Evaluation of gastric lavage eff ciency and utility using a rapid quantitative method in a swine paraquat poisoning model
Yun-fei Jiang, Jian Kang, Pei-pei Huang, Jia-xi Yao, Zhong-he Wang, Lei Jiang, Jun Wang, Li Qiao, Bao-li Zhu, Hao Sun, Jin-song Zhang
1 Department of Emergency, the First Aff liated Hospital of Nanjing Medical University, Nanjing, China
2 Department of Emergency, Nanjing Drum Tower Hospital, the Aff liated Hospital of Nanjing University Medical School, Nanjing, China
3 Key Lab of Modern Toxicology, Ministry of Education and Department of Hygienic Analysis and Detection, School of Public Health, Nanjing Medical University, Nanjing, China
4 Key Lab of Modern Toxicology, Ministry of Education and Department of Toxicology, School of Public Health, Nanjing Medical University, Nanjing, China
5 Department of Occupational Disease Prevention, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China
KEY WORDS: Acute paraquat poisoning; Gastric lavage; Sodium dithionite test; Swine model
INTRODUCTION
Paraquat (1,1'-dimethyl-4,4'-bipyridiniu m dichloride, PQ) is widely used as an effective herbicide in most developing countries.[1,2]However, the intentional ingestion of PQ is a frequent method of suicide in China; more than 10,000 such cases occurred between 2002 and 2011.[3]The most common route of PQ poisoning is through the digestive tract (81.29%)[3]in which it is primarily absorbed in the human jejunum (5%-15%)[4], and the peak plasma concentration reaches 1-4 hours after PQ ingestion.[5-7]With no known antidote, the PQ poisoning results in a high mortality rate. Therefore, its treatment relies primarily on the removal of PQ from patients via extra-corporeal and intra-corporeal elimination,[8]and the main measures used to prevent the absorption of this toxic substance include emetics, gastric lavage (GL), adsorption, and catharsis, with GL being the most commonly recommended treatment modality for extracorporeal elimination.[8,9]According to current guidelines and expert recommendations,[10]an adequate GL method involves GL within 4-6 hours of poisoning, the use of clean water as the general GL fl uid using a volume of no less than 5 L, with the repetition of GL until the eluate is colorless and odorless. However, in clinical practice, controversies remain with regards to the individualized selection of the timing of lavage and the most suitable eluate volume.
As such, rapid and accurate detection of the PQ level within the gastric juice or eluate samples will allow for rapid assessment of routine clinical GL parameters based on the history of PQ ingestion and thus facilitate proper decision making regarding the GL treatment strategy, reduce GL-related complications and improve patient survival rate. In recent decades, a number of analytical methods have been reported for the identification and quantitation of PQ in biological samples.[11-14]The traditional sodium dithionite test, which is inexpensive and simple to perform, has been widely used as a semiquantitative method for PQ detection in blood and urine samples.[11,12]However, this method is not well suited for directly testing gastric juice samples due to the strong acidic matrix effects and relatively high PQ concentrations of the gastric juice.[15]Here, we develop an effective and rapid quantitative method that uses a UV spectrophotometer and a chromogenic reaction. We also improve the sample pre-processing, optimize the detection steps and eliminate limitations to enable the successful application of this method as a diagnostic technology for quantitative PQ detection in both gastric juice and eluate samples.
In the present study, using an animal poisoning model, we aimed to evaluate the efficiency of this method after certain time points and at different dosages (eluate volumes) of GL, and to observe the relationship between the GL and the physiological changes. We propose that the data from our animal experiments provide additional information about reasonable GL time points and appropriate eluate volumes and may have a certain value for clinical GL practices in patients with acute PQ poisoning.
METHODS
Animal procedure and sample preparation Experimental animals
Eighteen female (67.5±2.5)-day-old pigs weighing 28±3.5 kg were purchased from the Jiangsu Academy of Agricultural Sciences and successfully passed inspection and quarantine tests. The animals were fasted with water access for 24 hours before the experiment and were then administered with 60 mg/kg dose of PQ solution intragastrically. The animals were randomly divided into a no treatment group, a GL with 20 liters of warm water at 1 hour (H1) group and a GL at 6 hours (H6) group (n=6 in each group). The observations ended at 24 hours (H24) after PQ ingestion. The vital signs and laboratory testing were recorded at H0 and H24. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of University (Permit Number: 2016-SR-237).
Reagents and apparatus for the animal model
A PQ solution with no added agents (40%, w/w) was supplied by the RedSun Company (Nanjing, China). Propofol, midazolam and xylazine hydrochloride were used for animal anesthesia and sedation. The animals were intubated and ventilated with a model AVEA ventilator (VIASYS, Washington, USA), and vital signs, including arterial pressure, were monitored with an ECG monitor (PHILIPS, Amsterdam, Holland) connected to a femoral artery catheter probe. A disposable gastric tube (model F26; Ling Ze, Beijing, China) and an automatic gastrolavage machine (model SC-II; TongYe, Tianjin, China) were prepared for the gavage and GL. The blood routine test was performed by Sysmex-2100 (Sysmex Corporation, Kobe, Japan); the biochemistry and electrolyte analysis was operated by Beckman-AU5800 (Beckman Coulter, CA, USA).
Sample collection and preparation
For quality control, 5 pigs that did not undergo PQ gavage were used for the collection of control gastric juice and eluate samples before the PQ experiment. In this study, the gastric juice was collected at H1 and H6 before GL and at H24 for PQ concentration detection. The initial 2-4 mL of gastric juice from the gastric tube was discarded, and the subsequent 5 mL of gastric juice were collected into polypropylene tubes. For the eluate samples, each 10 L of eluate was collected and homogenized sufficiently. Five-milliliter fluid samples were extracted and stored at -80 °C until testing. Both the gastric juice and eluate samples required centrifugation (12,000 g) at room temperature for 10 minutes. Only the supernatants (1,800 μL) were transferred to the test tubes for further testing. We retained the blood samples at H1, H6 and H24 to determine their PQ concentration. They were centrifuged (3,000 rpm) at room temperature for 10 minutes. Finally, they were transferred to the test tubes and frozen at -80 °C for further testing.
Reagent preparation and apparatus for PQ detection
Rapid quantitative method
Paraquat dichloride hydrate, Na2S2O4and NaOH were purchased from Sigma-Aldrich (St. Louis, MO, USA). A 2 mg/mL paraquat stock quality control (QC) solution was prepared via the dissolution of PQ powder in mixed blank gastric juice samples or GL liquids obtained from control swine (pre-obtained, see M & M 1.3). Working QC solutions with three concentrations (50 μg/mL, 200 μg/mL and 500 μg/mL) were prepared by diluting the stock solution with the samples. All QC solutions were aliquoted into polypropylene tubes and stored at -20 °C. For the detection solution, NaOH (1.5 g) was dissolved in deionized water (20 mL), Na2S2O4(1.5 g) was added, and the liquid was mixed gently by hand. Na2S2O4is unstable, so the detection solution was prepared within 10 minutes of its use.[16]All spectrum measurements were performed using a model UV-6100 spectrophotometer (YuanXi, Shanghai, China) with matched quartz cells. The water used in the experiment was deionized and purified with a Milli-Q system (Bedford, MA, USA).
Mass spectrometry analysis
The blood concentration is the most important clinical index for evaluating the GL efficiency and must be measured precisely. Mass spectrometry is the traditional method for measuring plasma concentration. According to our previous research,[17]the quantification of PQ in the plasma was performed using ultra performance liquid chromatography tandem high resolution mass spectrometry (UPLC-ESIHRMS/MS) method. Essentially, PQ in the plasma sample was prepared by protein precipitation with acetonitrile (Merck, Darmstadt, Germany). The PQ was separated with a HILIC (Hydrophilic Interaction Liquid Chromatography) column (Waters Corp, MA, USA), followed by detection which was carried out using Q Exactive Orbitrap mass spectrometer (ThermoFisher, MA, USA) in Targeted-MS/MS scan mode.
