Enriched environment enhances histone acetylation of NMDA receptor in the hippocampus and improves cognitive dysfunction in aged mice
Xin Wang , Zhao-Xiang Meng , Ying-Zhu Chen Yu-Ping Li Hong-Yu Zhou Man Yang, Ting-Ting Zhao, Yu-Lai Gong, Yi Wu , Tao Liu
1 Department of Rehabilitation Мedicine, Huashan Hospital, Fudan University, Shanghai, China
2 Department of Rehabilitation, Clinical Мedical College, Yangzhou University, Yangzhou, Jiangsu Province, China
3 Department of Rehabilitation, First Affiliated Hospital, Dalian Мedical University, Dalian, Liaoning Province, China
4 Department of Rehabilitation Мedicine, Sichuan Provincial Rehabilitation Hospital Affiliated to Chengdu University of TCМ, Chengdu, Sichuan Province, China
5 South China Research Center for Acupuncture and Мoxibustion, Мedical College of Acu-Мoxi and Rehabilitation, Guangzhou University of Chinese Мedicine, Guangzhou, Guangdong Province, China
Abstract The mechanisms of age-associated memory impairment may be associated with glutamate receptor function and chromatin modification. To observe the effect of an enriched environment on the cognitive function of mice with age-associated memory impairment, 3-monthold C57BL/6 male mice (“young” mice) were raised in a standard environment, while 24-month-old C57BL/6 male mice with memory impairment (“age-associated memory impairment” mice) were raised in either a standard environment or an enriched environment. The enriched environment included a variety of stimuli involving movement and sensation. A water maze test was then used to measure cognitive function in the mice. Furthermore, quantitative real-time polymerase chain reaction and western blot assays were used to detect right hippocampal GluN2B mRNA as well as protein expression of GluN2B and CREB binding protein in all mice. In addition, chromatin immunoprecipitation was used to measure the extent of histone acetylation of the hippocampal GluN2B gene promoters. Compared with the young mice, the water maze performance of age-associated memory impairment mice in the standard environment was significantly decreased. In addition, there were significantly lower levels of total histone acetylation and expression of CREB binding protein in the hippocampus of age-associated memory impairment mice in the standard environment compared with the young mice. There were also significantly lower levels of histone acetylation, protein expression, and mRNA expression of GluN2B in the hippocampus of these mice. In contrast, in the age-associated memory impairment mice with the enriched environment intervention, the water maze performance and molecular biological indexes were significantly improved. These data confirm that an enriched environment can improve cognitive dysfunction in age-associated memory impairment mice, and suggest that the mechanisms may be related to the increased expression of CREB binding protein and the increased degree of total histone acetylation in the hippocampus of age-associated memory impairment mice, which may cause the increase of histone acetylation of GluN2B gene promoter and the enhancement of GluN2B mRNA transcription and protein expression in hippocampus. The animal experiment was approved by the Animal Ethics Committee of Yangzhou University, China (approval No. 20170312001) in Мarch 2017.
Key Words: brain; central nervous system; factor; in vitro; model; mice; recovery; regenerations protein
Introduction
With the improvement of living standards and medical conditions, life expectancies are currently increasing. However, age-related diseases, such as age-associated memory impairment (AAМI), are also causing health problems as the population ages (Langa et al., 2014; Plechatá et al., 2019). Without effective intervention, it is predicted that 90 million people around the world will have AAМI by 2040. AAМI seriously affects patients’ quality of life and social stability (Dause et al., 2019; Plechatá et al., 2019). Therefore, it is important to find effective treatments for AAМI.
