Linagliptin-Associated Bullous Pemphigoid: The First Case in China
Yang-Chun Liu, Wen-Ling Zhao,2, Li Li,*
1Department of Dermatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China; 2Department of Dermatology, Shunyi Maternal and Children's Hospital of Beijing Children's Hospital, Beijing 101300, China.
Introduction
Bullous pemphigoid (BP) is the most common subepidermal bullous disease and involves an immune response directed against two hemidesmosome components in basal keratinocytes: BP antigen 180 (BP180) and BP antigen 230.The major epitope in patients with BP is the noncollagenous 16A (NC16A) domain of BP180.1BP most commonly occurs in people of advanced age,especially those aged>70 years.The worldwide incidence of BP ranges from 12 to 66 cases per million people per year, and the disease prevalence increases with age.2Degenerative neurological diseases, psychiatric disorders,and the chronic use of neuroleptics or spironolactone are known independent risk factors for the development of BP.3
Dipeptidyl peptidase 4 (DPP-4) inhibitors are a drug class that was first introduced into the market in 2006 to treat type 2 diabetes mellitus.4In recent years, an increasing body of evidence has suggested that DPP-4 inhibitors may be implicated in the development of BP.5Current knowledge regarding the association between DPP-4 inhibitor intake and BP is based mainly on case reports.5We herein report the first case of DPP-4 inhibitorassociated BP in China, and the DDP-4 inhibitor in this case was linagliptin.
Case report
A 74-year-old woman was admitted to Peking Union Medical College Hospital because of a 20-day history of widespread erythematous tense bullae over her whole body.She had a history of type 2 diabetes mellitus,hyperlipidemia,arterial hypertension,hyperuricemia,and moderate chronic kidney disease.She had used linagliptin for 15 months before the onset of the skin lesions,and she was still using it upon presentation.
Physical examination showed mild erythema and small tense blisters over the trunk and extremities along with extensive erosions and excoriations; however,this patient had less severe erythema than that seen in patients with typical non-drug-induced BP (Fig.1A).Adjunctive laboratory tests showed the following results:leukocytes, 10.44×109/L; serum C-peptide, 6.26μg/L;glycated hemoglobin,8.7%;fasting glucose,7.9mmol/L;2-hour postprandial glucose, 10.4mmol/L; glycated albumin, 22.9%; serum creatine, 121μmol/L; serum urea,8.71mmol/L;and urinary albumin:creatinine ratio,44mg/g.
A skin biopsy showed a subepidermal blister and eosinophil infiltration in the blister fluid.Direct immunofluorescence showed linear deposition of immunoglobulin G and C3 along the basement membrane zone.Her antibasement membrane immunoglobulin G titer(as detected by indirect immunofluorescence) was 1:40 (Fig.1B).The index of anti-BP180 antibody detected by ELISA was 48 IU/mL.The BP disease area index score was 116.According to her clinical manifestations, immunologic findings,and pathologic results,a tentative diagnosis of BP was made.
The patient had been taking linagliptin for 15 months before the eruption of skin lesions.She had also been taking atorvastatin for treatment of hyperlipidemia as well as losartan potassium and metoprolol for treatment of cardiovascular disease.However, we considered that her skin lesions had been induced by linagliptin for the following two reasons.First, the patient had taken atorvastatin, losartan potassium, and metoprolol for 6 years, whereas she had taken linagliptin for only 15 months before eruption of the skin lesions.Second, the skin lesions rapidly improved after she discontinued the linagliptin while continuing the other drugs.
The patient was treated with topical halometasone,which mostly controlled the skin lesions; only sporadic blisters reappeared.Her condition significantly improved after discontinuation of linagliptin.
