Network pharmacological approach to explore the mechanisms of Lianhua Qingwen capsule in coronavirus disease 2019
Wen-Li You,Jin-E Wan, Hai-Rui Gao, Peng-Lin Liu, Li-Ze Zhang, Dan-Dan Wang, Gang Zhao*
1Department of Traditional Chinese Medicine,the Affiliated Hospital of Qingdao University, Qingdao 266000,China;
2Department of Hyperditional Oxygen,the Affiliated Hospital of Qingdao University,Qingdao 266000,China.
Abstract
Background: This study aimed to explore the molecular mechanisms of the active compounds of Lianhua Qingwen capsule in the treatment of coronavirus disease 2019 by using systemic pharmacology approach. Methods: In this study, network pharmacology methodology was applied, including active chemical component screening, target gene prediction, herbal-compound and compound-target gene network construction, gene enrichment analysis, pathway enrichment analysis and network analysis. Results: Network analysis showed that 3 bioactive ingredients (quercetin,kaempferol, AC1LIUG4) were screened as pivotal ingredients. 30 target genes were identified as the anti-coronavirus disease 2019 of Lianhua Qingwen capsule. Among the targets, tumor necrosis factor, JUN (transcription factor AP-1), interleukin 6, vascular endothelial growth factor A, interleukin 1B, interleukin 2, mitogen-activated protein kinases 1 were regulated by various compounds and screened as the core genes of protein-protein interaction network.Nineteen signaling pathways screened by Kyoto Encyclopedia of Genes and Genomes pathway enrichment (P < 0.05)were enriched on various inflammatory signaling pathways such as interleukin 17 signaling pathway, NF-κB signaling pathway, tumor necrosis factor signaling pathway and C-type lectin receptor signaling pathway.Conclusion: The bioactive compounds in Lianhua Qingwen capsule may have a therapeutic effect on coronavirus disease 2019 by inhibiting cytokine storms by regulating multiple inflammatory signaling pathways.
Keywords: COVID-19, Lianhua Qingwen capsule, Network pharmacology, Cytokine storm
Background
An unexplained pneumonia epidemic broke out in Wuhan, China in December 2019. Coronavirus disease 2019 (COVID-19) caused by new coronavirus (SARSCoV-2) infection in 2019 has the characteristics of rapid spread, high infectivity, and general susceptibility to various types of populations. Some researchers have estimated that the primary reproductive number (R0) of the epidemic even reached 3.28 [1]. Numerous studies have found that viral RNA could be recognized by infected cells through numerous recognition receptors,and severe cytokine release could cause a “cytokine storm” [2]. It has been suggested as the reason for the fatal clinical symptoms such as dyspnea, respiratory distress syndrome, or septic shock [3]. At present, no effective anti-viral therapy has been confirmed for COVID-19, and symptomatic supportive treatment and comprehensive intervention are mainly used in clinical practice. Drawing on the critical role played by the combination of traditional Chinese medicine (TCM)and western medicine in the prevention and control of epidemics during the SARS and H1N1 influenza outbreak, it is of considerable signification to investigate the efficacy of TCM in preventing and treating COVID-19.
Lianhua qingwen capsule (LHQW-C) is an innovative Chinese patented medicine for the treatment of influenza, which is based on the TCM theory of plague. It has been widespread applied in the clinical for influenza and pneumonia for decades since passed the new drug approval green channel during SARS in 2003[4, 5]. Reports showed that LHQW-C has broadspectrum antiviral, immune-modulating, and other systemic intervention effects on viral respiratory infectious diseases, meanwhile, it is provided with significantly inhibit avian influenza virus H7N9 [6]. A retrospective study involving 42 COVID-19 cases showed that LHQW-C was superior to oseltamivir capsules in reducing fever, relieving cough, muscle soreness, and fatigue. However, the exact clinical efficacy needs to be further confirmed by randomized controlled clinical studies [7].