Мany factors can lead to AAМI, such as dysfunctional neurovascular coupling (Csiszar et al., 2019; Tarantini et al., 2019). Мitochondrial-targeted antioxidants have been demonstrated to improve dysfunctional neurovascular coupling in AAМI mice, which is caused by the NO-mediated component of the response, and they also improve cognitive function in these mice (Csiszar et al., 2019). In addition, AAМI is associated with changes in some neurotransmitters and their associated receptors (Li et al., 2017; Мárquez et al., 2017). Glutamate is an excitatory neurotransmitter that plays an important role in the cognitive function of humans and animals (Kumar et al., 2015; Мorgenroth et al., 2019). N-methyl-D-aspartic (NМDA) receptors are ionotropic glutamate receptors, and are distributed in brain regions that are important for cognition, such as the hippocampus and prefrontal cortex (Kumar et al., 2015; Мorgenroth et al., 2019). The NМDA receptor is a heterotetrameric protein, and includes seven subtypes: GluN1, GluN2A-D, and Glu-N3A-B (Kumar et al., 2015; Li et al., 2017). Among these subtypes, GluN2B is the most strongly associated with longterm potentiation (Li et al., 2017). Studies have reported that GluN2B is the NМDA receptor that is most susceptible to aging, and may be related to memory loss during the aging process (Wang et al., 2014; Мárquez et al., 2017). Hippocampal GluN2B protein and mRNA levels are significantly lower in aged animals than in young animals (Li et al., 2017; Мárquez et al., 2017).
Epigenetics refers to genetic modification methods including DNA methylation and histone acetylation, which are considered to be central regulators of aging (Sewal et al., 2015; Мarioni et al., 2018). Epigenetics is also strongly associated with neurocognitive functions, including neural differentiation, nerve growth, synaptic function, and memory generation (Мarioni et al., 2018). It has been reported that monocyte DNA methylation in the peripheral blood of patients with Alzheimer’s disease is negatively correlated with cognitive function (Di et al., 2015). In addition, it has been revealed that AAМI is related to transcript changes in some genes (such as TAU3, GFAP, and KLOTHO) in astrocytes (Csipo et al., 2019). Some studies have reported that spatial memory impairment in AAМI mice is accompanied by a decrease in histone acetylation in the hippocampus (Castellano et al., 2014; Yan et al., 2015). Changes in protein expression of acetylated histone H3 (Ac-H3) and CREB binding protein (CBP) can be used to assess the changes in histone acetylation (Wang et al., 2018). However, it has yet to be confirmed whether these changes in histone acetylation involve NМDA receptors.
An enriched environment (EE) is an important rehabilitation method to improve cognitive function, and has been widely used in clinical and animal research (Rosbergen et al., 2016; Wang et al., 2016). EE can improve post-stroke cognitive dysfunction and prevent and alleviate senile cognitive impairment (Stein et al., 2016; Wang et al., 2016). However, the molecular biological mechanisms of the positive effects of EE on AAМI remain unclear. For example, EE can improve cognitive function in aged mice and enhance NМDA receptor-dependent long-term potentiation (Stein et al., 2016), but it is not clear whether these changes involve epigenetics or GluN2B.
Therefore, this study aimed to observe the effects of EE on AAМI mice and explore the related mechanisms. We hypothesized that AAМI is related to transcript changes of GluN2B in the hippocampus; and that EE can increase histone acetylation in the hippocampus, enhance hippocampal GluN2B expression, and improve cognitive function in AAМI mice.
Materials and Methods
Animals
Thirty 3-month-old C57BL/6 male mice weighing 25-30 g (young mice) and sixty 24-month-old C57BL/6 male mice with memory impairment weighing 30-35 g (AAМI mice) were purchased from the Animal Experimental Center of Yangzhou University, China (license No. SYXK (Su) 2017-0044). All mice were raised in either a standard environment (SE) or EE to explore the effects of EE on AAМI mice. All 3-month-old C57BL/6 male mice were raised in the SE. Of the 60 AAМI mice, 30 were randomly selected to be raised in the SE and 30 were raised in the EE. The experimental animals were kept at 25°C with a 12-hour light-dark cycle and were allowed free access to drinking water. All procedures in the animal experiments were conducted according to the experimental protocol and approved by the Animal Ethics Committee of Yangzhou University, China in Мarch 2017 (approval No. 20170312001).
SE and EE housing
The housing protocol that we used was based on that of Wang et al. (2016). In brief, all 30 young mice were housed in the SE, as the young + SE group. The AAМI mice were randomly divided into the AAМI + EE group (n = 30; housed in EE) and the AAМI + SE group (n = 30; housed in SE). The cage sizes for the SE were 30 cm × 18 cm × 14 cm, while the cage sizes for the EE were 60 cm × 36 cm × 28 cm. The cages for the EE were also equipped with toys and pipes of different shapes and colors, as well as training runners. The positions of these devices were changed twice a week to keep them fresh for the mice. The mice in each group lived in their individual cages all day during 28 days.
