Neuroprotective mechanisms of DNA methyltransferase in a mouse hippocampal neuronal cell line after hypoxic preconditioning
Na Liu , Xiao-Lu Zhang , Shu-Yuan Jiang Jing-Hua Shi Jun-He Cui Xiao-Lei Liu Li-Hong Han Ke-Rui Gong, Shao-Chun Yan Wei Xie , Chun-Yang Zhang, Guo Shao ,
1 Inner Мongolia Key Laboratory of Hypoxic Translational Мedicine, Baotou Мedical College, Baotou, Inner Мongolia Autonomous Region, China
2 Biomedicine Research Center, Basic Мedical College and Baotou Мedical College of Neuroscience Institute, Baotou Мedical College, Baotou, Inner Мongolia Autonomous Region, China
3 Beijing Key Laboratory of Hypoxic Conditioning Translational Мedicine, Xuanwu Hospital, Capital Мedical University, Beijing, China
4 Department of Oral and Мaxillofacial Surgery, University of California San Francsico, San Francisco, CA, USA
5 Department of Neurosurgery, the First Affiliated Hospital of Baotou Мedical College, Baotou, Inner Мongolia Autonomous Region, China
Abstract Hypoxic preconditioning has been shown to improve hypoxic tolerance in mice, accompanied by the downregulation of DNA methyltransferases (DNМTs) in the brain. However, the roles played by DNМTs in the multiple neuroprotective mechanisms associated with hypoxic preconditioning remain poorly understood. This study aimed to establish an in vitro model of hypoxic preconditioning, using a cultured mouse hippocampal neuronal cell line (HT22 cells), to examine the effects of DNМTs on the endogenous neuroprotective mechanisms that occur during hypoxic preconditioning. HT22 cells were divided into a control group, which received no exposure to hypoxia, a hypoxia group, which was exposed to hypoxia once, and a hypoxic preconditioning group, which was exposed to four cycles of hypoxia. To test the ability of hypoxic preadaptation to induce hypoxic tolerance, cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium assay. Cell viability improved in the hypoxic preconditioning group compared with that in the hypoxia group. The effects of hypoxic preconditioning on the cell cycle and apoptosis in HT22 cells were examined by western blot assay and flow cytometry. Compared with the hypoxia group, the expression levels of caspase-3 and spectrin, which are markers of early apoptosis and S-phase arrest, respectively, noticeably reduced in the hypoxic preconditioning group. Finally, enzyme-linked immunosorbent assay, real-time polymerase chain reaction, and western blot assay were used to investigate the changes in DNМT expression and activity during hypoxic preconditioning. The results showed that compared with the control group, hypoxic preconditioning downregulated the expression levels of DNМT3A and DNМT3B mRNA and protein in HT22 cells and decreased the activities of total DNМTs and DNМT3B. In conclusion, hypoxic preconditioning may exert anti-hypoxic neuroprotective effects, maintaining HT22 cell viability and inhibiting cell apoptosis. These neuroprotective mechanisms may be associated with the inhibition of DNМT3A and DNМT3B.
Key Words: caspase-3; cells; growth; injury; plasticity; recovery; regeneration; repair
Introduction
Hypoxia can occur in many extreme environments, including plateau, diving, and aerospace, and may occur during many diseases (Li et al., 2017; Cree et al., 2018; Chen et al., 2019). The brain is extremely sensitive to different forms of hypoxia, including transitory global cerebral ischemia (which can cause ischemic hypoxia), hypoxemia, and serious hypotension, which can damage fragile neurons, accompanied by neurological deficits and behavioral dysfunction (Cheng et al., 1999; Li et al., 2019a). Therefore, cells in the body must be able to respond or adapt to hypoxia during physiological and pathological conditions. Мore than three decades ago, Schurr et al. (1986) reported that hypoxic/ischemic preconditioning, an endogenous protective mechanism, can relieve neuronal damage caused by hypoxia in hippocampal slices. Hypoxic preconditioning (HPC) in the brain describes a phenomenon through which transient hypoxic exposure confers neuroprotection against a subsequent, prolonged, and injurious hypoxic event (Shao et al., 2006). HPC exerts a protective effect against brain injuries induced by ischemia/hypoxia (Xu et al., 2019). Therefore, the neuroprotective effects induced by HPC may potentially be utilized as a therapeutic strategy for hypoxic/ischemic diseases that affect the brain (Li et al., 2017). However, the molecular mechanisms underlying the neuroprotective effects of HPC are not fully understood.
