有氧运动对高脂膳食大鼠脂肪组织COX2的影响
龚丽景 付鹏宇 王孝强
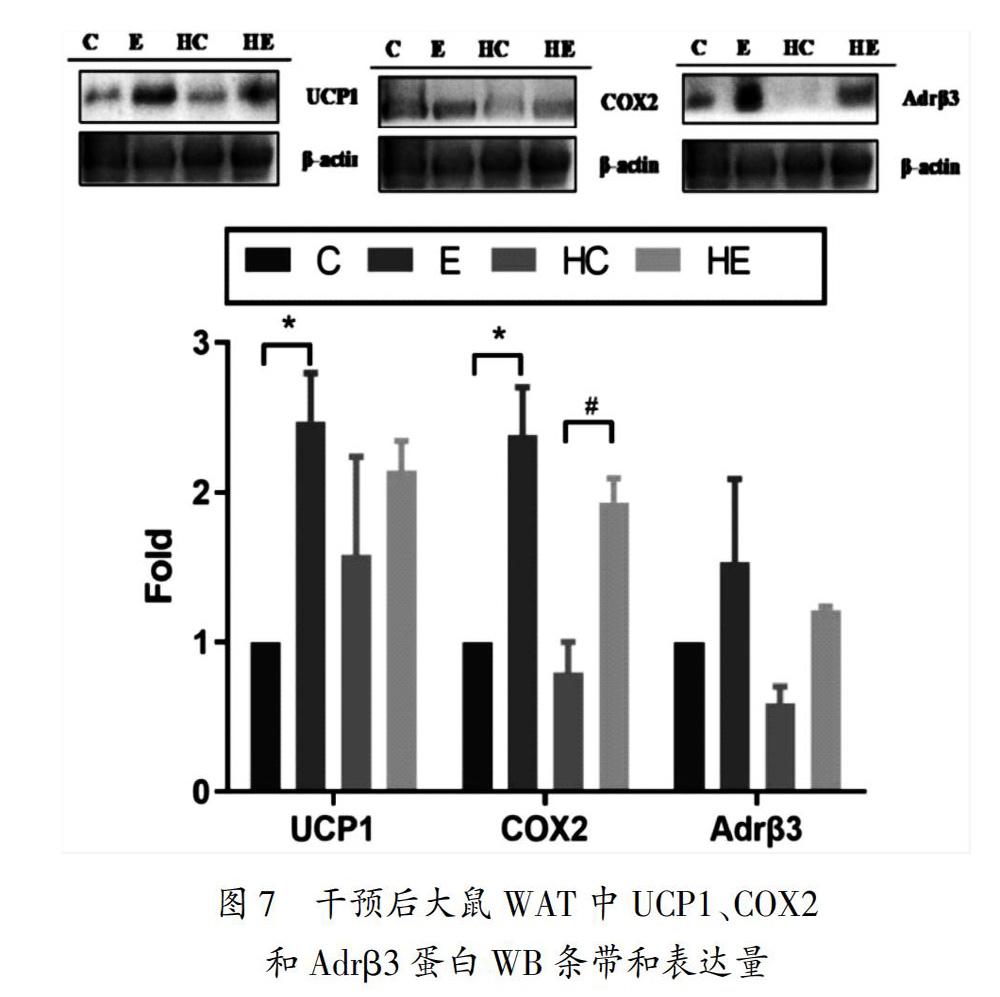

摘 要:目的:旨在观察有氧运动对高脂膳食大鼠白色脂肪组织(WAT)和棕色脂肪组织(BAT)中环氧合酶2(COX2)及其相关基因的影响,以探讨有氧运动促进WAT棕色化及BAT活化以抵抗高脂膳食对机体血脂和体重不利影响的可能机制。方法:雄性SD大鼠随机分为普通对照组(C组)、普通运动组(E组)、高脂对照组(HC组)和高脂运动组(HE组),每组7只。E组和HE组进行中等强度跑台训练。8周干预结束后,计算各组大鼠Lees指数,测量体成分,称量WAT(肾周和附睾处)和BAT(肩胛处)湿重;测试血清甘油三酯(TG)和总胆固醇(CHO)含量;RT-qPCR检测BAT中COX2 mRNA的相对表达量;Western blot法检测脂肪组织中肾上腺素能受体β3(Adrβ3)、COX2和解偶联蛋白(UCP1)蛋白含量。结果:1)饲喂第2周起高脂膳食大鼠体重较普通膳食大鼠显著升高,运动第3周起,HE组体重较HC组均显著降低;运动后E组和HE组大鼠的Lees指数分别较C组和HC组显著性下降;2)高脂膳食大鼠脂肪含量显著增加,运动后出现显著性下降;与C组相比,HC组WAT湿重显著增加,运动组WAT湿重显著降低,E组BAT%较C组显著升高;3)有氧运动降低高脂膳食大鼠血清CHO和TG水平;4)运动组BAT中COX2 mRNA表达显著高于对照组;5)E组BAT中UCP1、COX2和Adrβ3蛋白表达显著高于C组,HE组WAT中COX2蛋白表达显著高于HC组。结论:有氧运动可降低高脂膳食机体的血脂水平,改善体脂含量和体重,该过程可能与运动激活WAT和BAT中Adrβ3以促进COX2的表达,增加WAT和BAT中UCP1的表达,以促进BAT的活化和WAT的棕色化有关。
关键词:有氧运动;棕色脂肪;白色脂肪棕色化;COX2
Abstract:Objective: To clarify the possible mechanism of aerobic exercise promoting brown adipose tissue (BAT) activation and white adipose tissue (WAT) browning in order to resist the adverse effects of high-fat diet on blood lipids and body weight, by detecting the changes of cyclooxygenase 2 (COX2) related genes in Rats' WAT and BAT. Methods: Male SD rats were randomly divided into
高脂的飲食模式和静坐少动的生活方式是诱发肥胖及相关疾病的重要原因,严重威胁人类的健康[1]。肥胖及其相关疾病的发生发展由多组织器官代谢异常所致,其中脂肪组织是最主要的参与者[2]。脂肪组织主要分储脂为主的白色脂肪组织(white adipose tissue,WAT)和耗脂的棕色脂肪组织(brown adipose tissue,BAT)。BAT细胞线粒体特异性表达的解偶联蛋白1(uncoupling protein 1,UCP1),是BAT发挥产热功能的标志性基因。