Effect of high flux hemodialysis on renal anemia and soluble transferrin receptor in hemodialysis patients
Xiang-Geng Chi, Wen-Bin Zhang, Qi Cai, Yuan-Zhuan Chen, De-Liang Ding
Department of Nephrology, the Affiliated Xiaolan Hospital of Southern Medical University, Zhongshan 528415, China
Keywords:
ABSTRACT
1. Introduction
The number of people with chronic renal failure (CRF) in China each year can reach 100 to 150 per million population. Statistics in 2018 show that the number of recorded hemodialysis is 636,000 [1]. Due to the extreme lack of kidney donors, hemodialysis is currently used to treat end-stage renal disease (ESRD), but hemodialysis can only replace some physiological functions of the kidney and cannot achieve the desired results. Low-flux hemodialysis (LFHD) only has a certain ability to remove small molecule toxins, and has very low ability to remove medium to large molecule toxins, and its dialysis membrane has poor biocompatibility and is prone to long-term complications; Although hemodiafiltration (HDF) can remove medium and large-molecule toxins and reduce the incidence of complications, its equipment is complicated, cumbersome to operate, and expensive, and it is not suitable for long-term application [2]. In recent years, high-throughput hemodialysis (HFHD) has gradually been used in clinical practice, and has been valued for its ability to reduce mortality and reduce the incidence of long-term complications. Iron deficiency is a secondary cause of renal anemia in hemodialysis patients. Clinically, renal anemia is mainly treated by iron and erythropoietin, but various factors such as iron deficiency can still affect its efficacy [3]. This article explores the effects of high-throughput hemodialysis on soluble transferrin receptor in hemodialysis patients and the effect of improving renal anemia.
2. Materials and methods
2.1 General Information
A total of 132 patients undergoing maintenance hemodialysis with renal anemia in our hospital from 2017-07 to 2019-07 were selected and divided into a control group and an observation group according to the random number table method, with 66 cases in each group. Observation group: 17 males and 49 females; aged 46 to 55 years, average (53.1 ± 1.9) years; dialysis age 2 to 5 years, average (4.1 ± 0.5) years; primary disease: chronic glomerulonephritis 28 There were 16 cases of diabetic nephropathy, 12 cases of hypertensive renal damage, 6 cases of polycystic kidney disease, and 4 cases of hyperuricemia nephropathy. Control group: 21 males and 45 females; aged 47 to 54 years, average (53.4 ± 2.1) years; dialysis age 1 to 6 years, average (4.6 ± 0.4) years; primary disease: chronic glomerulonephritis 27 There were 18 cases of diabetic nephropathy, 13 cases of hypertensive renal damage, 5 cases of polycystic kidney disease, and 3 cases of hyperuricemia nephropathy. Inclusion criteria: 18 to 70 years of age; with renal anemia; regular hemodialysis for> 6 months; autologous arteriovenous fistula or artificial blood vessel as the dialysis vascular access, receiving erythropoietin treatment for ≥1 month. Exclusion criteria: those with severe malnutrition or infection; those with severely impaired heart, lung, liver and other organ functions; those with hypotension; those with a tendency to bleeding; those with iron allergy. There was no significant difference in gender and age between the two groups (P> 0.05). See Table 1.
2.2 Method
All patients underwent routine hemodialysis before enrollment. The control group used Fresenius F6 polysulfone membrane dialyzer (surface area 1.3 m2filtration coefficient 5.5 ml/h·mm Hg); The observation group used FX60 polysulfone membrane highthroughput dialyzer (surface area 1.3m2, filtration coefficient 46mL/h·mmHg); The flow of dialysate is 500 mL / min, the blood flow is 250-300 ml/min, and the dialysis path: autologous arteriovenous fistula or artificial blood vessel, 4 h/time, 3 times/week. In the observation group during the first two weeks of high-throughput dialysis treatment, 10 patients were found to be unsuitable for high-throughput dialysis, 7 were excluded, and 3 were transferred to the control group. Low-molecular-weight heparin was used for anticoagulation, and erythropoietin was given according to the condition. Both groups were treated for 6 months. After the study, the patients chose the follow-up dialysis method. The amount of dialysate flow, blood flow, time, anticoagulant, iron, and erythropoietin usage were unchanged before and after the test. Collect 4 mL of blood in the arterial end of the dialysis circuit before the first dialysis and 10 minutes before the end of the dialysis (blood flow rate: 50 mL / min). Serum β2 microglobulin (β2-MG) and serum creatinine (Scr) were measured. , Blood urea nitrogen (BUN), anemia and iron metabolism indicators.