A prescription plays a comprehensive and holistic role in regulating the body through multi-components and multi-targets on the molecular level. Network pharmacology is an important tool for studying the mechanism of action of such prescriptions [8]. It is beneficial to drug development in clinical trials with a higher success rate and lower cost by analyzing complex multi-layer networks to analyze the potential molecular mechanisms of multiple compounds on an overall level.
In this study, a network pharmacological analysis was utilized to explore the potential pharmacological mechanism of the anti-COVID-19 effect of LHQW-C.Firstly, download the bioactive ingredients of 13 herbs in LHQW-C, then they were entered into PubChem to obtain the compound structure, and the compound targets were downloaded from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP). In addition, known target genes for COVID-19 and viral pneumonia were obtained from the GeneCards database. Drug and disease overlapping targets were analyzed for proteinprotein interaction (PPI), gene ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG).The workflow was presented in Figure 1.
Materials and methods
Active compounds of LHQW-C
The chemical composition of Lianqiao (Forsythiae fructus), Jinyinhua (Lonicerae japonicae flos),Mahuang (Ephedra herba), Kuxingren(Armeniacae semenamarum), Banlangen(Isatidisradix),Guanzhong(Fortunes bossfern rhizome), Yuxingcao(Houttuyniae herba), Guanghuoxiang(Pogostemonis herba), Dahuang(Rhei radix et rhizoma), Bohe(Menthae haplocalycis herba), Gancao (Glycyrrhizae radix et rhizoma), Shigao(Gypsum fibrosum) as well as Hongjingtian(Rhodiolae crenulatae radix et rhizoma) was collected from TCMSP [9](http://tcmspw.com/tcmsp.php). The foremost pointers for evaluating ADME (absorption, distribution,metabolism and excretion) are oral bioavailability and drug-likeness. The screening criteria are oral bioavailability ≥ 30% and drug-likeness ≥ 0.18. Then,we obtained the standard names and structures of the compounds in the PubChem database [10](https://pubchem.ncbi.nlm.nih.gov/). In the end, due to the lack of precise structural information, only 153 compounds were recruited.
Compound targets of LHQW-C
Download the predicted targets of the screened ingredients included in DrugBank [11] from TCMSP.The standard gene name of the target was obtained by UniProt [12] (https://www.uniprot.org/). After removing the duplication, 137 candidate target genes of LHQW-C were obtained.
COVID-19 target gene
COVID-19-related genes were downloaded from the GeneCards database [13] ((https://www.uniprot.org/),and the selection condition relevance score ≥10. The union of both COVID-19 and viral pneumonia was considered the target gene of COVID-19. To obtain LHQW-C candidate targets for COVID-19, we integrated the predicted compound target genes with the target genes of COVID-19 and selected the intersection of these genes. In the end, 30 overlap target genes were identified.
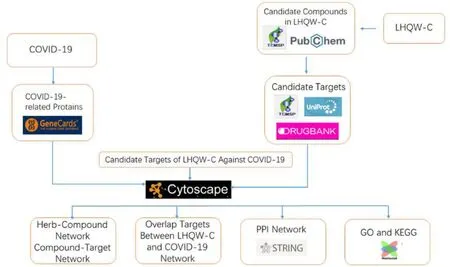
Figure 1 The technical strategy of the systems biology-based methodologies for unraveling the pharmacological basis of LHQW-C action in COVID-19. LHQW-C, Lianhua Qingwen capsule; COVID-19,coronavirus disease 2019; PPI, protein-protein interaction; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO,gene ontology.
PPI network
STRING database [14] was employed to build the PPI network (https://string-db.org/cgi/input.pl). The overlap targets were imported with its species limited to “Homo sapiens” and the confidence score > 0.9. The PPI data of the pivotal targets were visualized by version 3.7.2 based on degree value.
Network construction
The networks were constructed as follows: (1) the network between the LHQW-C herbs and the active compounds; (2) the network between the active compounds and the target genes; (3) the network of overlap targets between LHQW-C compounds and COVID-19; (4) PPI network of core targets. Network analysis software Cytoscape version 3.7.2 [15](https://cytoscape.org/) was utilized to visualize the network. Nodes represent herbs, targets and compounds,respectively, and edges indicate interactions.