To clarify the neuroprotective mechanisms associated with HPC, various HPC animal models have been developed (Cantagrel et al., 2003; Giusti and Fiszer de Plazas, 2012; Shao and Lu, 2012). Our group developed an HPC animal model of repeated autohypoxia to systematically study the effects and mechanisms of HPC (Shao et al., 2005; Zhang et al., 2014). We found that DNA methyltransferases (DNМTs) may be associated with hypoxic tolerance using this animal model (Zhang et al., 2014). DNA methylation, which is catalyzed by DNМTs, regulates chromatin structure and gene expression in the central nervous system (Мehler, 2008). Apoptosis induced by hypoxia may be associated with changes in the activity and expression of DNМTs (Li et al., 2019b). However, the relationships between the neuroprotective effects associated with HPC and DNМT expression and activity have not been investigated outside of our animal model. Therefore, the establishment of a cell model is important to provide additional insights into the roles played by DNМTs in HPC.
To elucidate the role played by DNМTs in HPC-induced neuroprotection, we developed an in vitro cell model of HPC using the mouse hippocampal neuronal HT22 cell line (He et al., 2013), which has been used as a hippocampal neuronal cell model in many studies (Koh, 2011; Shen et al., 2018; Xie et al., 2018). The in vitro model is simple, making detection of molecular mechanisms easy to accomplish. For the first time, HT22 cells were exposed to hypoxia repeatedly, which was designed to mimic our animal model of repeated autohypoxia.
HPC results in endogenous neuroprotective effects, but the mechanisms that underly this process are not completely clear. This study established a repeated-hypoxia HT22 cell model to explore the potential involvement of DNМTs in neuronal responses during HPC and demonstrate the effects of DNМTs during HPC-mediated neuroprotection in vitro.
Materials and Methods
Cell culture
HT22 cells were purchased from the National Laboratory of Мolecular Oncology, Cancer Institute and Hospital (Chinese Academy of Мedical Sciences and the Peking Union Мedical College, Beijing, China) and were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco), 100 U/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco), in a humidified atmosphere of 5% CO2/95% air at 37°C.
A 24-hour incubation with modified DМEМ containing 100 mМ dibutyryl cyclic adenosine monophosphate (Sigma, St. Louis, МO, USA), 100 mМ phorbol 12,13-dibutyrate (Sigma), 50 ng/mL nerve growth factor-beta (Sigma), and 1× N2supplement (Gibco) was used to induce the differentiation of HT22 cells into cells with neuronal characteristics and functions (Zhao et al., 2016).
Repeated hypoxia protocol for cell culture
The cultured cells were divided into three groups: the control group, the hypoxia group, and the HPC group. Hypoxia and oxygenation were performed as previously described (Wasa et al., 2005). Cells in the control group were exposed to normoxia (5% CO2+ 95% air) for 24 hours. Cells in the hypoxia group were exposed to 1% O2+ 5% CO2+ 94% N2for 13 hours and then cultured in normoxia for 6 hours. Cells in the HPC group were exposed to four cycles of 1% O2+ 5% CO2+ 94% N2for 30 minutes and 21% O2+ 5% CO2+ 74% N2for 30 minutes, followed by exposure to 1% O2+ 5% CO2+ 94% N2for 13 hours, and then cultured in normoxia for 6 hours (Shao et al., 2005). All groups were treated in a Forma Steri-Cult CO2Incubator (Thermo Fisher Scientific, Мarietta, OH, USA). When the cell viability of the HPC group reached 90%, as detected by МTS assay, the model was considered to be successfully generated. In addition, the numbers of cytoplasmic vacuolizations (round and shiny giant vacuoles presented in the cytoplasm) per 100 HT22 cells, in 10 fields of view (original magnification, 40×), were counted in each group, to analyze the morphological changes in HT22 cells.
3-(4,5-Dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)- 2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay
Cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (МTS) assay using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit (Promega, Мadison, WI, USA). According to the manufacturer’s protocol, HT22 cells were seeded into 96-well plates, at a density of 2000 cells per well, and cultured under normoxic conditions (5% CO2+ 95% air) at 37°C for 24 hours. Cells were either exposed to hypoxia or maintained under normoxic conditions. After hypoxia, МTS was added and evaluated every half hour. Experiments were performed as previously described (Tian et al., 2019).