2.3 Observation indicators
(1) β2-MG, Scr, BUN; (2) Anemia indicators: hemoglobin (Hb), hematocrit (HCT), reticulocyte percentage (Ret%); (3) iron metabolism indicators: serum ferritin (SF ), Serum sTfR, hepcidin (Hepc) level, transferrin saturation (TSAT); (4) dialysis adequacy index Kt / v value; (5) adverse reactions.
2.4 Statistical processing
SPSS 22.0 software was used to statistically analyze the data. The measurement data were expressed as mean ± standard deviation. The t test was used. The count data were expressed as frequency and number. The χ2test was used. P <0.05 was considered statistically significant.
3. Results
3.1 Comparison of β2-MG, Scr, BUN levels and kt/v values between the two groups before and after treatment
Comparison of β2-MG, Scr, BUN levels between the two groups before treatment (P> 0.05). After treatment, the levels of Scr and BUN in the two groups were significantly reduced (P <0.05), but compared between the two groups (P> 0.05); β2-MG levels in the observation group were lower than those in the control group (P <0.05). The kt v value in the observation group was greater than that in the control group (P <0.05). See Table 2.
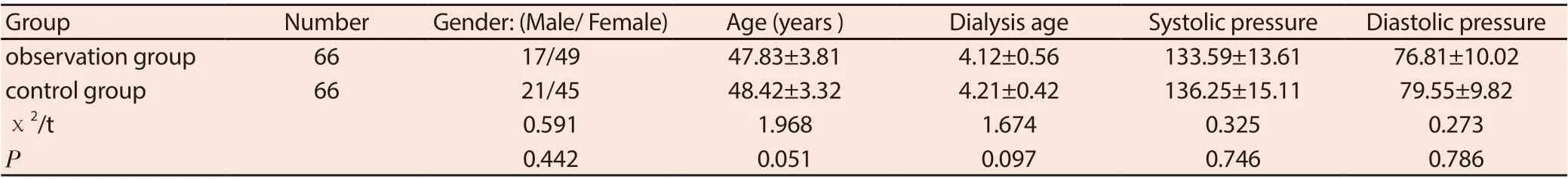
Table 1 General information comparison
Table 2 Comparison of β2-MG, Scr, BUN levels before and after treatment between the two groups (±s)
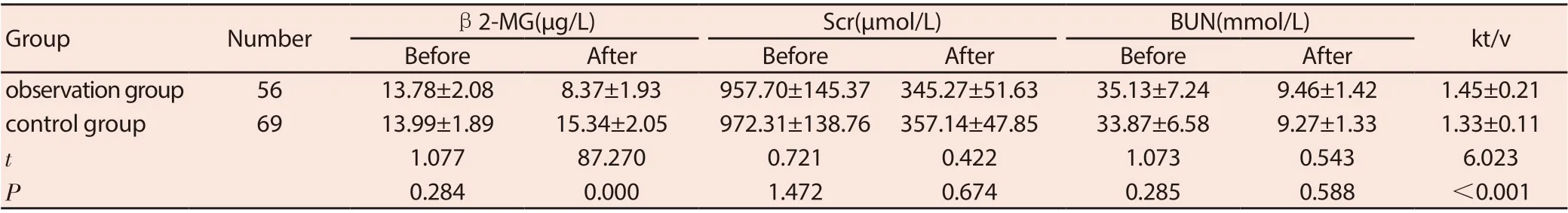
Table 2 Comparison of β2-MG, Scr, BUN levels before and after treatment between the two groups (±s)
GroupNumberβ2-MG(μg/L)Scr(μmol/L)BUN(mmol/L)kt/v BeforeAfterBeforeAfterBeforeAfter observation group5613.78±2.088.37±1.93957.70±145.37 345.27±51.6335.13±7.249.46±1.421.45±0.21 control group6913.99±1.8915.34±2.05972.31±138.76 357.14±47.8533.87±6.589.27±1.331.33±0.11 t 1.07787.2700.7210.4221.0730.5436.023 P 0.2840.0001.4720.6740.2850.588<0.001
Table 3 Comparison of anemia index levels between the two groups before and after treatment (±s)
