褐藻胶寡糖对D-半乳糖诱导小鼠白内障的影响
冯美苹 冯文静 胡松 毛拥军

[摘要] 目的 探討褐藻胶寡糖(AOS)对D-半乳糖诱导C57BL/6J小鼠白内障的影响及其机制。
方法 将45只雄性C57BL/6J小鼠随机分为对照组(A组)、D-半乳糖组(B组)、D-半乳糖+AOS低剂量组(C组)、D-半乳糖+AOS中剂量组(D组)和D-半乳糖+AOS高剂量组(E组),每组9只。A组小鼠注射无菌注射用水(5 mL·kg-1·d-1)8周,其余各组小鼠注射D-半乳糖(200 mg·kg-1·d-1)8周;并于第5周开始,A、B两组小鼠给予蒸馏水10 mL/(kg·d)灌胃处理4周,C、D、E组小鼠分别给予AOS 50、100、150 mg·kg-1·d-1灌胃处理4周。实验结束后摘除小鼠眼球,解剖显微镜下分离晶状体,评定各组小鼠晶状体混浊度得分,苏木精-伊红(HE)染色观察各组小鼠晶状体组织病理学改变,Western blot检测各组小鼠凋亡相关蛋白Bax、Bcl-xl和caspase-3表达。
结果与对照组比较,D-半乳糖组晶状体混浊度评分明显升高,差异有统计学意义(F=4.347,P<0.05);与D-半乳糖组相比,AOS干预各组晶状体混浊度评分明显降低,并且呈浓度依赖性降低,差异有统计学意义(P<0.05)。HE染色结果显示,对照组小鼠晶状体上皮细胞单层排列,整齐有序;D-半乳糖组晶状体上皮细胞稍肿胀,细胞大小及排列不均匀;AOS 150 mg·kg-1·d-1灌胃处理显著改善了晶状体上皮细胞的形态结构及排列紊乱。Western blot结果显示,与对照组相比较,D-半乳糖组小鼠晶状体Bcl-xl蛋白表达明显减少,Bax、caspase-3蛋白表达明显增加,差异有统计学意义(F=6.62~9.16,P<0.05);与D-半乳糖组相比,AOS干预各组小鼠晶状体Bcl-xl蛋白表达明显增加,Bax、caspase-3蛋白表达明显减少,差异有统计学意义(P<0.05);不同浓度AOS干预组小鼠晶状体Bcl-xl蛋白表达呈浓度依赖性增加,E组与C组比较差异有统计学意义(P<0.05);不同浓度AOS干预组小鼠晶状体Bax、caspase-3蛋白表达呈浓度依赖性减少,E组与C、D组差异有统计学意义(P<0.05)。
结论 AOS可以延缓D-半乳糖诱导C57BL/6J小鼠白内障的发生,其机制可能与抑制晶状体细胞凋亡有关。
[关键词] 白内障;半乳糖;细胞凋亡;衰老;晶体;上皮细胞
[中图分类号] R329.25
[文献标志码] A
[文章编号] 2096-5532(2020)03-0256-05
doi:10.11712/jms.2096-5532.2020.56.096
[开放科学(资源服务)标识码(OSID)]
[网络出版] http://kns.cnki.net/kcms/detail/37.1517.R.20200519.1445.016.html;2020-05-20 17:09
EFFECT OF ALGINATE OLIGOSACCHARIDES ON D-GALACTOSE-INDUCED CATARACT IN MICE
\ FENG Meiping, FENG Wenjing, HU Song, MAO Yongjun
\ (Department of Geriatric Medicine, The Affiliated Hospital of Qingdao University, Qing-
dao 266000, China)
\; [ABSTRACT]\ Objective\ To investigate the effect of alginate oligosaccharides (AOS) on D-galactose-induced cataract in C57BL/6J mice and its mechanism of action.