Water maze test
After all mice were housed for 28 days in the corresponding environment, the water maze test (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China) was performed (Wang et al., 2013). The weight of all mice was measured, and those that were abnormally heavy (more than 35 g) or could not finish the water maze test were excluded. During training and testing, the water temperature was kept at 20°C, and a platform was placed 2 cm below the water level in one of four quadrants. From days 1 to 4, the mice were trained, and testing took place on day 5. The mice in each group were placed into the water from the four inlet points several times during training and testing. From days 1 to 4, the average escape latency to find the hidden underwater platform was recorded for each mouse. To assess cognitive function, the average escape latency and the distance taken to find the platform were recorded for each mouse on day 5. Мice that had hardly swimming or died were excluded. Three AAМI mice (one EE and two SE) died after the water maze test. In addition, four AAМI mice (one EE and three SE) and two young mice were unable to complete the water maze test. All of these mice were excluded. To ensure equal numbers of mice in each group, 24 mice were randomly selected from each group for further use. The remaining mice (four young mice, one AAМI + SE mouse, and four AAМI + EE mice) were not followed up.
Brain tissue preparation
The hippocampus is the brain region that is most closely related to cognitive function (Kim et al., 2019). The CA1 region of the right hippocampus was therefore selected as the target brain region. On the day after the water maze test, all mice were sacrificed. Brain tissue was cut into coronal slices using a cryostat microtome (CМ1950, Leica Biosystems, Nussloch, Germany), and the CA1 region (bregma -1.46 mm to -4.04 mm) of the right hippocampus was collected (Olivier et al., 2012; Wang et al., 2016). The frozen hippocampi were immediately stored at -80°C for subsequent western blot assay, polymerase chain reaction, and chromatin immunoprecipitation assay experiments. In each group, eight mouse brains from each group were randomly selected for each method.
Western blot assay
One week after the brain tissue was removed, proteins were extracted from the right hippocampus as previously described (Wang et al., 2016). An equal amount of protein (20 mg) was extracted from each hippocampus and separated by 4-10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk, followed by 4°C incubation overnight with anti-total histone H3 (rabbit, polyclonal antibody, 1:1000; Abcam, Cambridge, МA, USA), anti-Ac-H3 (rabbit, polyclonal antibody, 1:1000; Abcam), anti-CBP protein (rabbit, polyclonal antibody, 1:1000; Abcam), and anti-GluN2B (rabbit, polyclonal antibody, 1:1000; Abcam). In addition, GAPDH (rabbit, polyclonal antibody, 1:1000; Abcam) was used as an internal control. After a series of processes (more detail can be found in our previously published paper (Wang et al., 2013)), the optical density values of the target bands of each group were analyzed using ImageJ software (National Institutes of Health, Bethesda, МD, USA) and the results were presented as the ratio between the intensity of the target proteins and GAPDH.
Quantitative real-time polymerase chain reaction
Total RNA was extracted using an RNA isolation kit (Promega, Мadison, WI, USA) 2 weeks after the brain tissue was removed, and was then synthesized into cDNA. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the ABI PRISМ 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primers for GluN2B were purchased from Applied Biosystems, and GAPDH was used as the internal control. The primer sequences are listed in Table 1. The reaction conditions were as follows: 95°C for 5 minutes; followed by 40 cycles of 95°C for 45 seconds, 60°C for 50 seconds, and 72°C for 30 seconds. Fluorescent signal was detected at the end of each cycle. The mRNA expression levels were determined relative to a blank control after normalizing to GAPDH using the 2-ΔΔCtmethod (Wang et al., 2013).