UCP1参与氧化磷酸化过程,可使游离脂肪酸(free fatty acids,FFA)转变为热能形式散发,增加机体能量消耗;WAT在一定条件下可向BAT转变,称为白色脂肪棕色化。这些方式均可有效抑制肥胖的发生与发展[3]。环氧合酶2(cyclooxygenase 2,COX2)在调节脂肪炎症和促进能量代谢中发挥着重要的作用,但它是否与肥胖及相关疾病的发展相关还不明确,在高脂膳食诱导肥胖的发展过程中控制COX2的信号和途径尚不明确[4]。COX2是UCP1合成的重要因子[5],交感神经被激活可上调肾上腺素能受体β3(adrenergic receptor β3,Adrβ3)而刺激COX2表达以增加UCP1活性,促进产热[6-7]。研究发现,有氧运动作为激活BAT和WAT棕色化的重要途径,其作用机制与交感神经的活性增加密切相关[8]。有氧运动是否通过激活Adrβ3以促进COX2的表达而促进BAT的活化和WAT的棕色化,以促进高脂膳食机体的能耗尚不明确。本研究拟通过8周有氧运动干预高脂膳食大鼠,观察其BAT和WAT中COX2相关基因的变化,以探明有氧运动促进BAT活化和WAT棕色化,以降低血脂、控制体脂和体重的可能机制。
1 材料与方法
1.1 实验对象分组与干预
SD大鼠28只,雄性,7周龄,购于北京维通利华实验动物技术有限公司(许可证号:SCXK(京)2015-0004),随机分为两组:普通膳食组(N组),饲喂普通维持饲料;高脂膳食组(H组),饲喂高脂饲料,每组14只。10周后,将N组随机分为普通对照组(C组,n=7)和普通运动组(E组,n=7),H组随机分为高脂对照组(HC组,n=7)和高脂运动组(HE组,n=7)。
1.2 饲料成分
普通饲料(中国军事医学科学院实验动物中心)和高脂饲料(60 kCal% fat,北京华阜康生物技术股份有限公司)的配方如图1所示。其中普通饲料的能量密度为334 kcal/100 g,高脂饲料的能量密度为524 kcal/100 g,即高脂饲料的能量较同等量的普通饲料高190 kcal/100 g。
1.3 干预与取材
E组和HE组施加跑台训练,训练方案为:速度20 m/min,坡度角0°,1 h/d/次,5次/周,共8周。每周称量各组大鼠体重。饲养和训练均在北京体育大学动物实验室内进行[许可证号:SYXK(京)2016-0033]。本研究伦理批准号为2015028(北京体育大学运动科学实验伦理委员会)。干预结束后,禁食12 h,使用2%戊巴比妥钠麻醉(50 mg/kg体重)大鼠,测量并记录体长、体重数据,计算反映成年大鼠肥胖情况的Lees指数[8],双能X射线(XR-46,Norland)扫描并分析大鼠体成分;腹主动脉取血,分离血清-20℃保存备用;取肾周、附睾周WAT和肩胛间BAT,称重后,液氮冷却后转移至-80℃储存。
3.2 有氧運动对高脂膳食机体BAT和WAT中COX2的调控作用
3.2.1 有氧运动促进Adrβ3和COX2的表达以增加BAT活性