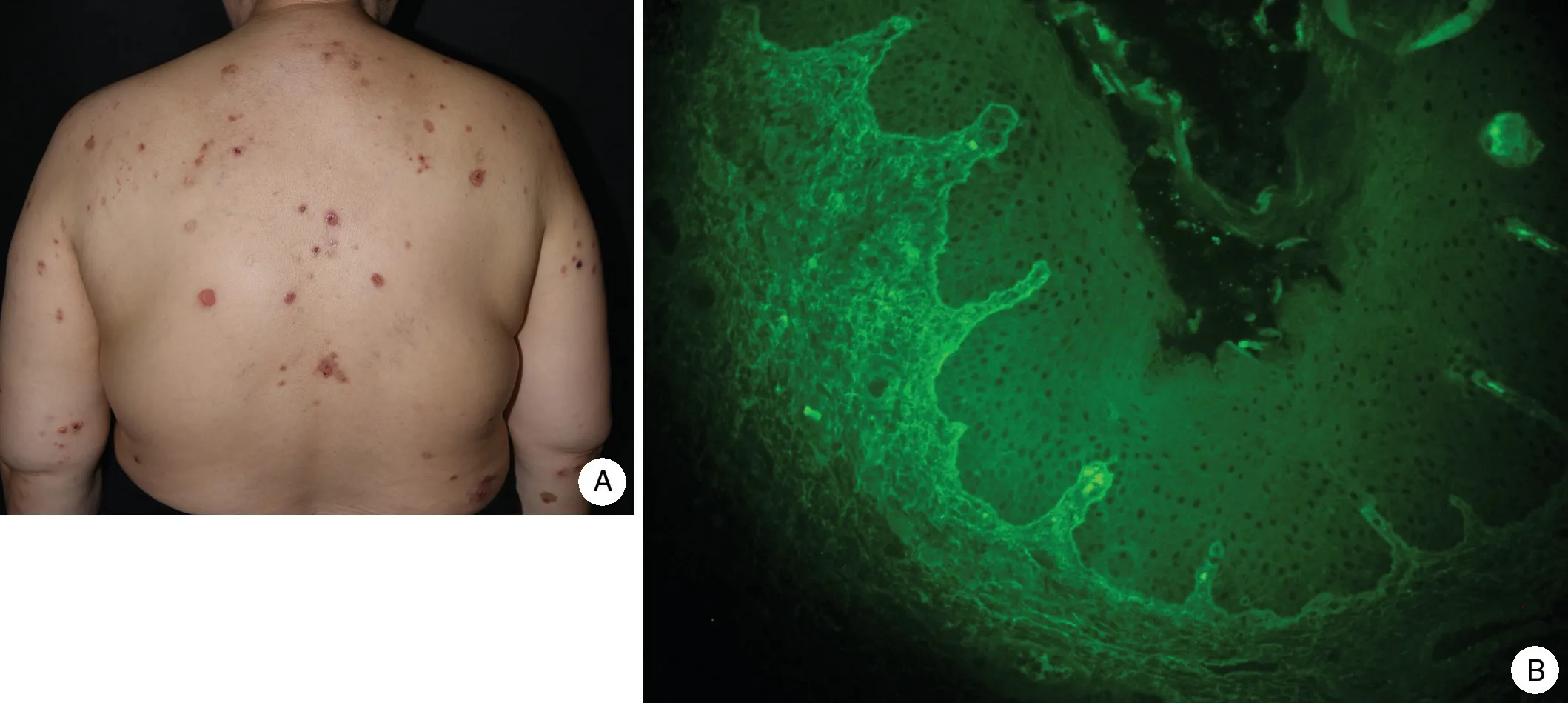
Figure 1.The clinical and pathological features of the patient with A:Physical examination showed widespread erythematous tense blisters and extensive erosions and excoriations over the trunk.B:Indirect immunofluorescence showed linear deposition of immunoglobulin G and C3 along the basement membrane zone.