GO and pathway analysis
To study the biological function of genes, GO enrichment analysis [16] was calculated and evaluated by Webgestalt Visualization and Integrated Discovery Database [17] (http://www.webgestalt.org/). The enrichment analysis of the KEGG signal pathway [18]was realized by ClueGO plug-in [19] of Cytoscape. The difference was considered statistically significant,P<0.05.
Result
Composite target network analysis
One hundred and fifty-three active compounds of 11 herbs in LHQW-C apart from Shigao(Gypsum fibrosum)and Hongjingtian(Rhodiolae crenulatae radix et rhizoma) have obtained from the TCMSP and PubChem databases. These compounds meet all the screening criteria. As shown in Figure 2, the herb-compound network consists of 164 nodes (11 herbal nodes, 153 active ingredient nodes). Beta-sitosterol, luteolin,kaempferol, quercetin, and stigmasterol shared in more than 4 herbs (Table 1). One hundred and thirty-seven potential targets for LHQW-C were recorded in DrugBank. The compound-target network consisted of 290 nodes (153 compound nodes, 137 target nodes,Figure 3). Most targets were hit by multiple compounds,but only 3 compounds (quercetin, kaempferol and AC1LIUG4) could affect more than 30 targets at the same time. More than 70 ingredients could adjust PTGS2, PTGS1, and Calmodulin (CALM).
Candidate targets for LHQW-C against COVID-19 Network
Thirty-six known COVID-19-related target genes and 394 known viral pneumonia-related target genes were obtained in the GeneCards database, and 404 target genes were obtained by combining. Among them, 30 common targets were identified between LHQW-C potential targets with known COVID-19-related target genes (Figure 4).
PPI network analysis
The PPI network can visualize and quantify the function of specific proteins in cells at the system level [20]. The STRING database was applied to build a PPI network with the common target of LHQW-C and COVID-19.The results included 30 nodes and 78 edges, and the figure shows the interacting proteins/genes (Figure 5A).Cytoscape software visualized the PPI network by import STRING data, where there was a significant dense interaction between tumor necrosis factor (TNF),JUN (transcription factor AP-1), interleukin (IL) 6,vascular endothelial growth factor A, IL1B, IL2, and mitogen-activated protein kinases 1 proteins (Figure 5B).
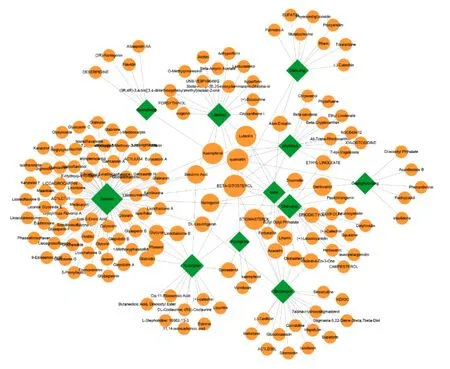
Figure 2 Herb-compound network of LHQW-C. LHQW-C, Lianhua Qingwen capsule.