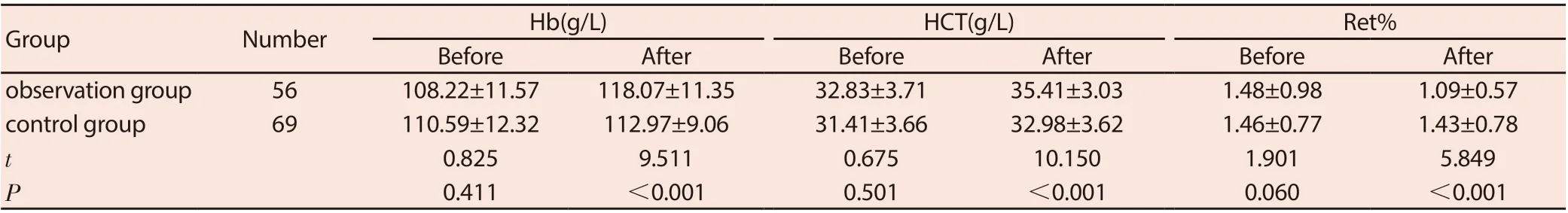
Table 3 Comparison of anemia index levels between the two groups before and after treatment (±s)
GroupNumberHb(g/L)HCT(g/L)Ret%BeforeAfterBeforeAfterBeforeAfter observation group56108.22±11.57118.07±11.3532.83±3.7135.41±3.031.48±0.981.09±0.57 control group69110.59±12.32112.97±9.0631.41±3.6632.98±3.621.46±0.771.43±0.78 t 0.8259.5110.67510.1501.9015.849 P 0.411<0.0010.501<0.0010.060<0.001
Table 4 Comparison of metabolic index levels between the two groups before and after treatment (±s)
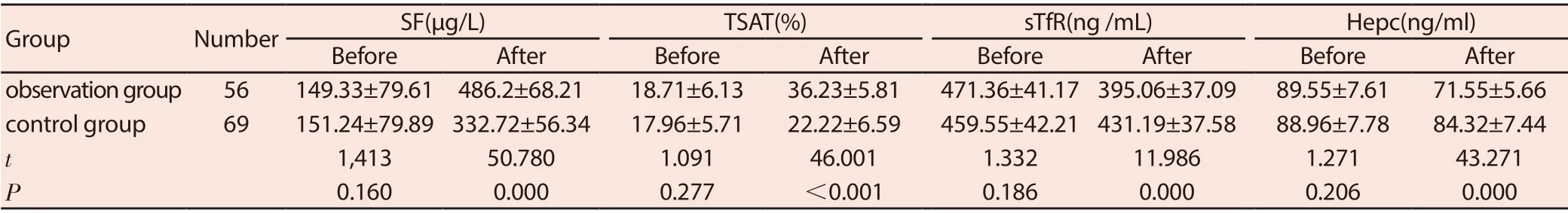
Table 4 Comparison of metabolic index levels between the two groups before and after treatment (±s)
GroupNumberSF(μg/L)TSAT(%)sTfR(ng /mL)Hepc(ng/ml)BeforeAfterBeforeAfterBeforeAfterBeforeAfter observation group56149.33±79.61 486.2±68.2118.71±6.1336.23±5.81471.36±41.17 395.06±37.0989.55±7.6171.55±5.66 control group69151.24±79.89 332.72±56.3417.96±5.7122.22±6.59459.55±42.21 431.19±37.5888.96±7.7884.32±7.44 t 1,41350.7801.09146.0011.33211.9861.27143.271 P 0.1600.0000.277<0.0010.1860.0000.2060.000
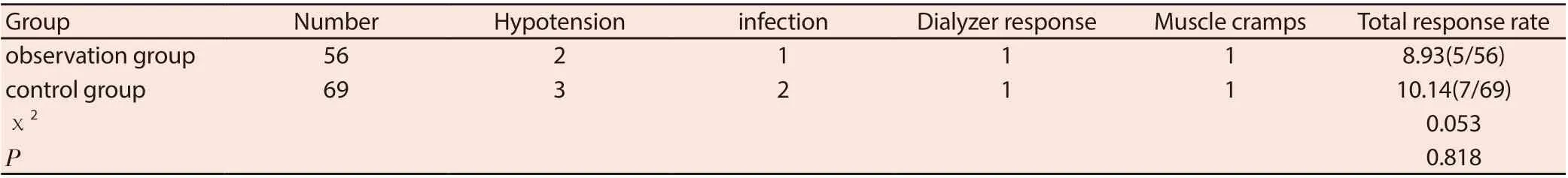
Table 5 Comparison of adverse reactions between the two groups (n/%)
3.2 Comparison of anemia index levels between the two groups before and after treatment
Before treatment, the Hb, HCT, and Ret% of the two groups were compared (P> 0.05); after treatment, the Hb and HCT levels in the observation group were higher than the control group, and the Ret% was lower than the control group (both P <0.05). See Table 3.
3.4 Comparison of iron metabolism indexes before and after treatment in the two groups
Before treatment, the levels of ST, TAST, sTfR, and Hepc were compared between the two groups (P> 0.05). After treatment, the levels of SF and TAST in the observation group were higher than those in the control group, and the levels of sTfR and Hepc were lower than those in the control group (P <0.05). See Table 4.