\ Methods\ A total of 45 male C57BL/6J mice were randomly divided into control group (group A), D-galactose group (group B), D-galactose+low-dose AOS group (group C), D-galactose+medium-dose AOS group (group D), and D-galactose+high-dose AOS group (group E), with 9 mice in each group. The mice in group A were injected with sterile water for injection (5 mL·kg-1·d-1) for 8 weeks; the mice in other groups were injected with D-galactose (200 mg·kg-1·d-1) for 8 weeks. From the fifth week, the mice in groups A and B were intragastrically administered distilled water (10 mL·kg-1·d-1) for 4 weeks, and the mice in groups C, D, and E were intragastrically administered 50, 100, and 150 mg·kg-1·d-1 AOS, respectively, for 4 weeks. At the end of the experiment, the eyeballs of the mice were removed, and the lens were dissected under a dissecting microscope to evaluate the lens turbidity scores of mice in each group. The histopathological changes of the lens of mice in each group were evaluated using HE staining, and the expression of apoptosis-related proteins, Bax, Bcl-xl, and caspase-3, of mice in each group was determined by Western blot.
\ Results\ Compared with the control group, the D-galactose group had a significantly increased lens turbidity score (F=4.347,P<0.05); compared with the D-galactose group, the AOS-treated groups had significant decreases in lens turbidity score in a concentration-dependent manner (P<0.05). HE staining showed that the lens epithelial cells of mice in the control group were orderly arranged in a single layer, and the lens epithelial cells of mice in the D-galactose group were slightly dilated with non-uniform cell size and arrangement; intragastric administration of AOS (150 mg·kg-1·d-1) significantly improved the mor-