BAT被毛细血管网包绕,丰富的血液供应和BAT细胞内部丰富的线粒体使BAT能够大量产热,增加机体能耗从而调节机体能量代谢平衡[15]。有研究发现,移植BAT可提高肥胖小鼠机体代谢水平,增加脂肪的氧化,降低肝脏TG水平[16]。BAT细胞表面广泛分布着Adrβ3,其与去甲肾上腺素(norepinephrine/noradrenaline,NE)结合后,能够促进脂类分解[17],激活UCP1表达,增加产热作用,激活BAT[18-19]。Adrβ3敲除可降低BAT的活性,增加WAT的储量[20],脂肪分化决定因子Shox2(short stature homeobox 2)敲除后可上调脂肪分解水平,并增加Adrβ3表达,表现出一定程度的肥胖抵抗性[21]。COX2作为BAT产热的重要参数,可评判机体能量代谢水平,与UCP1、磷酸鸟苷(guanosine diphosphate,GDP)、碘甲腺原氨酸脱碘酶Ⅱ(deiodinase iodothyronine 2,DIO2)等指标反映BAT的整体功能状态[22]。Adrβ3的激活被认为是寒冷刺激募集BAT和诱导WAT棕色化所必需的[23],有氧运动作为另一种激活BAT的有效手段,其发挥作用的机制也与Adrβ3的激活密切相关。本研究中,有氧运动干预后BAT中Adrβ3、COX2和UCP1的表达均增加,BAT%增加,血脂水平降低,Lees指数下降,体脂和体重减少。说明有氧运动可通过上调交感神经活性,激活Adrβ3-COX2通路以增加BAT活性。研究显示,运动能够增加小鼠BAT前体细胞的募集并增加BAT内UCP1的表达[24],而手术去除交感神经后,模拟运动干预BAT细胞,Adrβ3表达不再增加,也不能产生促进UCP1表达的效果[25],提示有氧运动对激活BAT中Adrβ3和COX2的表达发挥重要作用。
3.2.2 有氧运动增加Adrβ3和COX2的表达促进WAT棕色化
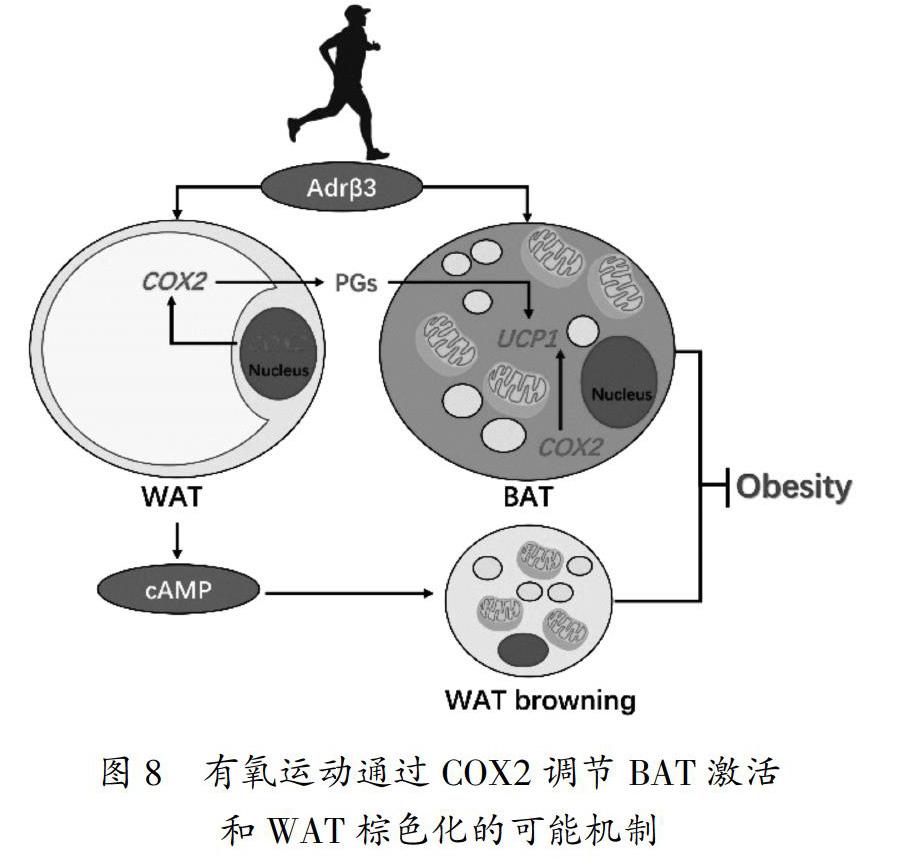
成人体内有活性的BAT数量少,且BAT激活受到遗传、生理状态、环境等多种因素的影响,较难发挥减脂控重的作用[26]。但是,肥胖机体贮存有大量WAT,WAT的棕色化对促进能量代谢平衡更具吸引力和可行性[27]。经典的肩胛BAT和WAT中的棕色脂肪样细胞具有不同的来源[28-29]。分化来源接近WAT细胞的Brite脂肪细胞,是一类BAT细胞,能被Adrβ3明显调控作用[30]。有研究显示,WAT中BAT细胞数量增加更有可能影响机体全身的能量平衡,同时更易诱发小鼠的肥胖抵抗。而在偏瘦大鼠脂肪细胞中异丙肾上腺素刺激的前列腺素E2(prostaglandin E2,PGE2)合成明显高于肥胖者[31]。而PGE2是COX2下游发挥产热功能的关键基因,提示COX2在能量平衡和肥胖的发生发展中起重要作用[32]。研究显示,寒冷可通过激活交感神经而发挥促进WAT棕色化的作用,而施加COX2的抑制剂或干预COX2敲除小鼠则抑制WAT中UCP1含量的增加[33]。运动干预可能通过提高交感神经兴奋性以增加WAT内Adrβ3数量,激活COX2的表达从而增加UCP1蛋白表达,诱导WAT内的新生棕色脂肪细胞募集产生UCP1阳性的脂肪细胞[6, 34]。本研究显示,有氧运动可显著增加WAT中Adrβ3、COX2和UCP1的表达,降低高脂膳食大鼠的血脂水平,提高能量代谢以达到降低体重和减脂的目的(见图8)。
4 结论
8周中等强度的有氧运动可降低高脂膳食大鼠的血脂水平,增加BAT百分含量,降低体脂含量和体重。有氧运动干预后WAT和BAT中Adrβ3、COX2和UCP1表达增加以促进WAT棕色化和BAT活化可能参与改善高脂膳食对机体的不良影响。
参考文献:
[1]Lau D C, Obesity Canada Clinical Practice Guidelines Steering C, Expert P. Synopsis of the 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children[J]. CMAJ, 2007,176(8):1103-1106.
[2]姜勇, 赵文华. 成人肥胖的评价方法、指标及标准在公共卫生中应用的研究进展[J]. 卫生研究, 2013(4):701-705.
[3]Cannon B, Nedergaard J. Brown adipose tissue:function and physiological significance[J]. Physiol Rev, 2004, 84(1):277-359.
[4]Zhang X, Luo Y, Wang C, et al. Adipose mTORC1 Suppresses Prostaglandin Signaling and Beige Adipogenesis via the CRTC2-COX-2 Pathway[J]. Cell Rep, 2018, 24(12):3180-3193.
[5]Preite N Z, Nascimento B P, Muller C R, et al. Disruption of beta3 adrenergic receptor increases susceptibility to DIO in mouse[J]. J Endocrinol, 2016, 231(3):259-269.
[6]Madsen L, Pedersen L M, Lillefosse H H, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity[J]. PLoS One, 2010, 5(6):e11391.
[7]Vegiopoulos A, Muller-Decker K, Strzoda D, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes[J]. Science, 2010, 328(5982):1158-1161.
[8]付鵬宇, 龚丽景, 朱镕鑫, 等. 有氧运动对肥胖小鼠棕色脂肪mRNA表达谱的影响[J]. 北京体育大学学报, 2016, 39(9):50-56.
[9]Smith W L. Prostanoid biosynthesis and mechanisms of action[J]. The American journal of physiology, 1992, 263(2 Pt 2):F181-191.
[10]龚文辉, 张素梅, 储珏. 有氧运动对饮食诱导的肥胖鼠下丘脑和褐色脂肪BMP7表达的影响[J]. 中国康复, 2016, 31(2):114-117.
[11]Lee M W, Lee M, Oh K J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes:Adipokines, Batokines and MicroRNAs[J]. Journal of clinical medicine, 2019, 8(6):3180-3190.
[12]Garcia-Alonso V, Claria J. Prostaglandin E2 signals white-to-brown adipogenic differentiation[J]. Adipocyte, 2014, 3(4):290-296.
[13]A B, A B, Mj Z, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor[J]. Cell, 1999, 96(6):857-868.
[14]Virtue S,Feldmann H,Christian M,et al. A new role for lipocalin prostaglandin d synthase in the regulation of brown adipose tissue substrate utilization[J].Diabetes,2012,61(12):3139-3147.
[15]Oelkrug R, Polymeropoulos E T, Jastroch M. Brown adipose tissue:physiological function and evolutionary significance[J]. Journal of comparative physiology B, Biochemical, systemic, and environmental physiology, 2015, 185(6):587-606.
[16]Liu X, Wang S, You Y, et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice[J]. Endocrinology, 2015, 156(7):2461-2469.
[17]Jia J J, Tian Y B, Cao Z H, et al. The polymorphisms of UCP1 genes associated with fat metabolism, obesity and diabetes[J]. Mol Biol Rep, 2010, 37(3):1513-1522.
[18]Azzu V, Brand M D. The on-off switches of the mitochondrial uncoupling proteins[J]. Trends Biochem Sci, 2010, 35(5):298-307.
[19]Yi C X, Tschop M H. Brain-gut-adipose-tissue communication pathways at a glance[J]. Disease models & mechanisms, 2012, 5(5):583-587.
[20]Susulic V S, Frederich R C, Lawitts J, et al. Targeted disruption of the beta 3-adrenergic receptor gene[J]. J Biol Chem, 1995, 270(49):29483-29492.
[21]Lee K Y, Yamamoto Y, Boucher J, et al. Shox2 is a molecular determinant of depot-specific adipocyte function[J]. Proc Natl Acad Sci U S A, 2013, 110(28):11409-11414.
[22]Ca T, T H, Mf H, et al. SERCA2a and mitochondrial cytochrome oxidase expression are increased in hearts of exercise-trained old rats[J]. American Journal of Physiology, 1996, 271(1 Pt 2):H68.
[23]Barbatelli G, Murano I, Madsen L, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation[J]. American journal of physiology Endocrinology and metabolism, 2010, 298(6):E1244-1253.
[24]Xu X, Ying Z, Cai M, et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue[J]. Am J Physiol Regul Integr Comp Physiol, 2011, 300(5):R1115-1125.
[25]Contreras G A, Lee Y H, Mottillo E P, et al. Inducible brown adipocytes in subcutaneous inguinal white fat:the role of continuous sympathetic stimulation[J]. Am J Physiol Endocrinol Metab, 2014, 307(9):E793-799.
[26]Madsen L, Pedersen L M, Lillefosse H H, et al. UCP1 Induction during Recruitment of Brown Adipocytes in White Adipose Tissue Is Dependent on Cyclooxygenase Activity[J]. PloS one, 2010, 5(6):e11391.
[27]Jan N, Barbara C. The browning of white adipose tissue:some burning issues[J]. Cell Metabolism, 2014, 20(3):396-407.
[28]Timmons J A, Kristian W, Ola L, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(11):4401-4406.
[29]Patrick S, Bryan B, Wenli Y, et al. PRDM16 controls a brown fat/skeletal muscle switch[J]. Nature, 2008, 454(7207):961-967.
[30]Granneman J G, Pipeng L, Zhengxian Z, et al. Metabolic and cellular plasticity in white adipose tissue I:effects of beta3-adrenergic receptor activation[J]. American journal of physiology Endocrinology and metabolism, 2005, 289(4):608-616.
[31]Gaskins H R, Hausman D B, Martin R J, et al. Evidence for abnormal prostaglandin synthesis in obese Zucker rat adipose cell cultures[J]. Journal of Nutrition, 1989, 119(3):458.
[32]Petersen R K, Claus J R, Rustan A C, et al. Arachidonic acid-dependent inhibition of adipocyte differentiation requires PKA activity and is associated with sustained expression of cyclooxygenases[J]. Journal of Lipid Research, 2003, 44(12):2320.
[33]Maria J, Giorgio B, Roberta A, et al. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat[J]. Febs Journal, 2003, 270(4):699-705.
[34]Garcia-Alonso V, Titos E, Alcaraz-Quiles J, et al. Prostaglandin E2 Exerts Multiple Regulatory Actions on Human Obese Adipose Tissue Remodeling, Inflammation, Adaptive Thermogenesis and Lipolysis[J]. PLoS One, 2016, 11(4):e0153751.

