Systematic Review and Meta-Analysis of Fuke Qianjin Tablets(妇科千金片) in the Treatment of Chronic Pelvic In flammation
GE Xiao-chen (葛晓晨), LV Jian (吕 健), SUN Meng-hua (孙梦华),XIE Yan-ming (谢雁鸣), SUN Lin-xi (孙粼希), ZHANG Li-dan (张利丹)
1. Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China 2. Shandong University of Traditional Chinese Medicine, Jinan 250000, China
ABSTRACT Objective:To systematically evaluate the ef ficacy and safety of Fuke Qianjin Tablets (妇科千金片) in the treatment of chronic pelvic inflammation.Methods:A systematically and comprehensively search was conducted in 4 Chinese databases of CNKI, VIP, WanFang and CBM and the foreign language databases of Pubmed, EMbase and The Cochrane Library. The retrieval time was from database establishment to March 2019. The randomized controlled trials of Fuke Qianjin Tablets (妇科千金片) in the treatment of chronic pelvic in flammation were selected according to the predetermined criteria. The quality of the included study was evaluated by Cochrane collaborative network bias risk evaluation tool, and the meta-analysis was performed by RevMan5.3 software.Results:A total of 1009 related literatures were searched. After initial screening and strict evaluation, 55 studies were included, with a total sample size of 6826 cases, including 3416 cases in the experiment group and 3410 cases in the control group. The results of meta-analysis showed that the total effective rate of Fuke Qianjin Tablets (妇科千金片) combined with antibiotics in the treatment of chronic pelvic in flammation was better than that of antibiotics alone (RR=1.20,95% CI [1.17, 1.22],P<0.00001). Fuke Qianjin Tablets (妇科千金片) combined with antibiotics was better than that of antibiotics alone in the improvement of abdominal pain symptoms (RR=1.40, 95% CI [1.04, 1.88],P<0.00001), leukorrhea abnormality (RR=1.38, 95% CI [1.16, 1.65],P<0.0004). In terms of safety, Fuke Qianjin Tablets (妇科千金片) combined with antibiotics could reduce the incidence of adverse reactions(RR=0.67, 95% CI [0.48, 0.93],P<0.02). The main adverse reactions were nausea and vomiting, bitterness and astringency in the mouth, rash and so on. All of them could be tolerated and the symptoms could disappear in the short term, and had no effect on the treatment.Conclusion:Fuke Qianjin Tablets (妇科千金片) combined with antibiotics in the treatment of chronic pelvic in flammation can improve the total effective rate, relieve abdominal pain and abnormal leukorrhea and other clinical discomfort symptoms, improve the quality of life of patients to a certain extent, and no serious adverse reactions are found. Due to the limitation of the quality and quantity of the included literature, the above conclusions need to be further studied and veri fied by high-quality research.
KEYWORDS Fuke Qianjin Tablets (妇科千金片); Chronic pelvic inflammation; Systematic evaluation;Randomized controlled trial; Meta-analysis
Chronic pelvic inflammatory disease, also known as chronic pelvic in flammatory disease (PID),is a group of infectious diseases mainly caused by female upper reproductive tract inflammation. PID is a common and frequently-occurring disease in gynecology. It has the characteristics of recurrent attacks and lingering dif ficulties[1]. If it is not treated in a timely, correct and active manner, in flammation will recur, and even cause tubal pregnancy, chronic pelvic pain and infertility, which will seriously affect women's physical and mental health, reduce the quality of life, and increase the economic burden on the family. Interpretation of2015 American Centers for Disease Control and Prevention's Diagnosis and Treatment of Pelvic Inflammatory Diseases[2]states that the current clinical treatments are mostly based on broad-spectrum, empirical antibiotic antiinfective treatments, covering possible pathogens of PID. Papers such asClinical Practice Guidelines for the Treatment of Common Infectious Diseases with Single/Combined Antibiotics in Traditional Chinese Medicine: Pelvic Inflammatory Diseases[3-5]pointed out that it is worth noting that the incidence of long-term sequelae is high, coupled with endlessly phenomenon of the abuse of antibiotics, prone to serious consequences such as dysbiosis, bacterial resistance, and super bacterial infection. The combination of traditional Chinese and western medicine has shown its unique advantages, which can alleviate the symptoms of discomfort and reduce the incidence of adverse reactions in a timely manner.Compared with monotherapy, it has enhanced the ef ficacy and reduced the incidence of drug resistance.In recent years, Chinese patent medicines, mainly for cooling blood and removing blood stasis and clearing heat and removing dampness, have also been widely used in the treatment of chronic pelvic in flammatory disease and have achieved good results.
Ancient medical books did not have the name of chronic pelvic inflammatory disease. According to their clinical characteristics, it can be classified as diseases such as "leukorrheal diseases","abdominal pain in women", "hot into the blood chamber" and so on. Traditional Chinese medicine(TCM) considers damp, heat, and toxin to be the key etiologies of chronic pelvic in flammatory diseases[6],so antipyretic and analgesic, antibacterial and antiinflammatory are taken as treatment principles.Chinese patent medicine Fuke Qianjin Tablets (妇科千金片) uses Qian Jin Ba (Flemingia philippinensis),Chuan Xin Lian (Herba Andrographis), Jin Ying Gen (Rosa Laevigata Radix) and other drugs as sovereign medicine, and Ji Xue Teng (Caulis Spatholobi) is minister medicine. The medicine instruction indicates that it has the functions of clearing heat and removing dampness, nourishing qi and resolving blood stasis, and is mainly used to treat discomfortable symptoms of chronic pelvic in flammatory disease, endometritis, chronic cervicitis and other diseases caused by dampness heat and blood stasis stagnation[7,8]. In clinical application and literature reports, they are basically consistent with the indications in the manual.
At present, there are still few relevant systematic reviews at home and abroad. 3 systematic reviews/meta-analysis[9-11]of Fuke Qianjin Tablets (妇科千金片) for chronic pelvic inflammatory disease were published after retrival. Through full-text analysis, these studies have certain limitations and deficiencies, mainly reflected in: ① the intervention measures were not uniform; ② the quality of the included literature wa uneven; ③ the inclusion and exclusion criteria were "complementary", which leaded to inaccurate research conclusions; ④ no subgroup analysis for different studies of specific antibiotic drugs or combined analysis after indicating clinical rationality, and the research results obtained are questioned; ⑤ different retrieval methods and methods leaded to incomplete retrieval and inaccurate research results; ⑥ the outcome evaluation indicator was single or did not analyze the included outcome indicators, and the authenticity of the research results were in doubt; ⑦ the time between retrival to publishing was too long, and the data was not updated in time. On the basis of reflecting the limitations and deficiencies in the above studies, this study comprehensively and systematically collected published or unpublished related studies, updated relevant clinical data in a timely manner, and used rigorous evaluation methods to screen out documents that met quality standards.Qualitative or quantitative analysis are performed to further systematically evaluate the effectiveness and safety of Fuke Qianjin Tablets (妇科千金片) in the treatment of chronic pelvic in flammatory disease, and provide more reliable evidence-based evidence.