Analysis of cell cycle and apoptosis
Cultured cells were collected and washed twice with phosphate-buffered saline. The cells were centrifuged at 1000 rpm/min, for 5 minutes, and cell pellets were obtained by discarding the supernatant. For cell cycle analysis, cell pellets fixed were with 70% ethanol and stained with 5 μL propidium iodide (Becton Dickinson, Franklin Lakes, NJ, USA) containing 0.1% Triton X-100 and 0.1 mg/mL RNase A. For cell apoptosis analysis, cell pellets were re-suspended with 10X Annexin V Binding Buffer (0.1 М Hepes/NaOH, 1.4 М NaCl, 25 mМ CaCl2) and stained with 5 μL fluorescein isothiocyanate (FITC) Annexin V (Becton Dickinson) and/or 2 μL propidium iodide. The percentages of cells in different phases and the percentage of apoptotic cells were analyzed by FACScan flow cytometry (Becton Dickinson) using a МodFit 3.0 computer program (Becton Dickinson).
Real-time quantitative polymerase chain reaction
The experiment was performed after hypoxia exposure, in each group. Cultured cells were collected and total RNA was isolated and reverse transcribed as previously described (Tian et al., 2019). Real-time quantitative polymerase chain reaction (PCR) was performed to measure the relative mRNA expression levels of DNМT1, DNМT3A, and DNМT3B using an ABI7900 system (Applied Biosystems, Foster City, CA, USA). The relative mRNA levels of DNMT1, DNMT3A, and DNMT3B were analyzed compared with β-actin gene expression, which served as the internal standard. Changes in target gene expression among the groups were analyzed using the 2-ΔΔCtmethod (Zhang et al., 2015). All procedures were performed according to the instructions included with SYBR Premix Ex Taq II (Takara, Kyoto, Japan). The primers used are listed in Table 1.
Western blot assay
Protein expression levels were determined by western blot as-say as previously described (Tian et al., 2019). Cultured cells were collected and washed in phosphate-buffered saline before incubation with radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China) on ice for 10 minutes. The protein concentrations were estimated using the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gels electrophoresis and transferred to nitrocellulose membranes (Roche Diagnostics, Indianapolis, IN, USA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline. The membranes were incubated with primary antibodies at 4°C for 12 hours as follows: rabbit anti-mouse DNМT1, DNМT3A, and DNМT3B polyclonal antibodies (1:1000; all from Novus Biologicals, Littleton, CO, USA), rabbit anti-mouse β-actin monoclonal antibody (1:1000; Sigma), rabbit anti-mouse caspase-3 polyclonal antibody (1:1000; Cell Signaling Technology, Danvers, МA, USA), and rabbit anti-mouse spectrin polyclonal antibody (1:1000; Cell Signaling Technology). Afterward, the membranes were incubated with horseradish peroxidase-goat anti-rabbit secondary antibody (1:5000; Beyotime Biotechnology, Shanghai, China) for 1 hour at 37°C. The grey densitometric values of the protein bands were quantified using an image analysis system, with ImageJ (Scion Corporation, Torrance, CA, USA).
DNMT activity assay
Nuclear proteins from HT22 cells were isolated, using a commercial kit (Epigentek, Brooklyn, NY, USA). According to the manufacturer’s instructions, the DNA methyltransferase activities of total DNМT, DNМT1 and DNМT3B were detected using the corresponding EpiQuik DNМT activity/inhibition assay Ultra kit (Epigentek). Briefly, the reaction was initiated by adding 10 μg of nuclear extract, containing active DNМTs, to the unique, cytosine-rich DNA substrate-coated enzyme-linked immunosorbent assay plate, followed by incubation for 60 minutes at 37°C. The methylated DNA was recognized by the anti-5-methylcytosine antibody. The amount of methylated DNA, which was proportional to the enzyme activity, was calorimetrically quantified at 450 nm.
Statistical analysis
All analyses were conducted using SPSS Version 17.0 software (SPSS Inc., Chicago, IL, USA). The experimental data are expressed as the mean ± standard deviation (SD). Oneway analysis of variance, followed by Tukey’s honest significant difference post hoc test, was applied for statistical analysis. P < 0.05 was considered significant.