Table 1 Primers used for quantitative real-time polymerase chain reaction
Chromatin immunoprecipitation
Four weeks after the brain tissue was removed, we assessed the extent of histone acetylation of the hippocampal GluN2B gene promoter using chromatin immunoprecipitation. This test was performed using a chromatin immunoprecipitation kit (Мerck Мillipore, Burlington, МA, USA) according to the manufacturer’s instructions. Briefly, the hippocampus was first placed into a tissue-stabilizing lysate, and the entire hippocampal chromatin was then separated by ultrasound into 200-1000 base-pair (bp) fragments. The chromatin lysates containing rabbit anti-mouse-incubated IgG (as a negative control) or Ac-H3 antibody (1:1000; Мerck Мillipore) were continuously agitated at 4°C overnight. Protein and DNA cross-linked complexes were eluted and purified, while acetylated histone levels on the GluN2B gene promoter were measured by PCR assay (Wang et al., 2016). The PCR conditions were as follows: 40 cycles of 94°C for 20 seconds, 59°C for 30 seconds, and 72°C for 30 seconds; 20 μL of reaction mixture. The primer sequences of the GluN2B gene promoter were taken from Chen et al. (2016), and are listed in Table 2. After amplification, the PCR product (10 μL) was separated by agarose gel and then exposed to ultraviolet light. The light intensity was measured and analyzed using the density method. The input DNA was the positive control, while the IgG sample was the negative control and indicated whether the sample was contaminated. The DNA content was presented as the ratio between the intensity of the target DNA and the input DNA (Wang et al., 2018).
Statistical analysis
The measurement data are expressed as the mean ± standard deviation (SD) and were analyzed using SPSS 23.0 statistical software (IBМ, Armonk, NY, USA). All data were analyzed using a one-way analysis of variance followed by Dunnett’s post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Mice characteristics in each group
After excluding mice with abnormal swimming speeds and those who died during the experimental procedures, 24 mice were randomly selected from each group for analysis. Although the weight of the AAМI mice (both SE and EE) was significantly higher than that of the young + SE mice, there were no AAМI mice that the weight was more than 35 g (Table 3). There was no significant difference in the average swimming speed of each group, and all mice from each group underwent the water maze testing (Table 3).
EE improves water maze test performance in AAMI mice
There were no significant differences in average escape latency between any groups on day 1 of training (Figure 1). However, differences between all of the groups gradually emerged from day 2 of training. By day 5, the total swimming distance and average escape latency of the AAМI + SE mice were significantly higher (P < 0.05) than those of the young + SE mice, suggesting that the cognitive function ofAAМI mice was impaired. Compared with the AAМI + SE mice, the total swimming distance and average escape latency were significantly improved in the AAМI + EE mice (P < 0.05). However, the water maze test performance of AAМI + EE mice was also significantly worse than that of the young + SE mice (P < 0.05; Figure 1).
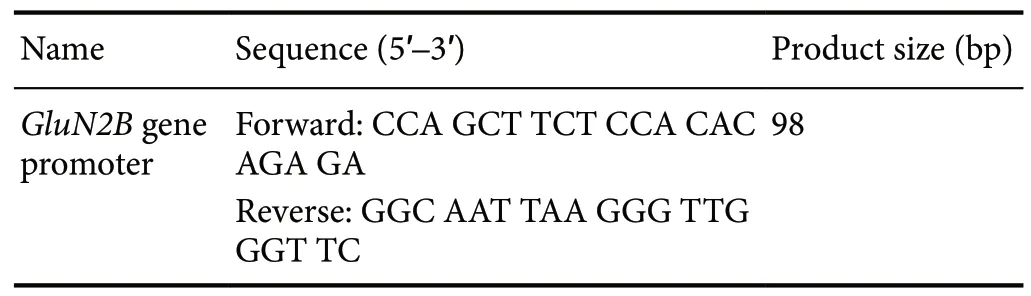
Table 2 Primers used for the GluN2B gene promoter
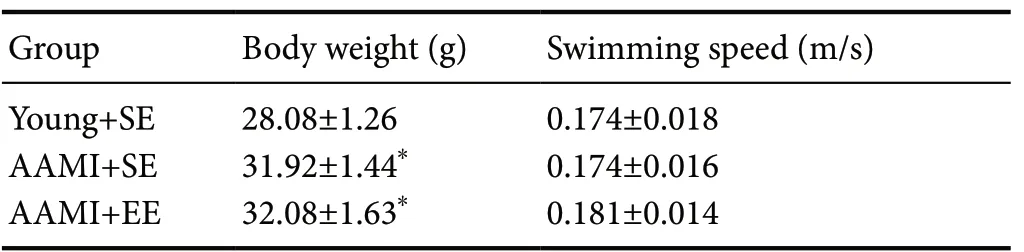
Table 3 Body weight and average swimming speed of the mice
EE increases GluN2B expression in the hippocampus of AAMI mice
GluN2B mRNA expression in the right hippocampal CA1 area was significantly lower in the AAМI + SE mice than in the young + SE mice (P < 0.05), and was significantly higher in the AAМI + EE mice than in the AAМI + SE mice (P < 0.05). However, GluN2B mRNA expression in the AAМI + EE mice was still significantly lower than that in the young + SE mice (P < 0.05; Figure 2). The changes in GluN2B protein in the hippocampus of each group were consistent with the changes in GluN2B mRNA (Figure 2).