MATERIALS AND METHODS
Screening Criteria
Design type: randomized controlled trials(RCTs) of Fuke Qianjin Tablets (妇科千金片) for chronic pelvic inflammatory disease, regardless of language and publication restrictions. Research subjects: Regardless of whether the diagnostic criteria for chronic pelvic inflammatory disease are described in detail in the literature, they can be included as long as they are described as being diagnosed with chronic pelvic in flammatory disease or chronic pelvic in flammatory disease. Intervention measures: The experiment group was Fuke Qianjin Tablets (妇科千金片) or combined with Fuke Qianjin Tablets (妇科千金片) on the basis of the control group; the control group was conventional treatment or antibiotics without Fuke Qianjin Tablets(妇科千金片). Antibiotics such as moxifloxacin,levofloxacin, metronidazole, ceftriaxone sodium,penicillin, and amoxicillin and clavulanate potassium are the main antibiotics. Conventional treatments include antibiotics, nutritional support, vitamins and corrective electrolyte disorders. The main outcome indicators: the effectiveness of improving the clinical symptoms of chronic pelvic in flammatory disease (cured, marked effective, and effective are considered effective), and the secondary outcome indicators: improvement of abdominal pain, normal time of leucorrhea, and adverse reactions.
For the literatures with serious errors in the research data, full text cannot be obtained,animal experimental research, non-randomized controlled trial literature, other Chinese herbs in the intervention measures (such as Chinese patent medicines, Chinese materia medica extract injections, decoctions), acupuncture, auricular points, Chinese medicine enemas, etc. as a means of adjuvant therapy were excluded. The data with duplicate data retains the most complete one.
Retrieval Strategy
Computer search of 4 Chinese databases of Chinese National Knowledge Infrastructure (CNKI),Chinese Science and Technology Periodical Database (VIP), Wangfang Data, China Biomedical Database (CBM), and foreign language database of PubMed, EMbase, The Cochrane Library.The retrieval time was from the time of database establishment to March 2019. The Chinese search terms were "妇科千金片", "千金片", "慢性盆腔炎", "慢性盆腔炎性疾病". The English database search was mainly based on the subject words combined with free words. The search terms were "Gynecologic Qianjin tablets", "Qianjin tablets", and "chronic pelvic inflammatory disease". In addition, grey literatures such as conference papers, dissertations, etc. were also searched.
Literature Screening and Data Extraction
A total of 2 researchers independently screened the literature and cross-checked the extracted data according to the developed screening criteria. In case of disagreement, it was discussed or decided by a third researcher. First of all, the initial screening was performed based on the titles and abstracts of the obtained documents. After excluding documents that obviously do not meet the inclusion criteria,further reading was performed on the full text of the documents that may meet the inclusion criteria and rescreening to determine whether to include them.The data were extracted using Excel to establish a data extraction table. The main items included investigator, study type, patient age, number of patients in the experimental group and the control group, interventions and outcome indicators of the 2 groups.
Processing of Multi-Arm Experiments
When the study has one or more common intervention groups, the methods recommended in the Cochrane Handbook were selected: group and merge to convert a multi-arm trial to a two-arm trial.
Methodological Quality Evaluation of Included Literature
A total of 2 researchers independently evaluated the methodological quality of the literature and discussed and resolved if disagreement occurred.The Cochrane collaborative network bias risk tools was used to evaluate the quality of the literature,including random sequence generation, allocation hiding, double-blind implementation and subject,blinding the outcome evaluator, incomplete result data, selective reporting, and other 7 entries for bias(eg, potential bias associated with a particular study design, statement of fraud, etc.). Finally, a total of 3 judgments of "low risk of bias", "high risk of bias", and"uncertain risk of bias" were made to the literatures.
Statistical Analysis
Statistical analysis was performed using RevMan5.3 software provided by the Cochrane collaboration network. Relative risk (RR) was selected for enumeration data or binary variables to represent the merge result. When the measurement data or continuous variables used the same unit of measurement, the mean difference (MD) is used, and expressed with effect size and 95%confidence interval (CI). The clinical and statistical heterogeneity between studies were determined.Clinical heterogeneity was determined based on the similarity of the research objects, interventions,controls, and outcome indicators between studies;statistical heterogeneity was measured using the Cochrane Q test, and its size was evaluated usingI2: ifP>0.1 andI250%, it is considered that the statistical homogeneity is good, and a fixed effect model is used. IfP0.1 andI2>50%, it indicates that statistical heterogeneity is large. A random effect model is used to perform sensitivity analysis or subgroup analysis to determine the homogeneity reasons. If the study does not meet the requirements of the meta-analysis, a descriptive analysis was performed. If there were10 articles included in an outcome indicator, a funnel plot was drawn to determine the publication bias of the included study.
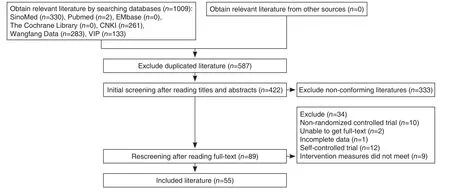
Figure 1. Literature Screening Process
RESULTS
Literature Search
A total of 1009 related papers were initially retrieved and imported into the document management software NoteExpress. The remaining 422 papers were performed duplicate checking.After reading the titles and abstracts, 89 papers were initially selected for full-text evaluation. After further reading the full-text, 55 papers were finally included, which were all written in Chinese. The literature screening process was shown in Figure 1.
Basic Characteristics of Included Studies
A total of 55 studies were included, including 56 trials. One of the studies[12]was a multi-arm trial(that is, it contained multiple interventions). There was a common intervention group, which was grouped into two-arm trials. The effect size was calculated by the method in the Cochrane manual.Except for a the total sample size was 6,826, the maximum sample size was 1,175, and the minimum sample size was 48. There were 3416 cases in the experimental group, and 3410 cases in the control group. The speci fic content was shown in Tables 1 and Table 2.
Quality Evaluation of Included Studies
None of the 55 studies reported research protocols, sample size estimates, and follow-up. 9 studies[14,15,17-19,21,30,33,43]reported using a random number table method, 2 studies[22,45]used a random order of visiting or admission, and 1 study[26]used the method of random color ball extraction, the rest of the studies did not report speci fic random implementation plan, only mention random. 9 studies[14,21,22,27,34,35,41,43,60]reported that all patients signed the informed consent.No study mentioned the use of blindness and the concealment of reporting random allocation schemes.No study did not fully report pre-speci fied indicators,and there was no case of selective reporting of results. Bias risk assessments included in the study were shown in Figure 2 and risk assessments were shown in Table 3.