phological structure and disorderly arrangement of the lens epithelial cells. Western blot results showed the following: compared with the control group, the D-galactose group had significantly reduced expression of Bcl-xl protein but significantly increased expression of Bax and caspase-3 proteins in the lens (F=6.62-9.16,P<0.05); compared with the D-galactose group, the AOS-treated groups had significantly increased expression of Bcl-xl protein but significantly reduced expression of Bax and caspase-3 proteins in the lens (P<0.05); the groups treated with different concentrations of AOS showed concentration-dependent increases in the expression of Bcl-xl protein, with a significant difference between group E and group C (P<0.05), and concentration-depen-
dent decreases in the expression of Bax and caspase-3 proteins in the lens, with significant differences between group E and groups C and D (P<0.05).
\ Conclusion\ AOS can delay D-galactose-induced cataract in C57BL/6J mice, which may be associated with the inhibition of lens cell apoptosis.
[KEY WORDS]\ cataract; galactose; apoptosis; aging; lens, crystalline; epithelial cells
白内障是由增龄、免疫和代谢异常、局部营养障碍、创伤等多种原因引起的晶状体代谢紊乱,使晶状体蛋白发生变性,最终导致晶状体混浊。白内障是导致老年人视力障碍的主要原因之一,位居我国致盲性眼病首位[1]。目前白内障的治疗仍以手术治疗为主,但药物治疗同样重要。研究发现,晶状体上皮细胞凋亡在白内障发生和发展过程中起重要的作用[2-3]。D-半乳糖介导的啮齿动物衰老模型已广泛应用于衰老相关性疾病及抗衰老研究。褐藻胶寡糖(AOS)属于海藻寡糖,具有抗氧化、抗肿瘤、抗炎、抑制凋亡等生物学活性。AOS对D-半乳糖诱导的白内障的作用鲜有报道。本实验通过D-半乳糖诱导制备C57BL/6J小鼠白内障模型,探讨AOS对D-半乳糖诱导C57BL/6J小鼠白内障的影响及其可能的作用机制,为白内障的药物治疗提供新思路。
1 材料与方法
1.1 实验材料
C57BL/6J小鼠购于济南鹏跃实验动物繁育中心;AOS购于青岛博智汇力生物科技有限公司;D-半乳糖、BCA蛋白浓度测定试剂盒购于北京索莱宝公司;Bax兔多克隆抗体、Bcl-xl和β-actin兔单克隆抗体、caspase-3鼠单克隆抗体购于美國Cell Signaling Technology公司;山羊抗兔IgG、山羊抗鼠IgG二抗购于武汉伊莱瑞特公司;RIPA裂解液购于上海碧云天公司。
1.2 实验方法