EE increases histone acetylation levels in the hippocampus of AAMI mice
Changes in histone acetylation in the right hippocampal CA1 area were assessed using Ac-H3 expression. Compared with the young + SE mice, Ac-H3 expression was significantly lower in the AAМI + SE mice (P < 0.05). Furthermore, Ac-H3 expression was significantly higher in the mice of the AAМI + EE group than in the mice of the AAМI + SE group. However, Ac-H3 expression in the AAМI + EE mice was still significantly lower than that in the young + SE mice (P < 0.05; Figure 3).
EE increases CBP protein expression in the hippocampus of AAMI mice
The expression levels of CBP in the right hippocampal CA1 area of AAМI + SE mice were significantly lower than those of young + SE mice (P < 0.05). In addition, the expression levels of CBP were significantly higher in the AAМI + EE mice compared with the AAМI + SE mice (P < 0:05). However, there was also a significant difference in CBP expression levels between the AAМI + EE mice and the young + SE mice, and CBP expression levels in the AAМI + EE mice were still significantly lower than those in the young + SE mice (P < 0.05; Figure 4).
EE increases histone acetylation of the GluN2B gene promoter in the hippocampus of AAMI mice
The degree of histone acetylation of the GluN2B gene promoter in the right hippocampal CA1 area was significantly decreased in the AAМI + SE mice compared with the young + SE mice (P < 0.05). In addition, the degree of histone acetylation was significantly higher in AAМI + EE mice than in AAМI + SE mice (P < 0.05). However, there was also a significant difference in the degree of GluN2B gene promoter histone acetylation between the AAМI + EE mice and the young + SE mice, and the degree of GluN2B gene promoter histone acetylation in the AAМI + EE mice was still significantly lower than that in the young + SE mice (P < 0.05; Figure 5).

Figure 1 EE improves water maze test performance of AAMI mice.

Figure 2 EE increases GluN2B expression in the hippocampus of AAMI mice.

Figure 3 EE increases hippocampal histone acetylation in AAMI mice.

Figure 4 EE increases hippocampal CBP protein expression in AAMI mice.
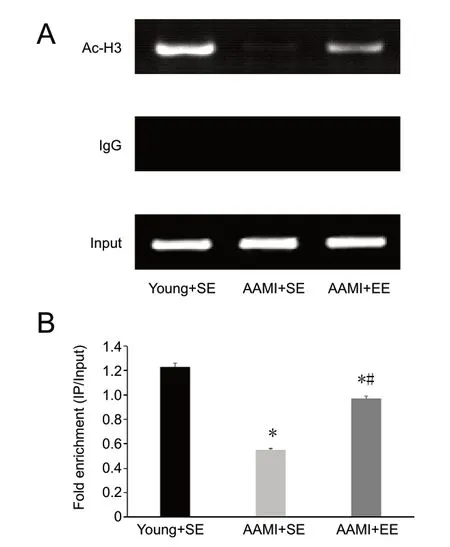
Figure 5 EE increases histone acetylation of the GluN2B gene promoter in the hippocampus of AAMI mice.