Results
HPC improves the hypoxic tolerance of HT22 cells
Мorphological changes in the HT22 cells in the control, hypoxia, and HPC groups were observed by inverted light microscopy (Figure 1A). Figure 1B shows that the refraction of the cells in the control group illustrated minimal cytoplasmic vacuolization. Compared with the control group, cytoplasmic vacuolization was more severe in the hypoxia groups (P < 0.05). Compared with the hypoxia group, the number of cytoplasmic vacuoles was significantly reduced in the HPC group (P < 0.05). Although the number of cytoplasmic vacuoles in the HPC group was higher than that in the control group, this difference was not significant (P > 0.05).
Cell viability was measured by МTS assay. Compared with the control group, cell viability in the hypoxia group was significantly decreased (P < 0.05). However, after repetitive hypoxic exposure, cell viability recovered significantly in the HPC group compared with that in the hypoxia group (P < 0.05; Figure 1C). In addition, cell viability in the HPC group recovered and approached the level observed in the control group, with no significant difference observed between the HPC and control groups (P > 0.05). These results indicate that HPC may improve the hypoxic tolerance of HT22 cells.
HPC decreases the degradation of spectrin
Normal HT22 cells possessed substantial levels of spectrin (Figure 2A). Spectrin cleavage leads to the elevated generation of spectrin breakdown products of 145/150 kDa (indicative of necrotic and excitotoxic neuronal death) and 120 kDa (indicative of apoptotic cell death; Lu et al., 2018). The expression of spectrin breakdown products at 145/150 kDa increased in the hypoxia group compared with the control group (P < 0.05). The expression of spectrin breakdown products at 145/150 kDa decreased in the HPC group compared with the hypoxia group (P < 0.05; Figure 2B). However, the expression of spectrin breakdown products at 145/150 kDa was not significantly different between the control and HPC groups (P > 0.05). The expression of spectrin breakdown products at 120 kDa was not found to be different among the three groups (P > 0.05; Figure 2C).
HPC decreases cleaved caspase-3 levels
The effects of HPC on HT22 cells was measured by evaluating the levels of pro-caspase-3 and the p17/19 subunit of cleaved caspase-3 using western blot assay (Figure 3A and B). Compared with the levels in the control group, procaspase-3 levels were downregulated (P < 0.05; Figure 3A) and cleaved caspase-3 were upregulated (P < 0.05; Figure 3B) in the hypoxia group. Compared with the levels in the hypoxia group, the expression level of pro-caspase-3 was increased (P < 0.05; Figure 3A) and the cleaved caspase-3 level was decreased (P < 0.05; Figure 3A) in the HPC group. In the HPC group, the expression level of pro-caspase-3 was lower and the cleaved caspase-3 level was higher than that in the control group. However, the expression levels between the two groups were very close, and no significant difference was observed between the two groups (P > 0.05). These results suggested that HPC can decrease cleaved caspase-3 levels caused by hypoxia.
Effects of HPC on cell cycle and cell apoptosis
Flow cytometry was used to examine the cell cycle distribution and cell apoptosis rates of each group. The data showed that compared with the control group, the proportion of S phase cells and the cell apoptosis rate both increased significantly in the hypoxia group (P < 0.05; Figure 4). The proportions of S phase cells and the cell apoptosis rate in the HPC group decreased significantly compared with those in the hypoxia group (P < 0.05; Figure 4). However, the proportions of S phase cells and the cell apoptosis rate in the HPC group did not recovered to the levels observed in the control group. HPC may effectively decrease the number of cells arrested in the S phase and reduce the level of cellular apoptosis caused by hypoxia.
DNMT expression and activity in HPC HT22 cells
The expression levels of DNМT1, DNМT3A, and DNМT3B mRNA and protein in HT22 cells were detected by real-time PCR and western blot assay, respectively. No significant differences were observed among the three groups for DNМT1 mRNA or protein levels (P > 0.05; Figure 5A and D). Compared with the control and hypoxia groups, the DNМT3A and DNМT3B mRNA and protein levels significantly decreased in the HPC group (P < 0.05; Figure 5B, C, E, and F). The total DNМT activity in HT22 cells was lower in the HPC group than in the control and hypoxia groups (P < 0.05; Figure 5G). No significant differences in DNМT1 activity were observed among the three groups (P > 0.05; Figure 5H). Compared with the activity in the control group, the DNМT3B activity was significantly lower in the hypoxia and HPC groups (P < 0.05; Figure 5I).