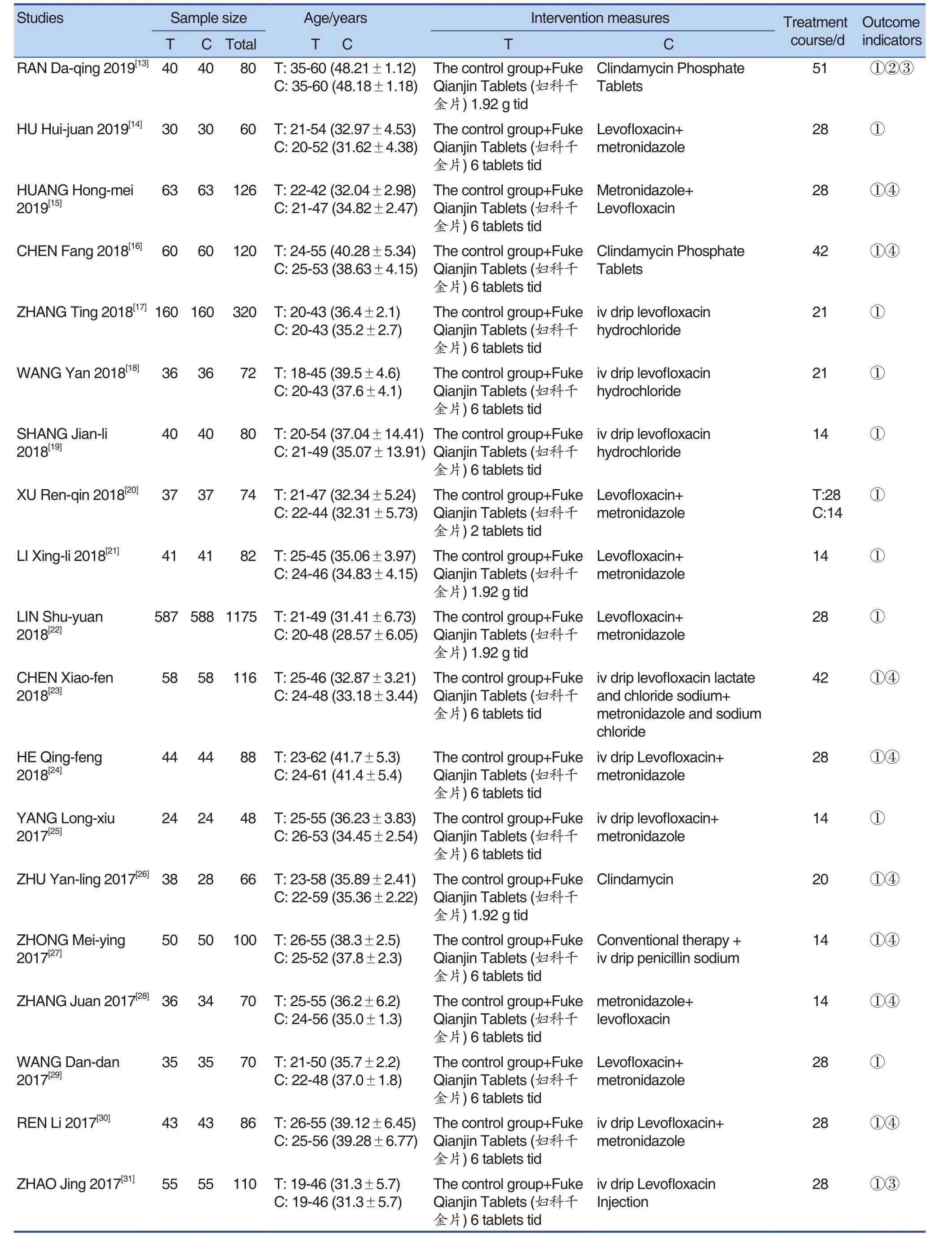
Table 1. Basic Information Included in the Study
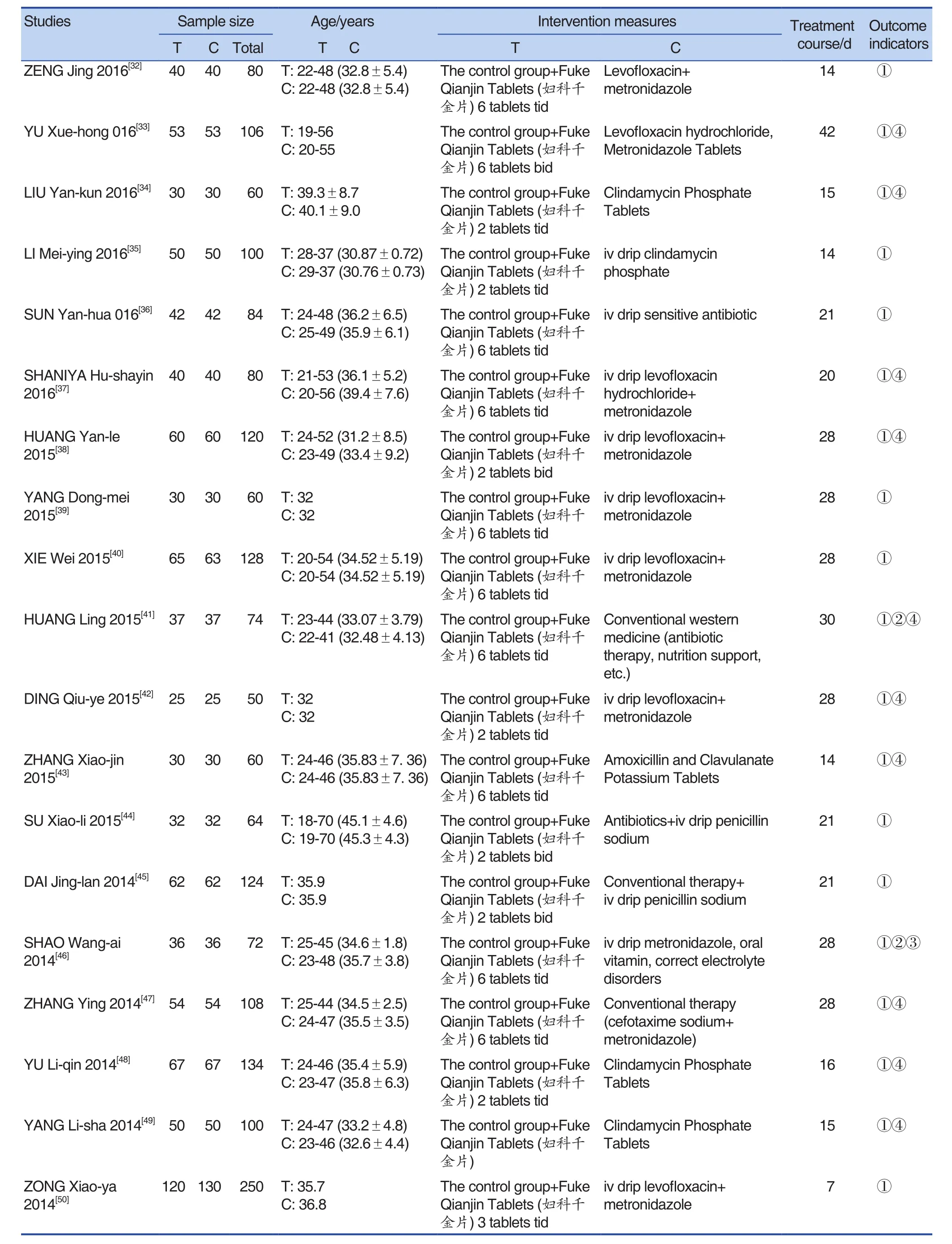
(Continued)
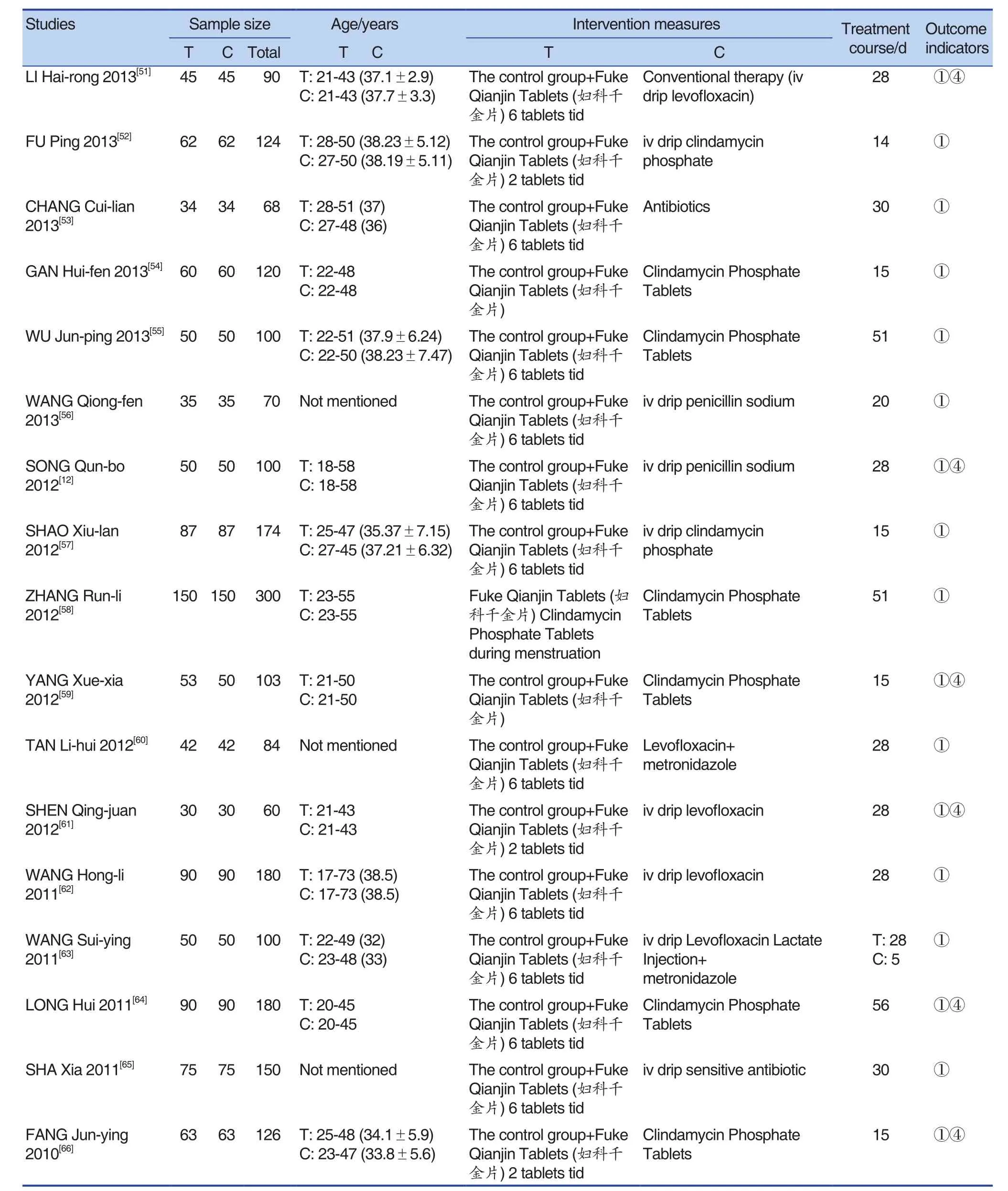
(Continued)
Meta-Analysis
Total effective rate
Clindamycin + Fuke Qianjin Tablets (妇科千金片) VS Clindamycin Clindamycin + Fuke Qianjin
Tablets (妇科千金片) VS Clindamycin were included in 15 studies[13,16,26,34,48,49,51,52,54,55,57-59,64,66]. Metaanalysis results showed that the heterogeneity test showed that the heterogeneity was small (P=0.68,I2=0%), so the fixed effect model was used to combine the effect size. Meta-analysis results showed that compared with the control group, the total effective rate of the Fuke Qianjin Tablets (妇科千金片)group was signi ficantly improved (RR = 1.20, 95% CI[1.16, 1.26],P<0.00001), as shown in Figure 3.

Table 2. Characteristics of Multi-Arm Test
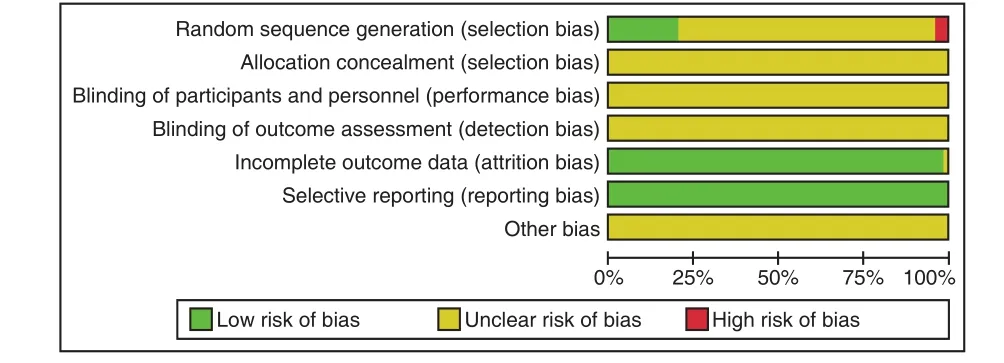
Figure 2. Percentage of Items Included in the Study with Risk of Bias
Penicillin + Fuke Qianjin Tablets (妇科千金片) VS Penicillin Penicillin + Fuke Qianjin Tablets(妇科千金片) VS Penicillin was included in 5 studies[12,27,44,45,56]. Meta-analysis results showed that heterogeneity test showed less heterogeneity(P=0.74,I2=0%), so the fixed effect model was used to combine the effect size. Meta-analysis results showed that compared with the control group, the total effective rate of the Fuke Qianjin Tablets (妇科千金片) group was signi ficantly improved (RR = 1.16,95% CI [1.08, 1.26],P<0.0001), as shown in Figure 4.
Amoxicillin + Fuke Qianjin Tablets (妇科千金片)VS Amoxicillin Amoxicillin + Fuke Qianjin Tablets(妇科千金片) VS Amoxicillin included 1 study[43].Due to the small sample size, a descriptive analysis was performed. The raw data were analyzed by RevMan software to get the correspondingPvalue,so as to determine whether the study had statistical differences. In this study, the control group was amoxicillin, and the experiment group was Fuke Qianjin Tablets (妇科千金片) + amoxicillin. The results showed no signi ficant statistical signi ficance (P=0.05).The total effective rate in the observation group was 93.33%, and the total effective rate in the control group It was 73.33%, indicating that the efficacy of Fuke Qianjin Tablets (妇科千金片) + amoxicillin may be better than that of amoxicillin. However, because the number of descriptive analyses is small, the results obtained do not provide sufficient clinical evidence, so they are only for clinical reference.