Table 1 Information for core bioactive components of LHQW-C

Table 1 Information for core bioactive components of LHQW-C (continued)
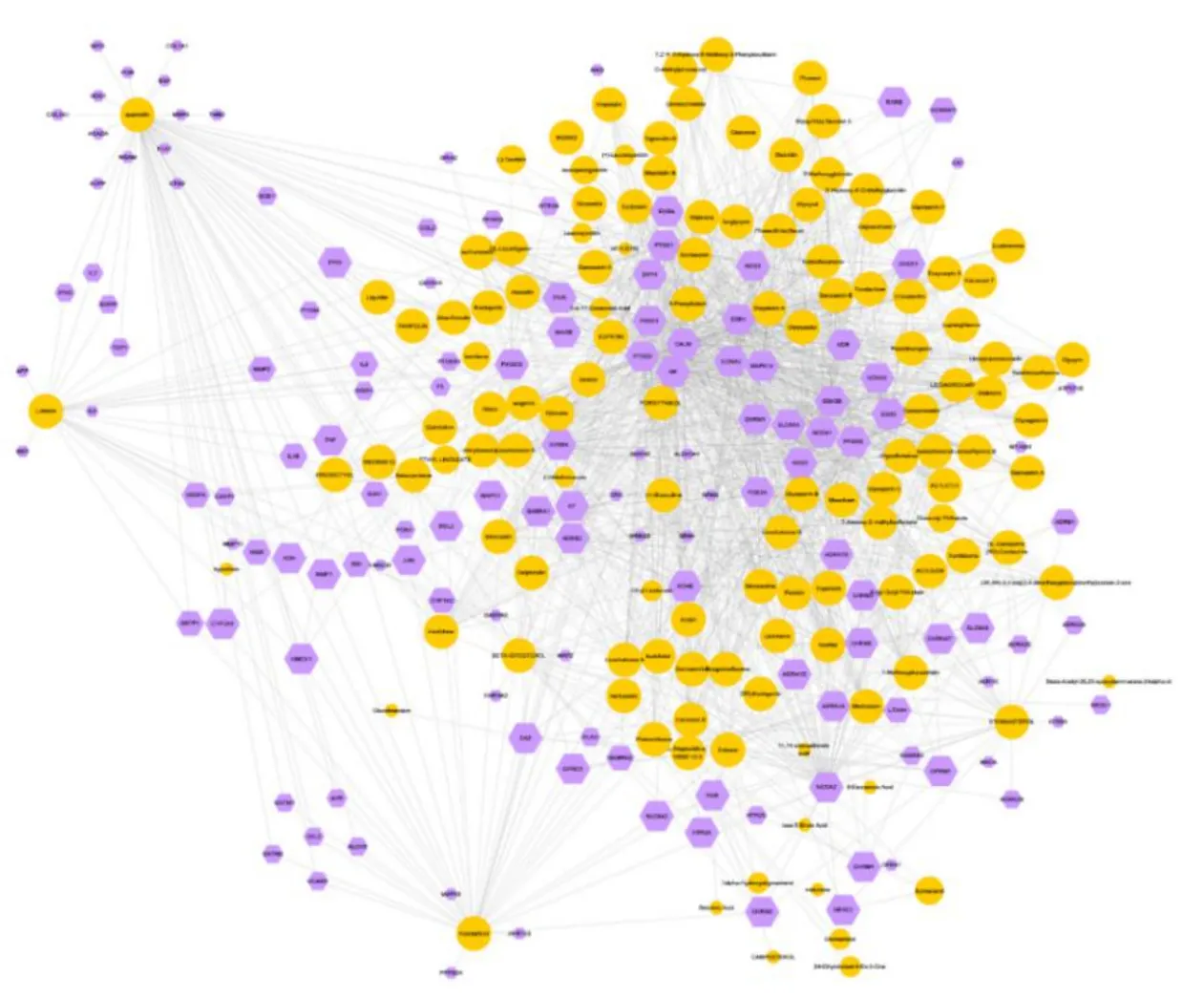
Figure 3 Compound-target network of LHQW-C. The yellow circles represent compounds; the purple hexagons represent targets. The bigger size of the nodes indicates the higher value of the degree. LHQW-C, Lianhua Qingwen
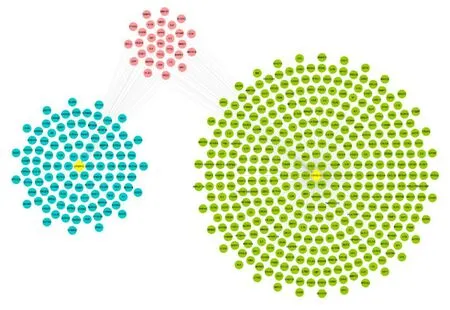
Figure 4 Overlapping targets network of LHQW-C and COVID-19. Blue circles represent targets of LHQW-C.Green circles represent known COVID-19-related target genes. Pink circles represent overlapping targets of LHQWC and COVID-19. LHQW-C, Lianhua Qingwen capsule; COVID-19, coronavirus disease 2019.
