic and freeing strangury.Folium Isatidis(Da Qing Ye) andHerba Taraxaci(Pu Gong Ying) both clear heat and remove toxicity, and helpFlos Lonicerae(Pu Gong Ying) andHerba Houttuyniaeto(Yu Xing Cao) clear the pathogen of heat and toxicity in lung-wei and throat heat. Modern pharmacological studies have shown that:Herba Taraxaci(Pu Gong Ying) andHerba Houttuyniaeboth(Yu Xing Cao)have antibacterial, antiviral, anti-inflammatory, and immune-enhancing effects[17];Lonicera japonica Thumb(Jin Yin Hua) has antiviral, antipyretic, antiinflammatory, antibacterial[18], and pharmacological effects that inhibit endotoxin[21].Folium Isatidis(Da Qing Ye) can be antibacterial and antiviral to improve immunity[22]. In addition, studies have shown that Jinye Baidu Granules (金叶败毒颗粒) have both direct anti-inflammatory effects and indirect anti-inflammatory effects by exciting the pituitary adrenal cortex system[27]. The antiviral, anti-infective and anti-inflammatory mechanisms of Jinye Baidu Granules (金叶败毒颗粒) in treating acute upper respiratory tract infection was explained from the perspective of pharmacology.
Safety of Jinye Baidu Granules (金叶败毒颗粒)in the Treatment of Acute Upper Respiratory Tract Infection
This study mainly re flected the safety of Jinye Baidu Granules (金叶败毒颗粒) in treating acute upper respiratory tract infection from the incidence of adverse reactions and the specific situation of adverse reactions. A total of 2 papers reported there were no related adverse reactions of drugs during the study. A total of 2 papers[8,12]reported adverse reactions during the study, one[8]of which specifically reported the experimental group:diarrhea, nausea, vomiting, and stomach pain;control: diarrhea, nausea, which disappeared after withdrawal, among which 1[12]paper reported the incidence of adverse reactions in the control group was 3.92%. The observation group was 5.88%.There was no significant difference between the 2 groups of data (P>0.05). However, there were the relatively few research literature included according to the standard, and the drug treatment course included in the study was generally 7 days, and the follow-up time was relatively short. Therefore, the long-term safety of Jinye Baidu Granules (金叶败毒颗粒) in the treatment of acute upper respiratory infections needs to be further verified by modern pharmacological research, with more large sample,multi-center, randomized, double-blind trials. And it was recommended that randomized controlled trials in the future should refer to the CONSORT standard first[28].
Limitations of the Research
Of the 4 clinical randomized controlled trial literature included in this study, 1 was specifically written in the random number table. No hidden grouping, blinding and lost follow-up were reported.It showed that when conducting clinical controlled trials (RCTs), researchers paid more attention to the implementation of random allocation schemes,but ignored other methodological quality aspects that may affected the ef ficacy. In addition, 2 of the 4 literature did not report adverse reactions in the trial. The inadequate reporting of adverse reactions will affect the clinical diagnosis and treatment of patients, indicating that researchers will pay more attention to adverse reactions when designing and reporting RCT.
Clinical Significance and Prospect of the Research
According to the results of this systematic review, Jinye Baidu Granules(金叶败毒颗粒)combined with conventional Western medicine for the treatment of acute upper respiratory tract infections need high-quality research evidence to con firm[28,29]. Based on this, the following prospects are proposed: first, the current clinical application of Jinye Baidu Granules(金叶败毒颗粒) is rarely used according to TCM theory for differentiation and classification, but only for diseases or symptoms (such as sore throat). This approach seems to expand the population. But in fact it is not conducive to highlighting the TCM characteristics of the drug. The systematic review found that this phenomenon needs to be improved through analysis. Because Chinese medicines ("Chinese Medicine" or "Traditional Chinese Medicine") refers to the guidance by TCM theory, and they have a unique theoretical system and application form.Chinese patent medicines also emphasize research,development and use under the guidance of TCM theory. Only with the accurate medication under the guidance of the theoretical system of TCM(derived from "Fifty-two Diseases") and syndrome differentiation (originated fromTreatise on Febrile and Miscellaneous Diseases) can the efficacy of this medicine be highlighted. In the future clinical application, it is recommended that clinicians strictly follow the instructions when using these medicines,especially Western medicine doctors diagnose the disease as acute upper respiratory infection, and in line with TCM wind-warmth lung heat disease (The syndrome differentiation belongs to heat in lungwei pattern). Secondly, I hope that future research will refer to the CONSORT standard[28], STRICTA statement[29], and the ROB tool entries[26]formulated by the Cochrane Collaboration Network, to report in detail the generation of random allocation sequences, allocation concealment, blindness of research objects and researchers, incomplete outcomes etc. Thirdly, I hope that researchers will formulate criteria for the inclusion in the diagnosis of diseases, and comprehensively consider all factors in determining the outcome indicators, TCM ef ficacy indicators, and fuzzy comprehensive evaluation of TCM clinical efficacy[30-31], in order to improve the clinical reference value of the study.
 World Journal of Integrated Traditional and Western Medicine2020年3期
World Journal of Integrated Traditional and Western Medicine2020年3期
- World Journal of Integrated Traditional and Western Medicine的其它文章
- INSTRUCTION FOR AUTHORS
- Expert Consensus of Guangdong Province on Prevention and Treatment of Coronavirus Disease 2019 with Integrated Chinese and Western Medicine(Trial Version I)
- Guangdong Association of Integrative Medicine Expert Consensus of Guangdong Province on Prevention and Treatment of COVID-19 with Integrated Chinese and Western Medicine
- Advantages and Suggestions of Scraping to Promote Rehabilitation of COVID-19 Patients in Convalescence
- Meditation-based Interventions Might be Helpful for Coping with the Corona Virus Disease 2019 (COVID-19)
- Systematic Review and Meta-Analysis of Fuke Qianjin Tablets(妇科千金片) in the Treatment of Chronic Pelvic In flammation