Antibiotics + Fuke Qianjin Tablets (妇科千金片)VS Antibiotics Antibiotics + Fuke Qianjin Tablets (妇科千金片) VS Antibiotics included in 2 studies[36,65].Meta-analysis results showed that the heterogeneity test showed less heterogeneity (P=0.50,I2=0%),so the fixed effect model was used to combine the effect size. Meta-analysis results showed that compared with the control group, the total effective rate of the Fuke Qianjin Tablets (妇科千金片) group was signi ficantly improved (RR = 1.33, 95% CI [1.16,1.54],P<0.0001 ), as shown in Figure 5.
Routine Therapy + Fuke Qianjin Tablets (妇科千金片) VS Routine Therapy Routine Therapy+ Fuke Qianjin Tablets (妇科千金片) VS Routine Therapy included 2 studies[41,47], and the metaanalysis results showed that heterogeneity test showed less heterogeneity (P=0.89,I2=0%), so the fixed effect model was used to combine the effect size. Meta-analysis results showed that compared with the control group, the total effective rate of the Fuke Qianjin Tablets (妇科千金片) group was significantly improved (RR = 1.32, 95% CI [1.15,1.52] ,P<0.0001), as shown in Figure 6.
Levofloxacin + Metronidazole + Fuke Qianjin Tablets (妇科千金片) VS Lefloxacin + Metronidazole Levo floxacin + Metronidazole + Fuke Qianjin Tablets(妇科千金片) VS Levofloxacin + Metronidazole included in 24 studies[14,15,20,21,23-25,28-30,32,33,37-39,40,42,50,51,53,60-63]. Meta-analysis results showed that heterogeneity test showed less heterogeneity
(P=0.23,I2=16%), so the fixed effect model was used to combine the effect size. Meta-analysis results showed that compared with the control group, the total effective rate of the Fuke Qianjin Tablets (妇科千金片)group was signi ficantly improved (RR = 1.22, 95% CI[1.19, 1.26],P<0.00001), as shown in Figure 7.
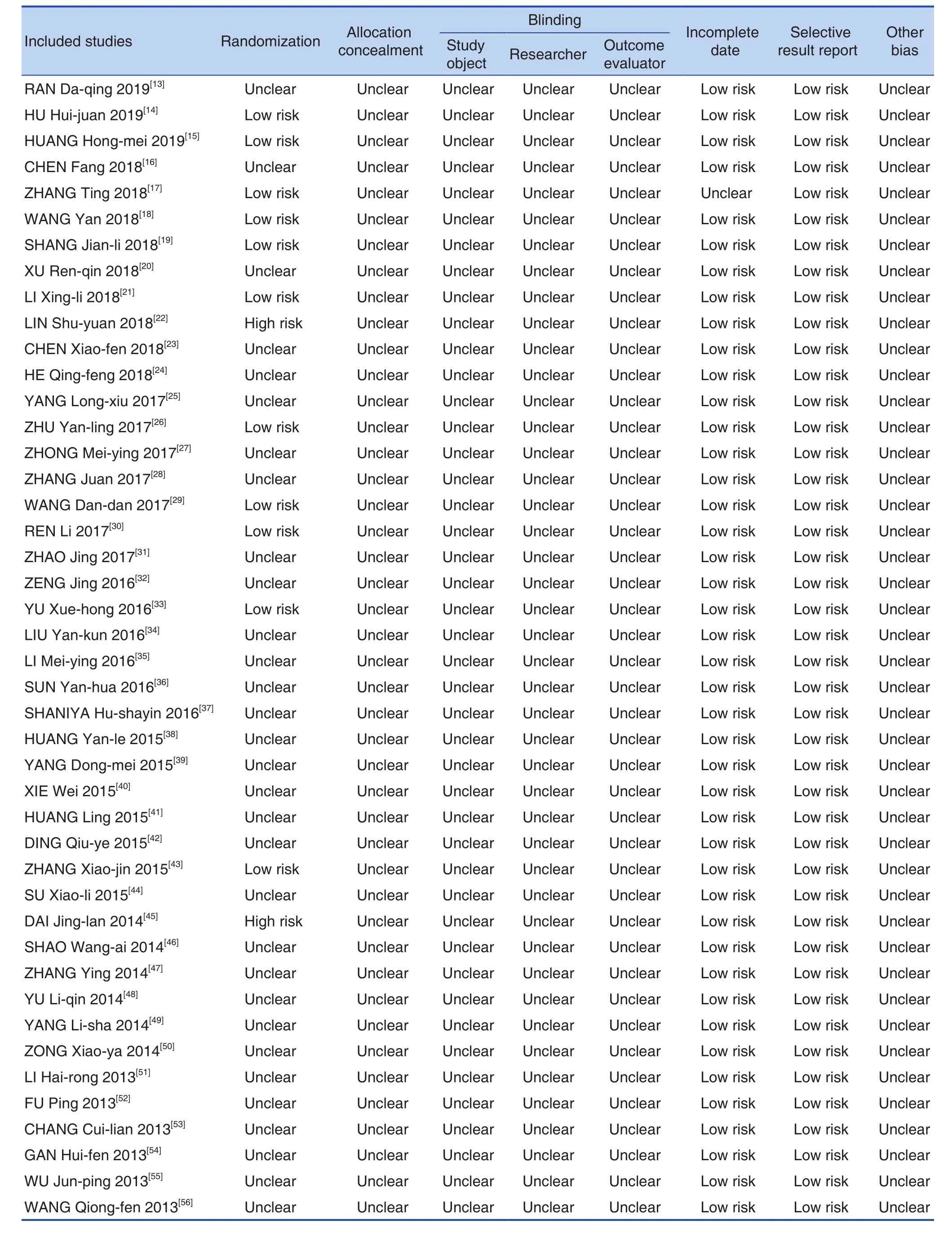
Table 3. Risk Assessment Form
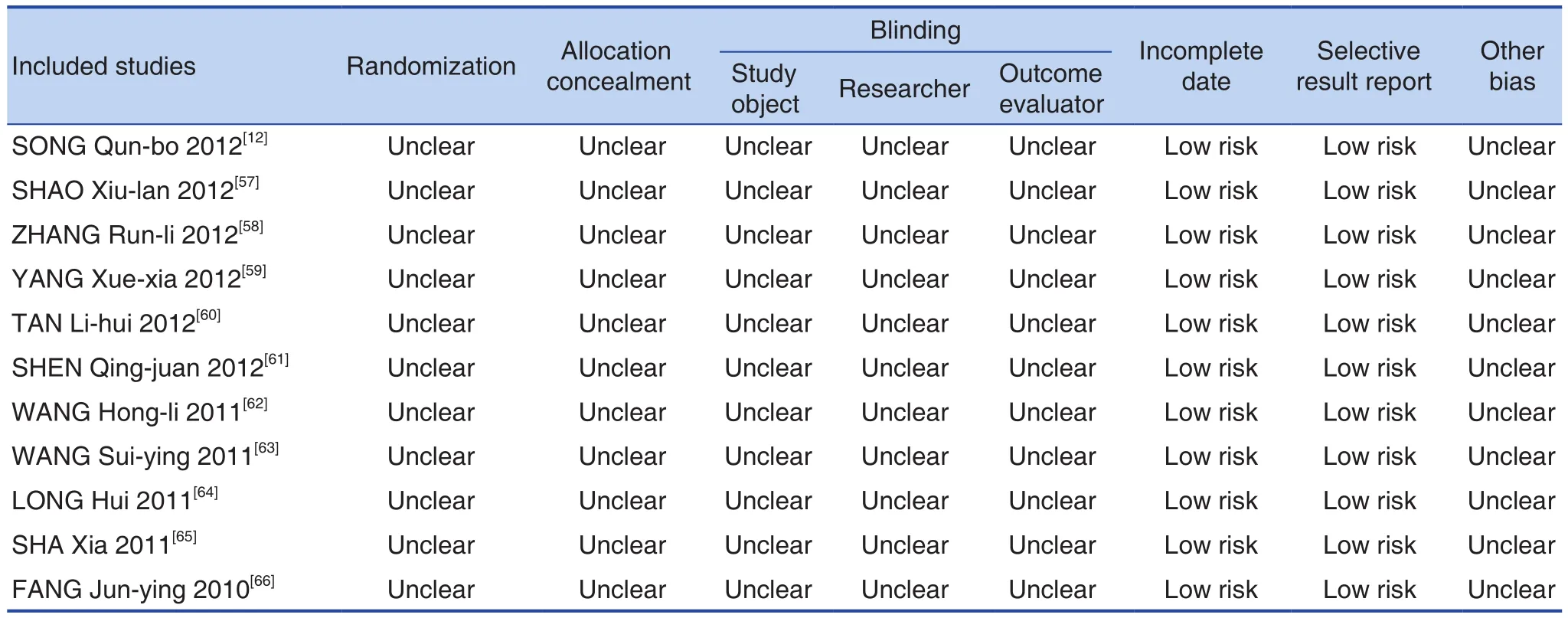
(Continued)

Figure 3. Total Effective Rate: Clindamycin + Fuke Qianjin Tablets (妇科千金片) VS Clindamycin Oral Administration/ Intravenous Drip
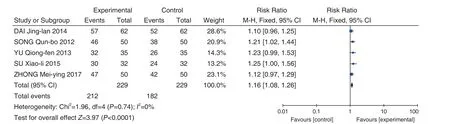
Figure 4. Total Effective Rate: Penicillin + Fuke Qianjin Tablets (妇科千金片) VS Penicillin Intravenous Drip
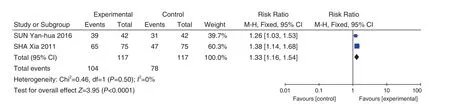
Figure 5. Total Effective Rate: Antibiotic + Fuke Qianjin Tablets (妇科千金片) VS antibiotic intravenous drip
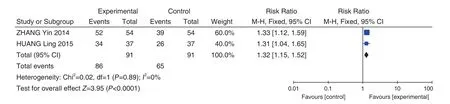
Figure 6. Total Effective Rate: Routine Therapy + Fuke Qianjin Tablets (妇科千金片) VS Routine Therapy

Figure 7. Total Effective Rate: Levo floxacin + Metronidazole + Fuke Qianjin Tablets (妇科千金片) VS Le floxacin + Metronidazole