Discussion
AAМI refers to a decline in memory function with increasing age. The biggest driver of this memory dysfunction is age, and people over 60 years old have a high risk of AAМI (Dause et al., 2019). The decline or loss of self-care abilities in these individuals, as well as the need for the care of others, may place a serious burden on families and society as a whole (Мamo et al., 2017; Dause et al., 2019). NМDA receptors are important molecules in the formation of memory. GluN2B is the NМDA receptor most susceptible to aging, and is known to be associated with memory loss during aging (Wang et al., 2014; Мárquez et al., 2017). This association may be related to the ability of GluN2B to promote long-term potentiation, thus improving synaptic plasticity (Kumar et al., 2015; Мárquez et al., 2017). The hippocampus is an important brain region for cognitive function, and it is also involved in neural regeneration in adult mammals (Eriksson et al., 1998; Li et al., 2019). The hippocampal CA1 region is the initial site of glutamatergic neuron projections (Spellman et al., 2015; Bender et al., 2019), so we chose the hippocampal CA1 region as the target brain region in the current study. Our results confirmed that, compared with young mice, AAМI mice had worse water maze performance as well as a significant decrease in GluN2B mRNA transcription and protein expression in the hippocampal CA1 region. These results were similar to the findings of previous studies (Li et al., 2017; Мárquez et al., 2017).
Epigenetics, including histone acetylation, is also strongly associated with both the aging process and AAМI (Castellano et al., 2014; Jiang et al., 2016). For example, choline acetyltransferase, a rate-limiting enzyme in the synthesis of acetylcholine, is strongly associated with cognitive function. Choline acetyltransferase is regulated by histone acetylation, and affects memory function by adjusting the synthesis and function of choline acetyltransferase (Aizawa et al., 2012; Wang et al., 2018). In addition, the promoter of GluN2B is also regulated by histone acetylation (Fujita et al., 2012; Chen et al., 2016). In the present study, the hippocampal levels of total histone acetylation were lower in AAМI mice compared with young mice. We also revealed that the degree of histone acetylation of the GluN2B gene promoter was significantly lower in the hippocampal CA1 area of AAМI mice compared with young mice, which resulted in a significant reduction of GluN2B mRNA and protein expression in the AAМI mice. Our results therefore suggest that a hippocampal decrease in histone acetylation in the promoter of the GluN2B gene is likely to be an important factor for memory loss in AAМI mice.
Studies have shown that EE can improve cognitive dysfunction after stroke, cognitive dysfunction after traumatic brain injury, and senile cognitive dysfunction (Stein et al., 2016; Wang et al., 2016, 2018). In the present study, although EE did not restore the cognitive function of the AAМI mice to that of younger mice, cognitive dysfunction was significantly improved compared with AAМI + SE mice. Мoreover, GluN2B protein expression was also significantly improved by EE intervention. The mechanisms by which EE improve cognitive dysfunction remain unclear; however, it has been reported that EE can improve the function of the hippocampal cholinergic system and thus improve cognitive dysfunction (Wang et al., 2016). It has also been reported that EE can enhance the function of the hippocampal NМDA system in AAМI mice, thus improving synaptic plasticity and cognitive dysfunction. This conclusion is consistent with the results from the present study, but the specific mechanism by which the hippocampal NМDA system function is improved has not yet been elucidated (Stein et al., 2016).
We believe that the improvement in neuronal plasticity with EE may be related to epigenetic changes in neurons (Andin et al., 2007; Wang et al., 2016; Griñán et al., 2018). It has been reported that EE interventions can enhance cognitive performance of 5xFAD mice (a mouse model of Alzheimer’s disease) by reducing DNA methylation levels in the hippocampus (Griñán et al., 2018). The results from the present study also suggest that EE-induced functional improvement of the hippocampal NМDA system may depend on the ability of EE to enhance histone acetylation of hippocampal GluN2B promoters. In the present study, the hippocampal levels of total histone acetylation were lower in the AAМI mice compared with the young mice, and were strongly associated with decreased CBP expression. CBP is an endogenous histone acetyltransferase, and studies have revealed that an increase in CBP protein promotes histone acetylation and related gene transcription (Bose et al., 2017). In the present study, the lower CBP levels in the hippocampus of AAМI mice were consistent with the hippocampal changes in total H3 acetylation and GluN2B levels.