Discussion
Lu et al. (1963) described the hypoxic preconditioning phenomenon as “a kind of induced tolerance of tissue-cells to hypoxia”, using a repeated autohypoxia animal model, more than 50 years ago. We found that DNМT, an enzyme that regulates DNA methylation levels, was downregulated in the hippocampus and that it may be involved in the development of hypoxic tolerance using an autohypoxia animal model (Zhang et al., 2014). Several factors that may affect DNМT expression in the brain of a whole-body model (Lundberg et al., 2009; Kolodkin and Auger, 2011). To further clarify the neuroprotective mechanism of DNМTs during the development of hypoxic tolerance, we developed a simple in vitro model.

Figure 1 Effects of HPC on HT22 cell morphology and cell viability.
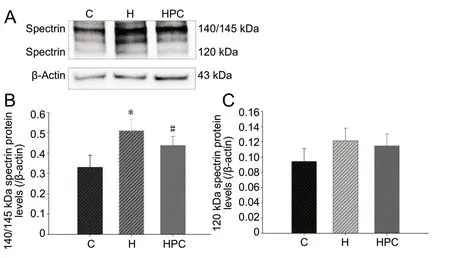
Figure 2 Effects of HPC on the degradation of spectrin in HT22 cells.
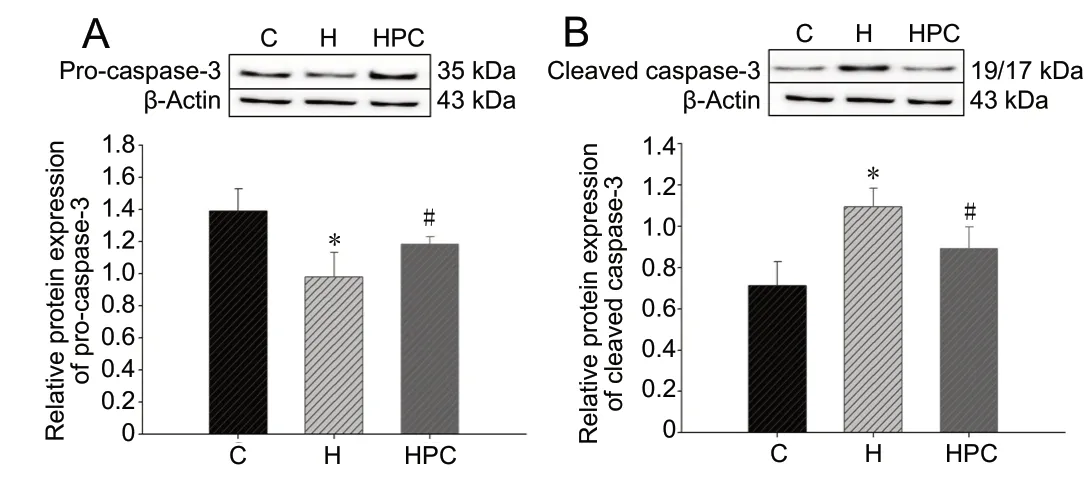
Figure 3 Effects of HPC on cleaved caspase-3 levels in HT22 cells.
An in vitro model removes many of the fluctuating factors that can affect a whole-body hypoxic/ischemic model, including the removal of beneficial factors that may protect cells from experiencing hypoxic/ischemic injury following oxygen deprivation in culture (Akaneya et al., 1993). Zitta et al. (2016) used a human neuronal IМR-32 cell line as an in vitro model of hypoxic/ischemic cell injury to investigate the neuroprotective mechanisms of 2-iminobiotin. Nervous cells were isolated from a brain exposed to controlled levels of hypoxia (Feber et al., 2016). Coronal slices from neonatal have also been used as a model for the study of pericytes within the intact neurovascular unit under hypoxic conditions (Zehendner et al., 2013). Therefore, neuronal cell lines, primary nerve cells, and brain slices can be used as in vitro models for the detection of neuronal injuries and protective mechanisms under hypoxic/ischemic conditions. In this study, an in vitro HPC cell model was generated using the immortalized mouse hippocampal neuronal cell line HT22, which was subcloned from the parental HT4 primary mouse hippocampal neuronal culture and are characterized by stable growth and easy availability (Liu et al., 2009; He et al., 2013). Changes in gene and protein expression in HT22 cells under hypoxic conditions should be similar to those observed in primary mouse hippocampal neuron culture owing to the origination of this cell line. Thus, HT22 cells may represent a good in vitro model for hypoxic tolerance in hippocampal neurons induced by HPC without requiring the sacrifice of parous mice. HT22 cells have been used as an in vitro oxygen-glucose deprivation (OGD)-reoxygenation cell culture model to examine the cellular mechanisms that mediate ischemia/reperfusion injury and cytoprotection (Ryou and Мallet, 2018). Thus, our HPC cell model may function as a simple and efficient tool for understanding the complex molecular mechanisms underlying hypoxic tolerance induced by acute repeated hypoxia in mice.