Levofloxacin + Fuke Qianjin Tablets (妇科千金片) VS Levofloxacin Levofloxacin + Fuke Qianjin Tablets (妇科千金片) VS Levofloxacin included in 4 studies[17-19,31]. Meta-analysis results showed that the heterogeneity test showed less heterogeneity (P=0.96,I2=0%), so the fixed effect model was used to combine the effect size. Meta-analysis results showed that compared with the control group, the total effective rate of the Fuke Qianjin Tablets (妇科千金片) group was signi ficantly improved (RR = 1.28, 95% CI [1.13,1.45],P<0.00001), as shown in Figure 8.
Improvement of abdominal pain
3 studies[13,41,46]reported the improvement of abdominal pain. After heterogeneity test, the heterogeneity among the studies was obvious(I2=71%), so the random effect model was used(RR = 1.40, 95% CI [1.04, 1.88],P=0.03). The analysis found no statistical difference between the 3 studies, as shown in Figure 9. After reading the original literature, it was found that the average course of disease had obvious differences between 1 study[41]and others 2 studies[13,46], which may be the main reason for the large heterogeneity. After excluding this study and re-analyzing the other 2 studies, the heterogeneity was signi ficantly reduced(P=0.26,I2=20%), so a fixed effect model was used.The results showed that the differences between the 2 groups were statistically significant (RR = 1.62,95% CI [1.27, 2.05],P<0.0001).
Normal Time of Leucorrhea
2 studies[13,46]reported improvements in leucorrhea. Heterogeneity tests showed less heterogeneity (P=0.87,I2=0%), so a fixed effect model was used for meta-analysis. The results showed that compared with the control group, the total effective rate of the Fuke Qianjin Tablets (妇科千金片) group was significantly improved and the difference was statistically significant (RR = 1.38, 95% CI [1.16,1.65],P=0.0004), as shown in Figure 10.

Figure 8. Total Effective Rate: Levo floxacin + Fuke Qianjin Tablets (妇科千金片) VS Levo floxacin
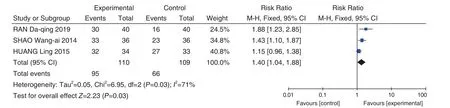
Figure 9. Forest Plot of Abdominal Pain Improvement
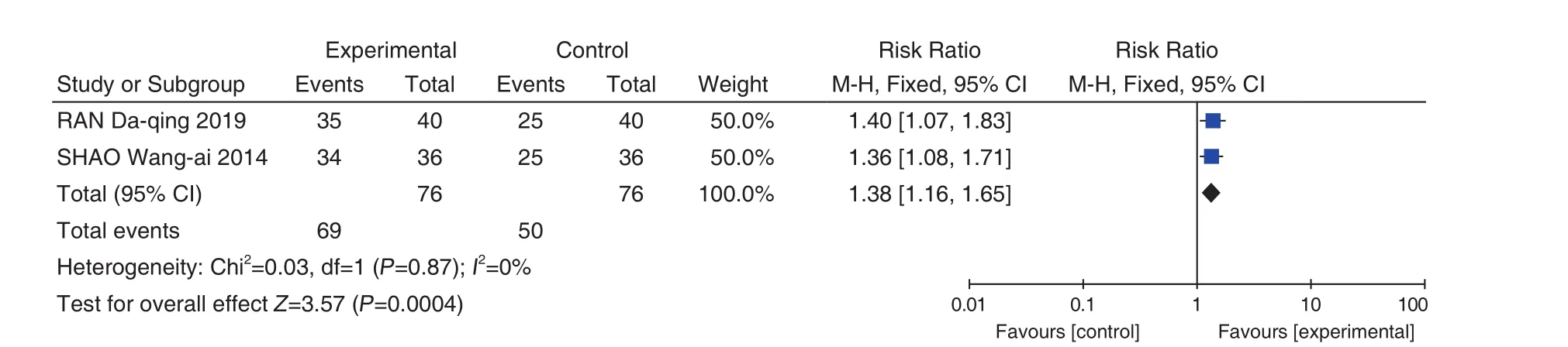
Figure 10. Forest Plot of Normal Time of Leukorrhea
Adverse Reactions
A total of 24 studies[12,15,16,23,24,26-28,30,33,34,37,38,41-43,
47-49,51,59,61,64,66]reported the occurrence of adverse reactions, of which 22 studies[12,15,16,23,24,26,28,30,33,34,37,38,41-43,47-49,51,59,61,66]occurred adverse reactions and were all relieved after discontinuation of the drug. No obvious adverse reactions were seen in the 2 studies.The speci fic conditions were shown in Table 4.