Discussion
BP is the most common autoimmune disease that involves blistering.It can be induced by diuretics,β-blockers, or antibiotics.3DPP-4 inhibitors are standard treatments for type 2 diabetes mellitus but are also causative agents for BP.Advanced age (60-70 years) is a risk factor for BP associated with use of DPP-4 inhibitors.1The mean time from introduction of DPP-4 inhibitors to BP onset is 10 months (range, 8 days to 37 months).The mean time to improvement after drug withdrawal is 10 days.6Although the pathogenic mechanism has not been elucidated, it might be related to a modified immune response and antigenic alteration of the epidermal basement membrane.Several studies have shown that patients in whom BP is induced by DPP-4 inhibitors usually have noninflammatory manifestations characterized by mild erythema, and these patients also usually have autoantibodies to the midportion of the extracellular domain of BP180 but not the common NC16A domain.6Hence,BP induced by DPP-4 inhibitors seems to be a different scenario from conventional BP.Although anti-BP180-NC16A antibodies may be absent, autoantibodies to full-length BP180 are present.This is because DPP-4 can activate plasminogen to form plasmin, an enzyme that usually cleaves BP180 within the immunodominant NC16A domain.Hence,plasmin inhibition by DPP-4 inhibitors can lead to antigenic alteration from the NC16A domain to outside the NC16A domain.5ELISA of full-length BP180 is useful for monitoring disease activity.After cessation of the use of DPP-4 inhibitors, the epitope can return to the NC16A domain.7
Some studies have also shown that DPP-4 inhibitors can promote eosinophil activation by a C-C motif chemokine-11/eotaxin-mediated mechanism and contribute significantly to blister formation.4Ujiie et al.8found that the frequency of carriers of certain human leukocyte antigen alleles (HLA-DQB1*03:01, HLA-DQA1*05:05, HLADRB1*11:01, and HLA-DRB1*12:01) was significantly higher among patients with BP using DPP-4 inhibitors(especially HLA-DQB1*03:01).Additionally, 86% of patients with noninflammatory DPP-4 inhibitor-associated BP carried HLA-DQB1*03:01.8Genomic tests revealed that our patient had the HLA-DQB1*03:01, HLADQA1*05:05, HLA-DQA1*06:01, HLA-DRB1*11:01,and HLA-DRB1*12:02 haplotypes, which is highly consistent with previous reports.Izumi et al.9demonstrated that DPP-4 inhibitors are involved in the development of noninflammatory BP and that patients with NC16Apositive BP usually present with much more severe erythema than patients with non-NC16A BP.Our patient presented with noninflammatory manifestations, exhibiting little erythema and pruritus; however, autoantibodies to BP180-NC16A were present, which differs from the findings of other studies.Moreover, some studies have shown that patients with DPP-4 inhibitor-associated BP usually have a high prevalence of mucosal involvement and low eosinophil count in peripheral blood.5Our patient had involvement of the vulvar mucous membrane,but the eosinophil count in peripheral blood was within the normal range.
Several cases of DPP-4 inhibitor-associated BP have been reported in recent years, but not in China.There are two possible reasons for the absence of such cases in China.First, there is a lack of concern about the association between use of DPP-4 inhibitors and BP among Chinese clinicians.Second, the autoantibodies in many patients with DPP-4 inhibitor-associated BP usually target the non-NC16A domain of BP180; thus, commercially available ELISAs for NC16A may misdiagnose these patients.Hence, application of ELISAs for full-length BP180 is expected in the future.For our patient,prompt withdrawal of the DPP-4 inhibitor achieved clinical remission within a short time.In conclusion,there is a possible link between the use of DPP-4 inhibitors and BP,which should be paid with more attention, and further studies are needed to demonstrate this correlation and underline mechanisms.
Acknowledgements
This work was supported by the National Key Research and Development Program of China Grant (No.2016YFC0901500), the National Natural Science Foundation of China (No.81972945), the Milstein Medical Asian American Partnership Foundation and Education Reform Projects of Peking Union Medical College (No.2016zlgc0106).
- 国际皮肤性病学杂志的其它文章
- Targetoid Hemosiderotic Nevus
- Eccrine Spiradenoma of the Forehead: A Case Report
- Lues Maligna in an Immune-Reconstituted Patient with Retroviral Infection: A Case Report
- Seborrheic Keratosis in a Young Woman:A Mimicker of Keratoacanthoma
- A Case of Condyloma Acuminatum on the Nipple Detected via Dermoscopy
- Azathioprine Hypersensitivity Syndrome in an Asian Woman