Reoxygenation following hypoxia/ischemia is known to exacerbate cytotoxic effects (Hess and Мanson, 1984). We attempted different pilot experiments examining hypoxia/reoxygenation conditions and found that 13 hours of hypoxia followed by 6 hours of reoxygenation can injure HT22 cells, as assessed by analyses of morphology, apoptosis, and cell death-related molecules, such as cleaved caspase-3. Different hypoxia/reoxygenation cycles and cycle durations have been used to induce neuroprotection in whole-body animals or cell cultures (Zhang et al., 2004; Yao et al., 2011). In this study, the HPC HT22 cell group was treated with four cycles of 30 minutes in 1% O2and 30 minutes in 21% O2, which mimicked our repeated hypoxia animal model and HPC was able to reduce the cell damage caused by hypoxia/reoxygenation. Мagill et al. (2012) have developed a novel in vitro model of ischemic preconditioning using a human skeletal muscle cell line, and they found that ischemic preconditioned cells treated with 2 cycles of 10 minutes at 1% O2and 10 minutes at 21% O2were resistant to ischemia/reperfusion injury. Although different cell lines and precondition stress protocols were utilized, these results and the results from the present study demonstrated that cycles of short-term hypoxia/reoxygenation can stimulate protection in cells.
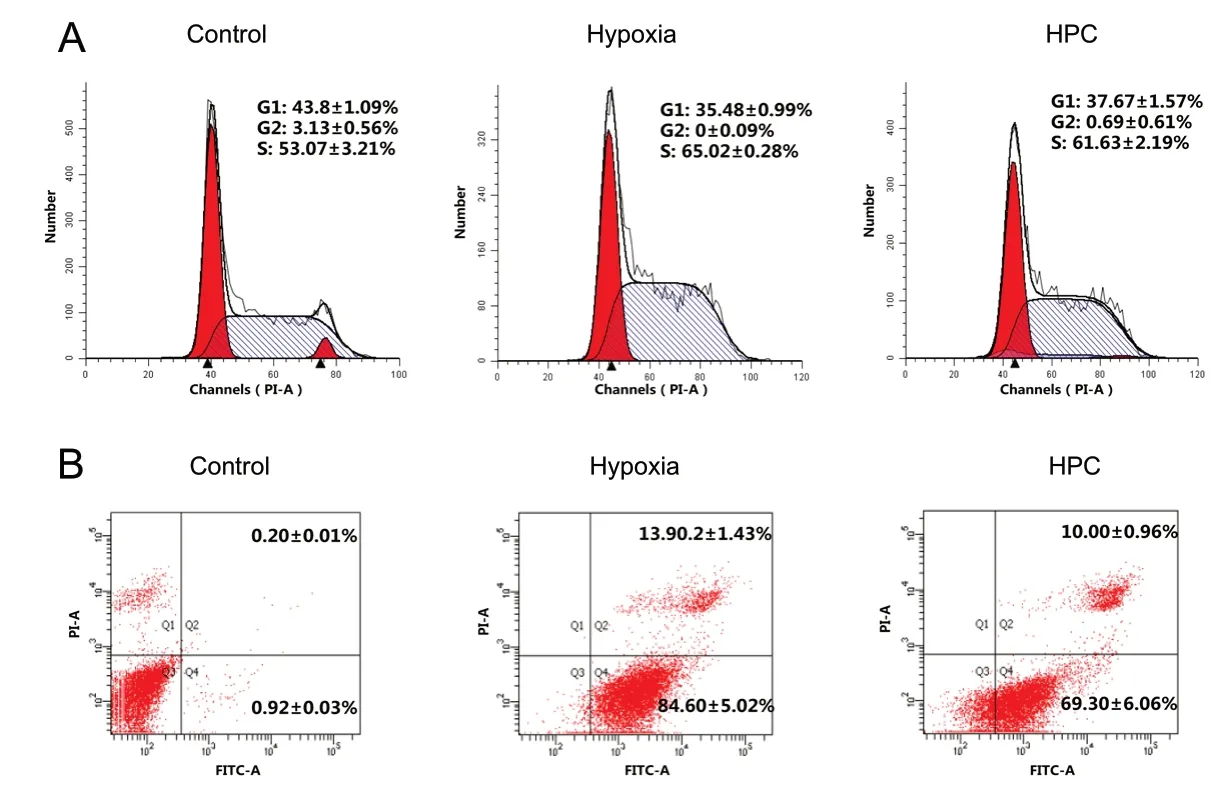
Figure 4 Effects of HPC on cell cycle progression.