1.2.1 小鼠分组及处理 45只8周龄C57BL/6J雄性小鼠于青岛大学实验动物中心(SPF级)适应性饲养1周后用于实验。实验期间保持室内温度18~22 ℃,相对湿度40%~60%,明/暗周期为12 h,小鼠自由进食和饮水。应用随机数字表法将45只小鼠分为对照组(A组)、D-半乳糖组(B组)、D-半乳糖+AOS低剂量组(C组)、D-半乳糖+AOS中剂量组(D组)和D-半乳糖+AOS高剂量组(E组),每组9只。A组小鼠于颈背部皮下注射灭菌注射用水(5 mL·kg-1·d-1)8周,其余各组小鼠均颈背部皮下注射D-半乳糖(200 mg·kg-1·d-1)8周。自实验第5周开始,A、B两组小鼠给予蒸馏水10 mL·kg-1·d-1灌胃处理4周,C、D、E组小鼠分别给予AOS 50、100、150 mg·kg-1·d-1灌胃处理4周。该动物实验经青岛大学动物福利和伦理管理委员会批准,且遵守《动物保护与使用指南》。
1.2.2 晶状体混浊度观察及评分 实验结束后,摘取小鼠眼球并在生理盐水中清洗3次,解剖显微镜下分离晶状体。根据SIPPEL[4]描述的晶状体混浊度评分标准,将晶状体混浊度分为0~5分。0分:晶状体透明,无空泡;1分:晶状体透明,空泡少于3个;2分:晶状体透明,空泡多于3个;3分:空泡覆盖整个晶状体表面;4分:晶状体部分混浊;5分:晶状体完全混浊。
1.2.3 苏木精-伊红染色(HE染色) 应用HE染色观察晶状体细胞形态变化。将摘取的眼球在生理盐水中清洗3次,迅速置于40 g/L甲醛缓冲溶液中固定,二甲苯脱蜡,梯度乙醇脱水,石蜡包埋,切片后经过脱水、HE染色、透明、封片处理后,应用光学显微镜观察晶状体上皮细胞形态结构变化。
1.2.4 Western blot方法检测晶状体Bax、Bcl-xl和caspass-3蛋白的表达 收集各组小鼠至少10个晶状体样本,应用4 ℃磷酸盐缓冲液冲洗3次,滤纸吸干后称质量,按质量体积比1∶9加入4 ℃的磷酸盐缓冲液,于冰上经研磨棒充分研磨后,制成100 g/L的晶状体匀浆,低温12 000 r/min离心5 min,留取上清液。加入RIPA裂解液提取蛋白,应用BCA试剂盒测定蛋白浓度。根据蛋白浓度加入晶状体蛋白进行SDS-PAGE凝胶电泳,当溴酚蓝到达底部时将分离胶转移至PVDF膜上(200 mA、90 min),封闭液封闭2 h,分别加入1∶1 000稀释的Bax、Bcl-xl和caspase-3一抗工作液,4 ℃孵育过夜;TBS-T洗膜1 h后加入1∶5 000稀释的二抗工作液,室温孵育2 h;TBS-T洗膜1 h,应用ECL发光液显影,并应用Quantity One 软件进行灰度分析。以目的蛋白与内参蛋白灰度值的比值表示各蛋白表达。
1.3 统计学方法
应用SPSS 21.0软件进行统计学分析,计量资料结果以±s表示,多组数据比较采用One-way ANOVA检验,组间两两比较采用LSD法。P<0.05为差异有统计学意义。
2 结果
2.1 各组小鼠晶状体混浊度得分比较
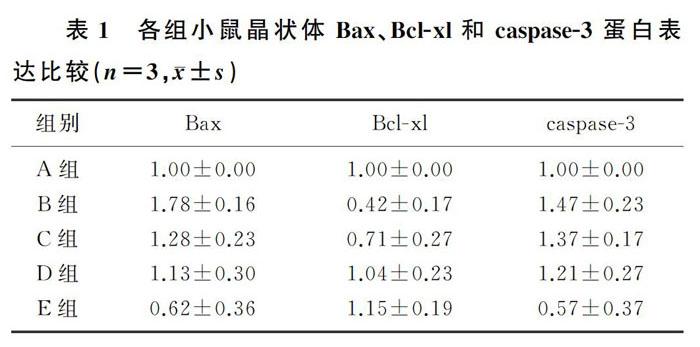
A组、B组、C组、D组、E组小鼠晶状体混浊度得分分别为:0.44±0.72、4.44±0.73、3.67±0.71、2.78±0.97、1.22±0.67。与对照组比较,D-半乳糖组晶状体混浊度评分明显升高,差异有统计学意义(F=4.347,P<0.05);与D-半乳糖组相比,不同浓度AOS干预组晶状体混浊度评分明显降低,并且呈浓度依赖性降低,差异有统计学意义(P<0.05)。
2.2 各组小鼠晶状体HE染色结果比较
对照组小鼠晶状体上皮细胞呈单层排列,整齐有序;D-半乳糖组小鼠晶状体上皮细胞稍肿胀,细胞排列不均匀;AOS 150 mg·kg-1·d-1灌胃处理显著改善了晶状体上皮细胞的形态结构及排列紊乱。见图1A~C。
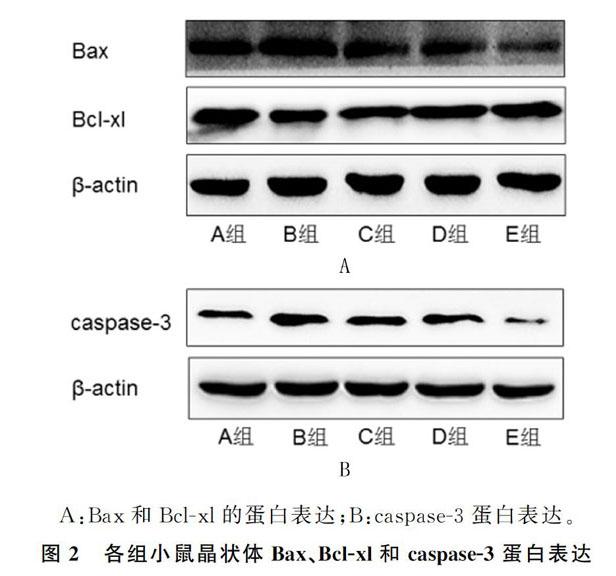
2.3 各组小鼠晶状体Bax、Bcl-xl和caspase-3蛋白表达比较
与对照组相比,D-半乳糖组小鼠晶状体Bcl-xl蛋白表达明显减少,Bax、caspase-3的蛋白表达明显增加,差异有统计学意义(F=6.62~9.16,P<0.05);与D-半乳糖组相比,D-半乳糖+AOS各剂量组(C、D、E组)小鼠晶状体Bcl-xl的蛋白表达明显增加,Bax、caspase-3的蛋白表达明显减少,差异有统计学意义(P<0.05);不同浓度AOS干预组小鼠晶状体Bcl-xl的蛋白表达呈浓度依赖性增加,E组与C组比较差异有统计学意义(P<0.05);不同浓度AOS干预组小鼠晶状体Bax、caspase-3蛋白表达呈浓度依赖性减少,E组与C组、D组差异有统计学意义(P<0.05)。见图2、表1。
3 讨论
白内障是老年人常见致盲性眼病,衰老是老年人白内障的重要病因之一。研究表明,晶状体上皮细胞受损与白内障的发生和发展有密切联系,除先
天性白内障外,晶状体上皮细胞凋亡在多种类型白内障发生中起重要作用[5-6]。
D-半乳糖诱导的亚急性衰老模型被广泛应用于构建衰老啮齿类动物模型,廣泛应用于增龄性听力下降[7]、白内障[8]、记忆障碍[9]、肾脏衰老[10]、心脏衰老[11]等研究。高剂量半乳糖可诱导产生过量活性氧,从而促进晶状体上
皮细胞的脂质过氧化[12]。此外,多项研究表明,D-
半乳糖通过损伤晶状体上皮细胞促进白内障形成[13-14]。晶状体上皮细胞凋亡是多种类型白内障发生的细胞学基础[6,15-16]。研究表明,经D-半乳糖处理后的动物表现为氧化应激损伤与糖基化反应同时存在,导致晶状体上皮细胞的细胞核和线粒体功能、结构损伤,进一步引起细胞凋亡[17-18]。此外,D-半乳糖在氧化酶的作用下分解形成木酮糖和CO2,并产生氧自由基和H2O2,导致晶状体氧化损伤,进一步导致晶状体混浊[19]。本实验结果表明,应用D-半乳糖处理后的小鼠晶状体混浊度评分明显增加,细胞凋亡相关蛋白Bcl-xl蛋白的表达明显降低,Bax、
caspase3蛋白表达明显增加,表明D-半乳糖可以诱导白内障的发生,其机制可能与细胞凋亡有关。
根据晶状体的混浊程度可进行白内障病情分级。晶状体混浊通常从周边开始出现空泡,逐渐向中心发展,最终出现核混浊[20]。病理改变主要表现为晶状体细胞结构破坏以及晶状体纤维水肿、崩解等[21-22]。AOS因其无免疫原性、无毒性、可生物降解等优点而被应用于生物医学领域。已有研究表明,AOS对肺动脉高压[23]、心血管疾病[24]、肾脏损伤[25]等具有重要保护作用,但其对白内障的影响却鲜有研究。本研究结果显示,D-半乳糖组小鼠晶状体混浊度评分明显升高,AOS干预显著改善了晶状体上皮细胞的形态结构及排列紊乱,表明D-半乳糖诱导了C57BL/6J小鼠白内障的发生,AOS显著延缓了D-半乳糖诱导的C57BL/6J小鼠白内障的进展。本研究显示,应用不同浓度的AOS干预后,小鼠晶状体混浊度呈浓度依赖性降低,高剂量组降低效果最为明显;晶状体上皮细胞的形态结构及排列紊乱得到明显改善;且细胞凋亡相关蛋白Bcl-xl表达呈剂量依赖性增加,而Bax、caspase3蛋白表达呈剂量依赖性减少,以高剂量组效果最为明显。表明AOS可以延缓白内障的发生,其机制可能与抑制细胞凋亡有关。
細胞凋亡是为了维持内环境稳定,在基因调控
下的细胞程序性死亡,受多种基因如Bcl-2家族
Bax、Bcl-xl以及caspase家族中的caspase-3等调控。Bax属于Bcl-2家族中重要的凋亡调节基因,Bax的过度表达可以拮抗Bcl-2的保护作用,从而促进细胞凋亡。Bcl-xl是Bcl-2家族基因中重要的拮抗细胞凋亡基因,可与Bax结合形成异二聚体,发挥抑制细胞凋亡的作用[26-28]。JING等[29]在研究过氧化氢诱导的人晶状体上皮细胞凋亡时发现,Bax、caspase-3的mRNA表达明显增加,表明其在细胞凋亡中发挥重要作用。caspase-3是近年来发现的在细胞凋亡中起关键作用的蛋白酶[30-31]。有研究表明,老年白内障病人和中青年白内障病人晶状体上皮细胞凋亡与caspase-3的表达水平呈正相关。本实验结果显示,与对照组相比较,D-半乳糖组小鼠晶状体Bcl-xl蛋白表达明显减少,Bax、caspase-3蛋白表达明显增加;与D-半乳糖组相比,AOS干预各组小鼠晶状体Bcl-xl的蛋白表达呈剂量依赖性增加,Bax、caspase-3蛋白表达呈剂量依赖性减少,以高剂量组效果最为明显,表明白内障的发生与细胞凋亡相关蛋白Bcl-xl、Bax和caspase-3的表达有关。
综上所述,AOS显著延缓了D-半乳糖诱导的C57BL/6J小鼠白内障的进展,其机制可能与AOS抑制晶状体细胞凋亡有关。但其具体分子调控机制仍需进一步研究。
[参考文献]
[1]PASCOLINI D, MARIOTTI S P. Global estimates of visual impairment: 2010[J]. The British Journal of Ophthalmology, 2012,96(5):614-618.