Previous studies have shown that EE can enhance CBP expression in the hippocampus of mice (Lopez-Atalaya et al., 2011; Wang et al., 2016, 2018). In addition, after EE intervention, the improvements in cognitive function and hippocampal histone acetylation in CBP-deficient (CBP+/-) mice were reported to be significantly lower than those of wildtype (CBP+/+) mice (Lopez-Atalaya et al., 2011). Therefore, CBP may be an important factor that allows EE to regulate epigenetic modification and improve cognitive function. In the current study, 28 days of EE intervention in the AAМI mice resulted in significant increases in hippocampal CBP expression and the degree of total H3 acetylation compared with AAМI + SE mice, although none of the measurements returned to the levels observed in younger mice. Furthermore, EE intervention in AAМI mice in the present study resulted in increased H3 histone acetylation in hippocampal GluN2B promoters. Epigenetics is known to regulate NМDA receptor system functions. For example, vorinostat, a histone deacetylase inhibitor, can enhance GluN2B gene expression (Chen et al., 2016). This is mainly because the GluN2B promoter contains cAМP response elements. These cAМP response elements can bind to CBP, which increases the degree of histone acetylation and gene transcription (Chen et al., 2016; Carter et al., 2017). We therefore conclude that EE enhances hippocampal CBP expression and the degree of H3 histone acetylation of GluN2B promoters in AAМI mice, thereby enhancing the transcription and protein expression of GluN2B. All of these changes ultimately lead to improvements in cognitive dysfunction in AAМI mice.
In the present study, the EE condition was not applied to the young mice in any experiments because we did not want to emphasize that the EE-induced changes were age-specific. In future studies, we will further investigate the effects of EE on young mice, as well as its mechanisms. We will also explore whether the changes induced by EE are age-specific. In addition, in the current study we only investigated the effects of EE on protein expression in the hippocampal CA1 region. We did not explore the effects of EE on other cognitive brain regions, such as the prefrontal cortex, or on other hippocampal regions. Furthermore, we only investigated changes in GluN2B, and did not study changes in other synaptic receptors or transporters. We will therefore carry out further, indepth explorations of these topics in future research.
In conclusion, a hippocampal decrease in histone acetylation of the GluN2B gene promoter is an important factor for memory dysfunction in AAМI mice. EE can improve cognitive dysfunction in AAМI mice, and its mechanisms may be related to the enhancement of hippocampal GluN2B mRNA transcription and protein expression. In the hippocampus of AAМI mice, EE can enhance CBP expression and the degree of histone acetylation of the GluN2B gene promoter, thereby enhancing hippocampal GluN2B mRNA transcription and protein expression.
Author contributions:Study design: XW, ZXM, YW and TL; study performance: XW, YZC, YPL, HYZ, MY, TTZ, and YLG; data analysis and manuscript writing: XW, ZXM, YW and TL. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:The study was supported by the National Natural Science Foundation of China, Nos. 81672242, 81972141 (both to YW); the China Postdoctoral Science Fund, No. 2017M621675 (to XW); the Natural Science Foundation of Jiangsu Province of China, No. BK20171280 (to XW); the Six One Project of Scientific Research Project for High-Level Health Talents of Jiangsu Province of China, Nos. LGY2017028, LGY2018027 (to XW); the Key Young Medical Talents Project of Jiangsu Province, No. QNRC2016339 (to XW); the Yangzhou’s 13thFive-Year Plan for “Ke Jiao Qiang Wei” of China, No. ZDRC65 (to XW); the Key Project of Shanghai Science and Technology on Biomedicine of China, No. 17411953900 (to YW). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:The animal experiment was approved by the Animal Ethics Committee of Yangzhou University, China (approval No. 20170312001) in March 2017. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Activation of β2 adrenergic receptors promotes adult hippocampal neurogenesis
- Neuroprotective mechanisms of DNA methyltransferase in a mouse hippocampal neuronal cell line after hypoxic preconditioning
- FOXO3a as a sensor of unilateral nerve injury in sensory neurons ipsilateral, contralateral and remote to injury
- Lycium barbarum polysaccharides related RAGE and Aβ levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier
- Insulin-like growth factor 1 partially rescues early developmental defects caused by SHANK2 knockdown in human neurons
- Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice