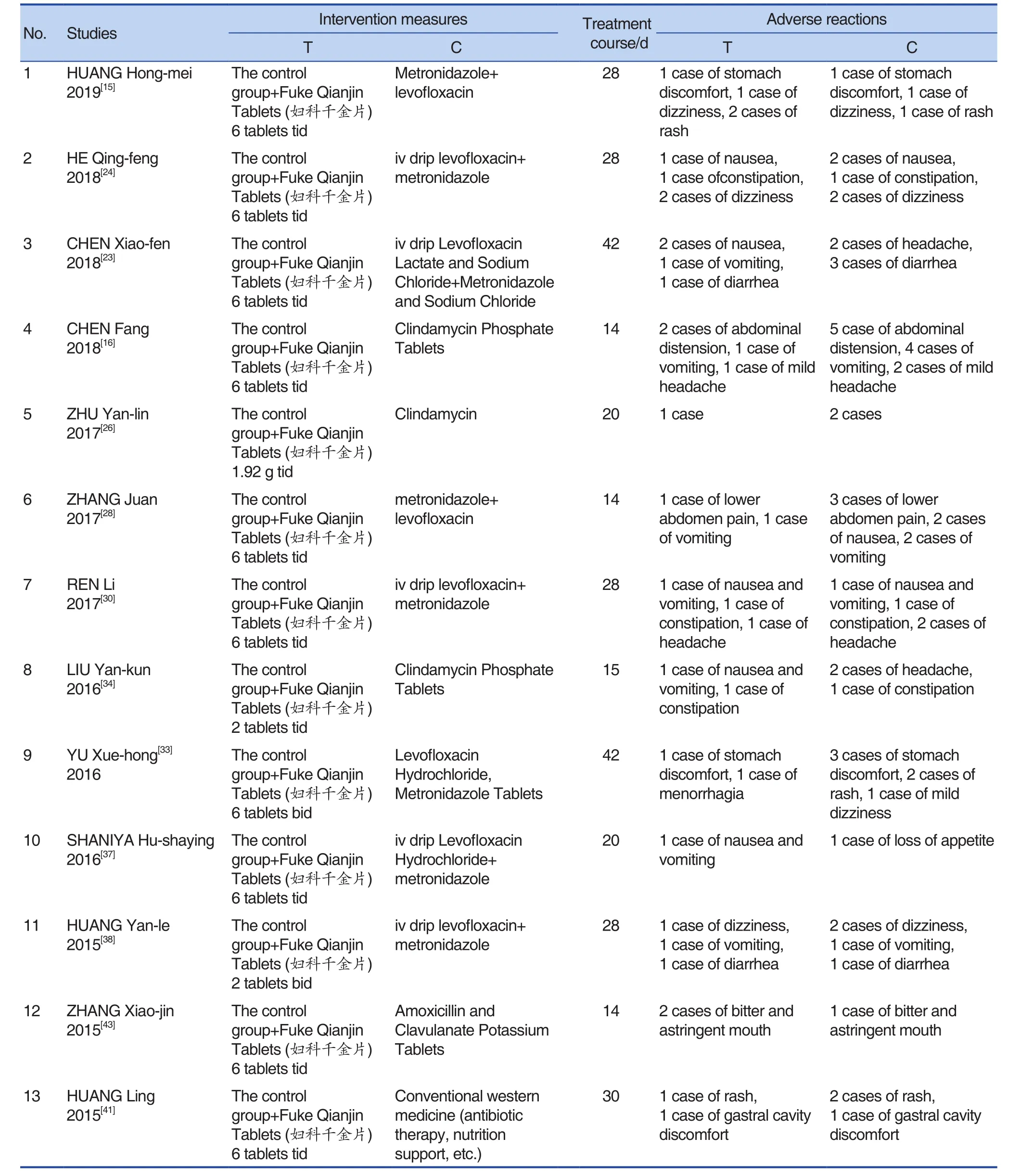
Table 4. Summary of Adverse Reactions
Publishing Bias
A publication bias analysis was performed on the total effective rate and adverse reactions of the 2 subgroups with the number of studies10, as shown in Figures 11 and Figure 12. As can be seen from the funnel plot, the asymmetry of the studies on both sides indicated that there may be potential publication bias, and the reasons for the publication bias were diverse. It may be that the negative results were not published, and some of the literature was of low quality and few samples included. The analysis studies were all in Chinese, so there may be factors such as language publishing bias.
(Continued)
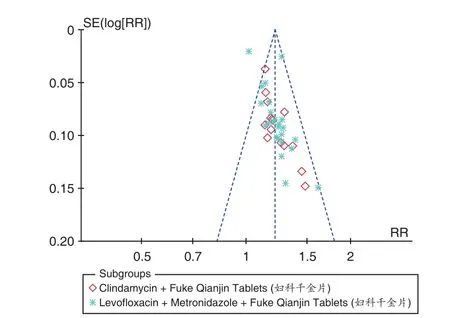
Figure 11. Funnel Plot of Total Effective Rate
DISCUSSION
Effectiveness of Fuke Qianjin Tablets (妇科千金片) in the Treatment of Chronic Pelvic In flammatory Disease
This study used rigorous inclusion and exclusion criteria to finally screen out 55 studies,which systematically evaluated the effectiveness of Fuke Qianjin Tablets (妇科千金片) in the treatment of chronic pelvic in flammatory disease from the total effective rate, improvement of abdominal pain, and normal time of leucorrhea. For analyzing the total effective rate, the study performed subgroup analysis based on different interventions; For relieving clinical symptoms (abdominal pain, leucorrhea abnormalities), qualitative analysis was used. The results showed that in the case of consistent ef ficacy standards, Fuke Qianjin Tablets (妇科千金片)combined with antibiotics (clindamycin phosphate tablets, penicillin, levo floxacin, metronidazole, etc.)were better than antibiotics alone in the treatment of chronic pelvic inflammation. On the basis of conventional treatment, the efficacy of Fuke Qianjin Tablets (妇科千金片) in the treatment of chronic pelvic in flammatory disease combined with antibiotics was also better than that of antibiotics alone. It also has obvious advantages in the relief of abdominal pain and leucorrhea, which can effectively relieve the clinical signs and symptoms of patients with chronic pelvic in flammatory disease.