[2]于春艳,于春荣,敬舒,等. 北五味子总木脂素对D-半乳糖诱导的小鼠脑衰老自噬和凋亡的影响[J]. 吉林大学学报(医学版), 2014,40(6):1210-1215.
[3]BIANCHI E, SCARINCI F, GRANDE C, et al. Immunohistochemical profile and VEGF, TGF-β and PGE2 in human pterygium and normal conjunctiva: experimental study and review of the literature[J]. International Journal of Immunopathology and Pharmacology, 2012,25(3):607-615.
[4]SIPPEL T O. Changes in the water, protein, and glutathione contents of the lens in the course of galactose cataract development in rats[J]. Investigative Ophthalmology,1966,5(6):568-575.
[5]HUANG Xiurong. Inhibition effects of compound leech eye drops on apoptosis of lens epithelial cells and expressions of Bcl-2 and Bax genes in rats[J]. Journal of Chinese Integrative Medicine, 2007,5(6):681-685.
[6]LI W C, KUSZAK J R, DUNN K, et al. Lens epithelial cellapoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals[J]. The Journal of Cell Biology, 1995,130(1):169-181.
[7]DU Z, YANG Q, LIU L, et al. NADPH oxidase 2-dependent oxidative stress, mitochondrial damage and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging rats[J]. Neuroscience, 2015,286(21):281-292.
[8]VINSON J A. Oxidative stress in cataracts[J]. Pathophysiology: the Official Journal of the International Society for Pathophysiology/ISP, 2006,13(3):151-162.
[9]SUNG N, SEO M, JIN-SEOK S, et al. Ascorbic acid mi-
tigates D-galactose-induced brain aging by increasing hippocampal neurogenesis and improving memory function[J]. Nu-
trients, 2019,11(1):176-193.
[10]LIU Buhui, TU Yu′e, HE Weiming, et al. Hyperoside attenuates renal aging and injury induced by D-galactose via inhibiting AMPK-ULK1 signaling-mediated autophagy[J]. Aging, 2018,10(12):4197-4212.
[11]BEI Y H, WU X T, CRETOIU D, et al. miR-21 suppression prevents cardiac alterations induced by D-galactose and doxorubicin[J]. Journal of Molecular and Cellular Cardiology, 2018,115(9):130-141.
[12]DAI Jie, ZHOU Jun, LIU Hongmei, et al. Selenite and ebselen supplementation attenuatesd-galactose-induced oxidative stress and increases expression of SELR and SEP15 in rat lens[J]. Journal of Biological Inorganic Chemistry. 2016,21(8):1037-1046.
[13]CHANG Yungming, CHANG Henhong, KUO Weiwen, et al. Anti-apoptotic and pro-survival effect of alpinate oxyphyllae fructus (AOF) in a D-galactose-induced aging heart[J]. International Journal of Molecular Sciences, 2016,17(4):466-485.
[14]XU Yao, LI Yong, MA Limei, et al. D-galactose induces premature senescence of lens epithelial cells by disturbing autophagy flux and mitochondrial functions[J]. Toxicology Letters, 2018,289(6):99-106.
[15]LONG A C, COLITZ C M H, BOMSER J A. Apoptotic and necrotic mechanisms of stress-induced human lens epithelial cell death[J]. Experimental Biology & Medicine, 2004,229(10):1072-1080.