GO, KEGG pathway enrichment analysis
To further analyze the mechanism of LHQW-C acting on COVID-19 from a comprehensive level, GO enrichment analysis of biological processes, molecular functions and cellular components of 30 selected targets was performed by Webgestalt (Figure 6A). The analysis results showed that LHQW-C against COVID-19 by regulating multiple biological processes (P< 0.05), of which the top 3 were metabolic process, response to the stimulus, and biological regulation. The top 3 molecular functions (P< 0.05) included protein binding, ion binding, and hydrolase activity. The top 3 cellular components (P< 0.05) were extracellular space,membrane, and endomembrane system. As shown in Figure 6B, 30 target genes were further mapped to the KEGG pathway (P< 0.05). The data indicated that the effect of LHQW-C on COVID-19 depends mainly on signaling pathways (SPs) related to inflammation regulation, such as NF-κB, TNF, IL17, and C-type lectin receptor SP.
Discussion
Although influenza is mostly a self-limiting disease,some can develop into severe conditions [21]. A few severe cases have rapid disease progression that may rapidly develop into interstitial lung disease in clinical manifestations. In severe cases, acute respiratory distress syndrome and multiple organ lesion, which means higher mortality [22]. The influenza virus invades respiratory epithelial cells and is swallowed by the latter. Viral RNA can infect cells through a variety of receptor recognition pathways, thereby activating the cellular and humoral immune responses and promoting the release of cytokines in large quantities, which can trigger the so-called “cytokine storm” [23], and eventually leading to extensive tissue damage, systemic inflammatory response syndrome, and multiple organ failure [24, 25]. Excessive inflammation associated with the uncontrolled release of pro-inflammatory cytokines is the leading cause of death from influenza virus infection. Therefore, more and more researches focus on immunomodulatory therapy besides antiviral therapy[26, 27]. However, there have been no reports of significant benefits of effective immunomodulators in the treatment of severe influenza viral pneumonia. In contrast, studies showed that using hormone therapy was not conducive to virus clearance [28]. The inflammatory response network is complex, and drugs targeting a single cytokine and a single process are difficult to achieve the desired therapeutic effect.Therefore, new drugs are urgently needed to suppress the inflammation caused by the influenza virus.
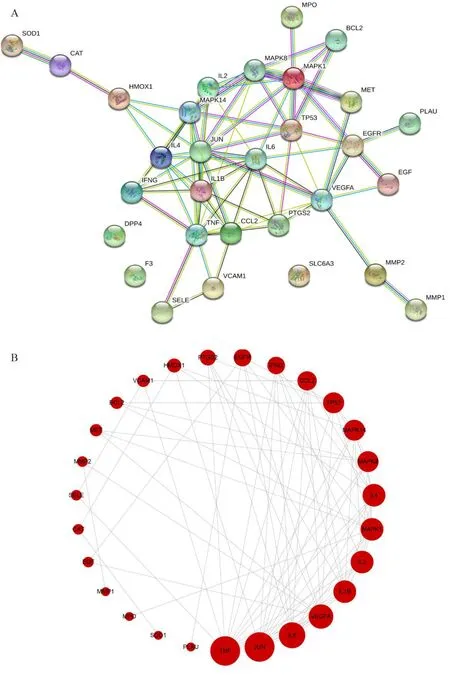
Figure 5 PPI network. (A) Interactive PPI network of 30 overlapping target protein/genes. (B) Interactive PPI network of top 27 protein/genes. The bigger size of the nodes indicates the higher value of the degree. PPI: proteinprotein interaction.