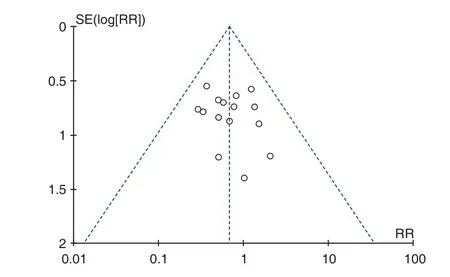
Figure 12. Funnel Plot of Adverse Reaction Rate
Safety of Fuke Qianjin Tablets (妇科千金片) in the Treatment of Chronic Pelvic In flammatory Disease
This study mainly systematically evaluated the safety of Fuke Qianjin Tablets (妇科千金片)in the treatment of chronic pelvic inflammation from the occurrence of adverse reactions. Metaanalysis results showed that the incidence of adverse reactions of Fuke Qianjin Tablets (妇科千金片) combined with antibiotics in chronic pelvic inflammation was significantly lower than that of antibiotics alone. It showed that the treatment of chronic pelvic inflammatory disease with Fuke Qianjin Tablets (妇科千金片) combined with antibiotics can not only play a synergistic effect, but also reduce the incidence of adverse reactions.
A total of 24 studies in this study reported adverse reactions after medication, of which 2 studies reported no adverse reactions in both groups, and 22 studies reported adverse reactions in the experiment group or the control group. Most of the symptoms were gastrointestinal discomfort,headache, dizziness, and rash, which did not affect the normal treatment. The patients could relieve themselves after stopping the drug without serious adverse reactions. Due to the combination of drugs, incomplete research information, and low methodological quality, its safety needs further research and clarification. This study recommends the standardized use of Fuke Qianjin Tablets (妇科千金片) for clinical monitoring and standardized records, and the combined use of drugs should be avoided as much as possible to reduce the risk of adverse drug reactions.
Methodological Quality of the Study
There were differences in the speci fic content of western medicine treatment among the studies included, which may lead to heterogeneity and affect the research results. For different interventions included in the study, subgroup analysis was performed, which reduced the heterogeneity between studies to a certain extent and improved the accuracy of the results.
From the randomized controlled trials of Fuke Qianjin Tablets (妇科千金片) for chronic pelvic inflammation included in this study, the limitations are mainly as follows: ① A total of 53 studies mentioned the random method, of which 9 studies showed that the random number table method was adopted. None of the studies mentioned the blind method and allocation concealment, and it was impossible to determine whether the random and blind methods were truly implemented, and it was prone to bias issues. ② All studies did not report lost follow-up; ③ No studies reported long-term followup. Chronic pelvic inflammatory disease is difficult to heal. Long-term follow-up are needed, but all the included literatures did not peform follow-up, so it is impossible to determine the long-term efficacy; ④Only 28 studied had sample size100 cases, 14 sample size70. Due to small sample size, some studies may result in unstable efficacy indicators and low test ef ficacy. ⑤ A total of 7 reports showed that patients have signed informed consent. ⑥ The total effective funnel chart showed an asymmetric distribution, which indicated that there was a publication bias. Subjective bias exaggerated the improvement of the experiment group on chronic pelvic in flammatory disease.
Compared with the previously published 3 systematic reviews/meta-analysis results of Fuke Qianjin Tablets (妇科千金片) for the treatment of chronic pelvic inflammatory disease, the results of this study are roughly the same in terms of total treatment effectiveness, but there are many limitations and shortcomings in the previous systematic review, of which the intervention measures (Fuke Qianjin Capsules (妇科千金胶囊)or Fuke Qianjin Tablets (妇科千金片)) of 1 study[10]were not unified. The intervention measures are too complicated, and there are large differences between studies, which cannot accurately reflect the effect of Fuke Qianjin Tablets (妇科千金片)on chronic pelvic inflammation; The intervention measures of 1 study[11]included applied 2 Chinese patent medicines at the same time, which led to inaccurate research conclusions, and the publication time was too long, and the data urgently needed to be updated. 1 study[9]was not comprehensively searched and only included 12 papers. The total sample size was small and the quality was low.The latest literature search of the above 3 articles was up to February 2017, and this study has been searched until March 2019, and 19 new studies that meet the criteria after February 2017 have been newly selected. At the same time, in order to avoid incomplete search, this study has developed a strict and comprehensive search strategy. The included studies used uni fied intervention measures to expand the search scope. A total of 55 studies were included in the study, with a total sample size of 6826 cases. The research results are more accurate, more representative, and extensive. This study more systematically and comprehensively evaluated the effectiveness and safety of Fuke Qianjin Tablets (妇科千金片) in the treatment of chronic pelvic in flammatory disease from the total effective rate, the relief of abdominal pain, the normal time of leucorrhea and the occurrence of adverse reactions, providing newer, more comprehensive and accurate information and evidence-based evidence for clinical practice.
Clinical Guidance Signi ficance
Based on the results of this study, Fuke Qianjin Tablets (妇科千金片) combined with antibiotics has a significant effect on chronic pelvic inflammatory disease, which can increase the total effectiveness and improve clinical symptoms and signs. In terms of total effectiveness, analysis showed that the results of the Fuke Qianjin Tablets (妇科千金片)combined with antibiotics are signi ficantly better than the antibiotic single-use group, which suggests that in clinical practice for patients with chronic pelvic inflammatory disease, we can consider adding Fuke Qianjin Tablets (妇科千金片) on the basis of antibiotics to improve the efficacy and reduce the incidence of adverse reactions. The use of Fuke Qianjin Tablets (妇科千金片) can help reduce the use of antibiotics, and can reduce the incidence of antibiotic abuse and antibiotic resistance to some extent. In view of the low overall quality of this included study, the results of the study need to be further confirmed, so clinicians need to take into account the actual situation of patients, carefully refer to the results of this study, follow treatment guidelines and combine clinical experience to integrate all aspects of social-physical-psychological conditions. Based on comprehensive consideration,this can guide clinical practice.
CONCLUSION
To sum up, based on the existing research data, evidence and methods, Fuke Qianjin Tablets(妇科千金片) combined with antibiotics or Fuke Qianjin Tablets (妇科千金片) can significantly improve the clinical efficacy in the treatment of chronic pelvic inflammatory disease, and can improve the discomfort such as abdominal pain and leucorrhea to a certain extent, improve the quality of life of patients, and have not found serious adverse reactions. However, because the methodological quality of the included studies is not high, and some of the evidence is of low grade, it is recommended to conduct long-term follow-up in future studies and carry out large-scale, multi-center randomized double-blind controlled trials to further verify the effectiveness and safety of Fuke Qianjin Tablets(妇科千金片) in the treatment of chronic pelvic in flammation, especially long-term ef ficacy, improve the quality of evidence.
 World Journal of Integrated Traditional and Western Medicine2020年3期
World Journal of Integrated Traditional and Western Medicine2020年3期
- World Journal of Integrated Traditional and Western Medicine的其它文章
- INSTRUCTION FOR AUTHORS
- Expert Consensus of Guangdong Province on Prevention and Treatment of Coronavirus Disease 2019 with Integrated Chinese and Western Medicine(Trial Version I)
- Guangdong Association of Integrative Medicine Expert Consensus of Guangdong Province on Prevention and Treatment of COVID-19 with Integrated Chinese and Western Medicine
- Advantages and Suggestions of Scraping to Promote Rehabilitation of COVID-19 Patients in Convalescence
- Meditation-based Interventions Might be Helpful for Coping with the Corona Virus Disease 2019 (COVID-19)
- Effectiveness and Safety of Jinye Baidu Granules(金叶败毒颗粒) for Acute Upper Respiratory Tract Infection:A Systematic Review and Meta-analysis