[16]LI W C, JEROME R K, WANG G M, et al. Calcimycin-induced lens epithelial cell apoptosis contributes to cataract formation[J]. Experimental Eye Research, 1995,61(1):91-98.
[17]林媛,黎秉富,呂俊华. 氨基胍对D-半乳糖致大鼠眼晶状体损伤的影响及其机制[J]. 中国病理生理杂志, 2009,25(5):1004-1008.
[18]张世平,谭海荣,潘竟锵,等. D-半乳糖致蛋白质糖基化动物模型的建立[J]. 基础医学与临床, 2006,26(1):92-95.
[19]武飞. 天葵提取物对D-半乳糖诱导的大鼠白内障的防治作用及对免疫功能影响的实验研究[D]. 贵阳:贵阳医学院, 2015.
[20]冯春燕,黄秀榕,祁明信,等. 异补骨脂素对氧化损伤的人晶状体上皮细胞的防护作用及其机制[J]. 中华眼科杂志, 2011,47(4):353-355.
[21]刘祁,李东旭,王阜蕾,等. 大鼠半乳糖性白内障晶状体上皮细胞的病理变化[J]. 眼科新进展, 2016,36(12):1101-1104.
[22]KUBO E, URAKAMI T, FATMA N, et al. Polyol pathway-dependent osmotic and oxidative stresses in aldose reductase-mediated apoptosis in human lens epithelial cells: role of AOP2[J]. Biochemical and Biophysical Research Communications, 2004,314(4):1050-1056.
[23]YI Hu, FENG Zhe, FENG Wenjing, et al. AOS ameliorates monocrotaline-induced pulmonary hypertension by restraining the activation of P-selectin/p38MAPK/NF-κB pathway in rats[J]. Biomedicine & Pharmacotherapy, 2019,109(3):1319-1326.
[24]GUO Junjie, XU Fengqiang, LI Yonghong, et al. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress-mediated apoptosis[J]. Drug Design Development and Therapy, 2017,11(11):2387-2397.
[25]TERAKADO S, UENO M, TAMURA Y, et al. Sodium alginate oligosaccharides attenuate hypertension and associated kidney damage in dahl salt-sensitive rats fed a high-salt diet[J]. Clinical and Experimental Hypertension, 2012,34(2):99-106.
[26]HUSKA J D, LAMB H M, HARDWICK J M. Overview of BCL-2 family proteins and therapeutic potentials[J].
Methods in Molecular Biology (Clifton, N.J.), 2019,1877(2):1-21.
[27]KOLLEK M, MLLER A, EGLE A, et al. Bcl-2 proteins in development, health, and disease of the hematopoietic system[J]. The FEBS Journal, 2016,283(15):2779-2810.
[28]JAE-SUN S, JI-HYANG H, SEUNG-WOOK C. Targeting of P53 peptide analogues to anti-apoptotic Bcl-2 family proteins as revealed by NMR spectroscopy[J]. Biochemical and Biophysical Research Communications, 2014,443(3):882-887.
[29]GAO Jing, WANG Tao, WANG Meng. Investigation of the anti-cataractogenic mechanisms of curcumin through in vivo and in vitro studies[J]. BMC Ophthalmology, 2018,18(1):48-56.
[30]李延輝,叶仕英,何小杰,等. 不同年龄白内障晶状体上皮细胞中caspase-3、Fas/FasL的表达及意义[J]. 中国当代医药, 2014,12(31):15-17.
[31]YANG Liuqin, FANG Dianchun, WANG Rongquan, et al. Effect of NF-κB, survivin, Bcl-2 and Caspase-3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand[J]. World Journal of Gastroenterology, 2004,10(1):22-25.
(本文编辑 黄建乡)
[收稿日期]2020-02-29; [修订日期]2020-03-28
[基金项目]国家自然科学基金面上项目(3157100089)
[第一作者]冯美苹(1990-),女,硕士研究生。
[通信作者]毛拥军(1964-),男,博士,教授,博士生导师。
E-mail:mmc168@126.com。