3.5 Comparison of adverse reactions between the two groups
Comparison of the total incidence of adverse reactions between the two groups (P> 0.05). See Table 5.
4. Discussion
Renal anemia is one of the major complications in patients with chronic kidney disease (CKD) and maintenance hemodialysis, and it is also an independent risk factor for cardiovascular disease in patients with CKD [4]. Decreasing erythropoietin is the leading cause of renal anemia, and improving the efficacy of erythropoietin is the focus of clinical research. It has been reported that improving dialysis adequacy can significantly reduce the amount of erythropoietin, and large, medium and small molecular toxins such as BUN, β2-MG in the blood of hemodialysis patients can affect red blood cell production and metabolism. In theory, as long as these are effectively removed, Molecular toxins can achieve the best dialysis results. The low-throughput F6 polysulfone membrane dialyzer has a small membrane pore size, which can only remove small molecule toxins such as Scr, BUN, etc. by diffusion, and the large molecule toxin such as β2-MG is not effective [5-7]. The high-throughput FX60 polysulfone membrane dialyzer has a large membrane pore size and high permeability, which can not only remove toxins through diffusion, but also have some convection effects. It can not only remove small molecule toxins, but also significantly accelerate the removal of medium and large molecule toxins[8].
The results of this study show that the levels of β2-MG, Scr, and BUN in the two groups after treatment are significantly lower than before treatment. However, there is no significant difference in the levels of Scr and BUN between the two groups, indicating that both methods can reduce the blood of hemodialysis patients. Small molecule toxins. β2-MG is a small-molecule globulin produced by lymphocytes, polymorphonuclear leukocytes and platelets, and belongs to the medium molecular substance [9]. The β2-MG level in the observation group was significantly lower than that in the control group after treatment, indicating that high-flux hemodialysis is better than low-flux hemodialysis in removing medium and large molecular toxins, suggesting that high-flux hemodialysis may affect red blood cells by removing blood The produced molecular toxins accelerate the production of red blood cells, thereby improving renal anemia. Higher Hb levels indicate better nutritional status of the body; HCT can reflect the increase or decrease of red blood cells [10]. The results of this study show that the Hb and HCT levels in the observation group after treatment are higher than those in the control group, indicating that high-flux hemodialysis can promote red blood cell production and improve the nutritional status of the body. SF is a complex of apoferritin and iron core Fe3+, which is a storage form of iron, which can be used as an effective indicator of whether the body is short of iron or overloaded [11]. TSAT is the ratio of the binding capacity of serum iron to transferrin. Its increase can be seen in aplastic anemia, megaloblastic anemia, hemolytic anemia, etc., and it can be seen in iron deficiency anemia, erythrocytosis, and inflammation. [12]. sTfR is produced by proteolysis of cell surface receptors. Serum sTfR and different transferrins exist as complexes. About 80% of serum sTfR is derived from early red blood cells. When red blood cell production activity increases, especially iron deficiency increases sTfR synthesis [13-14]. Hepc Hepcidin is a cysteine-rich antibacterial peptide synthesized and secreted by liver cells, which can control duodenal iron absorption and macrophage iron release and negatively regulate iron balance in the body [15]. Stem cells store excess iron in the organism and are transported to transferrin via iron membrane export protein 1 (FPN1), which is discharged with the intestinal epithelial cells that have fallen off. Hepc competitively binds with (FPN1) in large quantities, preventing iron from entering the hematopoietic process, which in turn Renal anemia [16]. The results of this study show that the levels of SF and TAST in the observation group after treatment are higher than those in the control group, and the levels of sTfR and Hepc are lower than those in the control group, indicating that high-flux hemodialysis to clear Hepc can increase iron utilization and thereby improve renal anemia. There was no significant difference in the overall incidence of adverse reactions between the two groups, indicating that highthroughput hemodialysis did not increase the incidence of adverse reactions.
In summary, high-throughput hemodialysis can significantly improve renal anemia, iron metabolism, reduce serum sTfR levels, and have fewer adverse reactions.
 Journal of Hainan Medical College2020年13期
Journal of Hainan Medical College2020年13期
- Journal of Hainan Medical College的其它文章
- Effects of lumbar sagittal balance remodeling on natural absorption after lumbar disc herniation
- Network pharmacology of threatened abortion treated by ShouTaiWan
- Characteristic changes of intestinal flora and its correlation with clinical indexes in patients with Behcet's disease based on TCM syndromes
- The analysis of acupoint selection rules for acupuncture treating functional constipation
- Meta analysis of clinical efficacy of combination of traditional Chinese and western medicine in the treatment of venous ulcer of lower extremities
- The correlation between different ABO blood group gene loci and the pathogenesis and prognosis of acute myocardial infarction