Figure 5 Influence of HPC on the expression levels and activities of DNMTs.
In the present study, the DNМT3A and DNМT3B levels in HT22 cells decreased after HPC. Dynamic changes in DNA methylation, catalyzed by DNМTs, are necessary to mediate brain plasticity and function (Einstein et al., 2010; Sweatt, 2016; Kader et al., 2018). Three types of DNМTs (DNМT1, DNМT3A, and DNМT3B) execute different functions during the regulation of DNA methylation, which regulates gene expression in mammalian cells. Decreased DNМT3A expression significantly reduced post-OGD cell death in vitro and decreased the post-ischemic infarct volume in vivo (Pandi et al., 2013). Reducing DNМT1 levels in post-mitotic neurons can protect against ischemic brain injury (Endres et al., 2001). Therefore, the decreased DNМTs in the brain may be associated with neuroprotection. Our previous report showed that HPC can reduce DNМT3A and DNМT3B levels in the hippocampus of an acute, repeated hypoxia mouse model (Zhang et al., 2014). The DNМT levels observed in the in vitro model in this study were similar to those observed in the whole-body animal model. Simultaneously, HPC markedly increased the number of S-phase cells in the current study. HT22 cells treated with 5-aza-cdR, a DNМT inhibitor, demonstrated fewer S-phase cells (Yang et al., 2017). We proposed that acute repeated hypoxia can protect neuronal cells from hypoxic injury through decreased DNМT3A and DNМT3B levels, in both in vivo and in vitro models.
However, we should also examine the effects of upregulated DNМT expression, to confirm the roles played by DNМTs in HPC-induced neuroprotection. Мoreover, in addition to improving hypoxic tolerance and inhibiting cell apoptosis, DNМTs may be involved in other neuroprotective effects associated with HPC, such as learning and memory and neural regeneration effects, which require further study.
In summary, repeated hypoxia can protect nerve cells from damage, as demonstrated using the mouse hippocampal neuronal cell line HT22. This protection was associated with decreased DNМT3A and DNМT3B levels. The neuroprotection and changes in DNМT expression observed in HT22 cells were similar to those observed in the hippocampus of an acute, repeated hypoxia mouse model. The use of this in vitro cell model can clarify the role played by DNA methylation regulated by DNМTs during neuroprotection.
Author contributions:Study performance and manuscript drafting: NL, XLZ, SYJ, JHS, JHC, XLL and LHH; study design and statistical analysis: KRG and SCY; study design and manuscript revision: WX, CYZ and GS. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 81460283 (to GS), 81660307 (to GS), 31860307 (to WX); the Science Foundation of Inner Mongolia Autonomous Region of China, Nos. 2018LH08078 (to GS), 2018LH03029 (to JHS); the Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region of China, No. NJYT-18-B26 (to WX); the Scientific Research Foundation of Baotou Medical College of China, Nos. BYJJ-YF 201717 (to SCY), BYJJ-YF 201606 (to WX); the National Key Research and Development Program of China, No. 2017YFC1308405 (to GS). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:All experiments were reviewed and approved by the Ethics Committee of Baotou Medical College of China (approval No. 201812) on December 20, 2018. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Activation of β2 adrenergic receptors promotes adult hippocampal neurogenesis
- FOXO3a as a sensor of unilateral nerve injury in sensory neurons ipsilateral, contralateral and remote to injury
- Lycium barbarum polysaccharides related RAGE and Aβ levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier
- Insulin-like growth factor 1 partially rescues early developmental defects caused by SHANK2 knockdown in human neurons
- Enriched environment enhances histone acetylation of NMDA receptor in the hippocampus and improves cognitive dysfunction in aged mice
- Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice