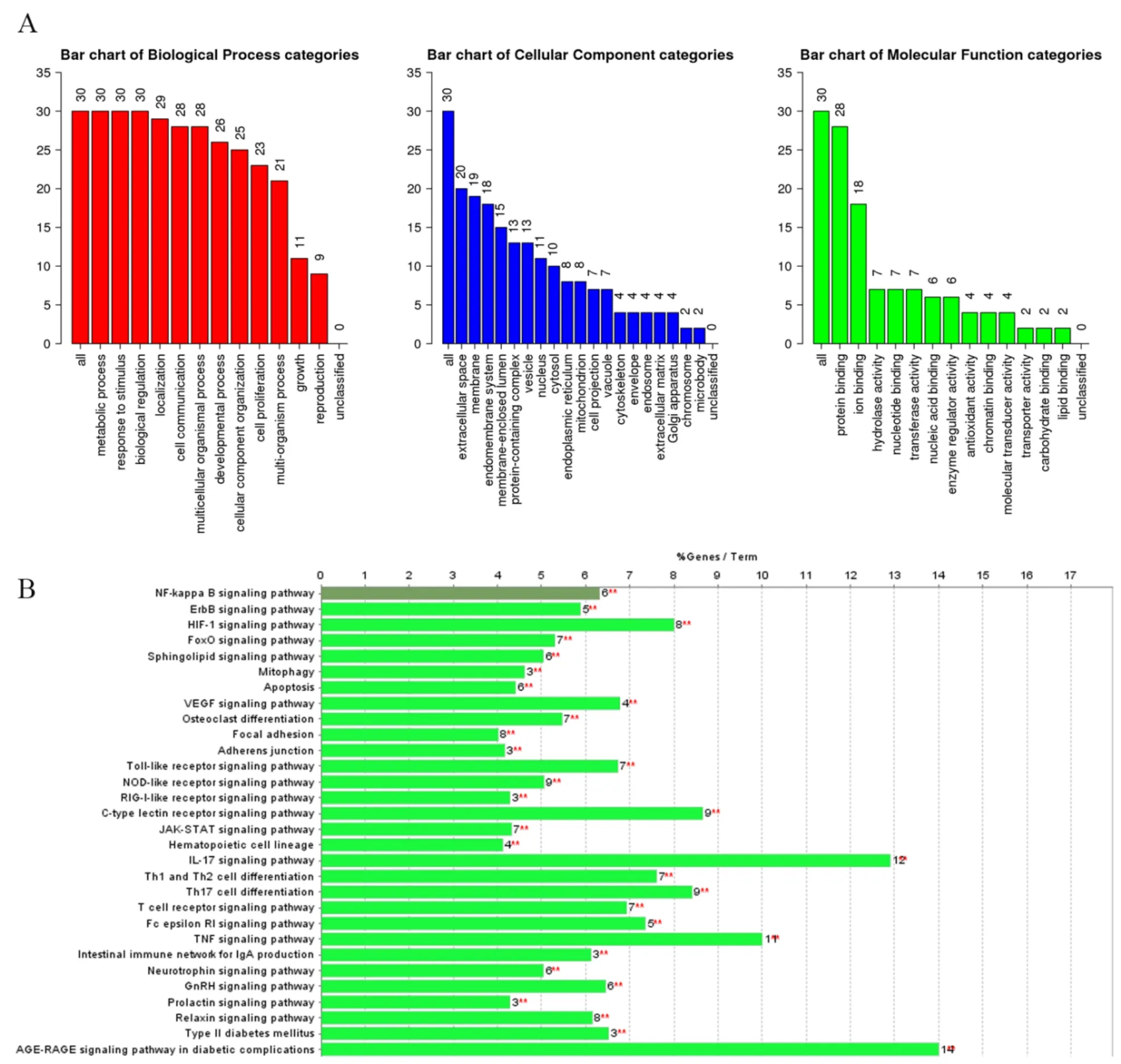
Figure 6 GO and KEGG pathway analysis of target genes of LHQW-C on COVID-19. (A) GO analysis of the 30 core targets (P < 0.05). The y-axis shows the number of genes related to these terms; the x-axis shows significantly enriched terms. (B) KEGG pathway analysis (P < 0.05). The y-axis shows significantly enriched terms; the x-axis shows the number of genes related to these terms. GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; LHQW-C, Lianhua Qingwen capsule;COVID-19, coronavirus disease 2019.
This study provides a novel idea for curing COVID-19 through the network pharmacology approach, which systematically analyzed the potential therapeutic mechanism of LHQW-C for COVID-19. The characteristics of “overall regulation and multi-target therapy” confirm the scientific of its theory and formula.
In this research, 153 active compounds and 137 targets in 11 herbs of LHQW-C were studied. The compound-target network showed that there was a phenomenon in which a compound interacts with multiple targets, and there were also different compounds acting on the same target. This reflects the mechanism of interaction between the multicomponents and multi-targets of TCM. Quercetin,kaempferol and AC1LIUG4 were screened as the key compounds. Four hundred and four known COVID-19 related targets were obtained, and there were 30 common targets between COVID-19 and LHQW-C. By analyzing the common target network, 27 key targets were identified. They were significantly enriched in numerous pro-inflammatory cytokines such as TNF, ILs(IL6, IL1B, IL2 and IL4), interferons and chemokine,which usually increased in critical viral pneumonia patients [29]. Functional enrichment analysis of these targets found that they were enriched in the immune system and various inflammatory SPs. Influenza virus infection causes a strong immune response and inflammatory storm, and a mass of cytokines are activated during the storm. The main function of Th17 cells is to promote the mobilization [30], recruitment and activation of neutrophils, and mediate the proinflammatory response. This study revealed that the targets of LHQW-C have regulatory effects on multiple cytokine-release-related pathways that could play important roles in the development of the COVID-19,including the IL17 SP, NF-κB SP, TNF SP, Toll-like receptor SP, VEGF SP, JAK-STAT SP, Th17 cell differentiation pathway, C-type lectin receptor, and FoxO SP (Figure 6B). It intimated that LHQW-C plays a role in inhibiting activated cytokines, alleviating excessive immune response, and eliminating inflammation potentially. It has been confirmed in previous studies [6] that LHQW-C can inhibit the proliferation of influenza virus in vitro and reduce the expression of IL6, IL8 and TNF-a genes induced by the virus. In vivo experiments [31] have shown that LHQWC can effectively reduce macrophage movementchemotactic potential. However, other results predicted by this study need to be further confirmed by experiments.
It is worth noting that more than 70 ingredients could adjust PTGS2, PTGS1, and CALM. PTGS1(prostaglandin-endoperoxide synthase-1, previously COX1) and PTGS2 (previously COX2) are the ratelimiting enzyme in the production of prostaglandins[32], have been found to contribute to T-cell development [33]. CALM has been found of widespread role in cellular signaling, which is essential to its property to stably bind to many of its downstream effectors [34]. Regrettably, the relevance between these targets and the pathogenesis of COVID-19 still lacks definitive evidence.
Conclusion
In this study, a network pharmacological method was adopted to systematically study the active chemical components and targets of LHQW-C. At the molecular level, the overall regulation mechanism of the prescription through multi-component and multi-target inhibition of inflammation on the body was explained.It provides new insights for the clinical application of LHQW-C in the treatment of COVID-19.
 Precision Medicine Research2020年2期
Precision Medicine Research2020年2期
- Precision Medicine Research的其它文章
- Screening key target genes for pulmonary arterial hypertension based on bioinformatics
- Screening key target genes for severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) based on bioinformatics and gene network
- A network pharmacology approach to investigate the mechanism of “Huangqi-Shanzhuyu” in the treatment of diabetic nephropathy
- Use of network pharmacology and molecular docking to explore the potential mechanism governing the efficacy of Jinhuaqinggan granules in the treatment of novel coronavirus-induced pneumonia