Condition optimization and validation for rapid quantitative method
Maximum absorption wavelength selection
The working QC solutions (1,800 μL) and blank gastric juice/eluate (1,800 μL) mixed with the detection solution (200 μL) were scanned with a spectrophotometer over a wavelength range of 290 to 450 nm. The maximum absorption corresponded to the optimal wavelength.
Detection time optimization
The scan wavelength was selected based on the optimal wavelength. The samples mixed with the working QC solutions were then scanned and detected 3 times per minute over ten minutes and again at 15 and 30 minutes.
Calibration curve
The stock QC solution was used to generate a calibration curve. For each test, a series of 9 different concentrations was prepared via dilution of the stock QC solution. The final concentration gradient included the following concentrations: 1, 2, 5, 10, 20, 50, 100, 200, and 500 μg/mL. The limits of detection (LOD) and quantification (LOQ) were evaluated according to previously published methods.[18]
Precision and accuracy
The intra-day and inter-day precisions were calculated based on 5 repeated tests of the QC solutions. The data was expressed as the relative standard deviations (RSDs), and the results indicated that the method was reliable both within the same day and across different days. The accuracy (recovery test) was calculated by comparing the actual results with the theoretical values of the QC solutions and was required to be within 80%-120% according to reference.
Dilution recovery
A dilution recovery test was performed using gastric juice samples with PQ concentrations of 5,000 μg/mL to detect the stability of the dilution method and to keep the samples within the detection range. A recovery test was performed using three different dilution ratios (i.e., 1:10, 1:20 and 1:40).
Data analysis
The experimental data was analyzed with the SPSS17.0 software package (SPSS, Chicago, IL, USA). The measurement data was expressed as the mean ± the standard deviation. Group comparisons were performed using t-tests or ANOVA. P values below 0.05 were considered to be statistically signifi cant.
RESULTS
Optimization of PQ detection using rapid quantitative method
The reaction conditions of the test were optimized for the two types of samples, and Figure 1 presents the optimal conditions for the gastric juice samples as an example. The optimized conditions included the absorption wavelength, detection time, composition of the detection solution, and detectable ranges. The maximum absorption wavelength appeared at 395 nm, and the blank gastric juice produced no endogenous interference effect (Figure 1A). The optimal proportions of NaOH (Figure 1B) and Na2S2O4(Figure 1C) in the detection solution were 5%-15% and 7.5%, respectively. The most apposite and stable time range for the test was within ten minutes (Figure 1D). The calibration curves of the different samples revealed favorable and reproducible linear relationships (R2>0.99) within the same ranges from 1 μg/mL to 500 μg/mL.
Method validation with the gastric juice and eluate samples
Precision (RSD%) and recovery tests were performed using the gastric juice and two parts of the eluate samples, respectively. For all QC samples, the intra-day and inter-day precisions were both less than 6%, and the recovery rates of the PQ were all between 80%-120%. These parameters all met the standard criteria (Table 1). For the high-PQ concentration gastric juice samples, the recovery tests for the three different dilution ratios were performed, the LOD and LOQ were evaluated (data not shown), and all results met the standard criteria.
Application to gastric juice samples
The proposed method was applied to detect the PQ concentrations in the gastric juice samples. There were no significant differences in the PQ concentrations in the gastric juices of each group at H1 before GL and the PQ concentrations was the highest at this time point. After GL, the PQ concentration in the gastric juice was obviously decreased when compared to that in the untreated group at the same time point. Furthermore, we found that the PQ concentration in the gastric juice was sustained at a relatively high level until H24 in the untreated group. These results are illustrated in Figure 2A.
Application to eluate samples
At the different GL time points, each 10 L eluate sample was collected and homogenized. The PQ concentrations were then detected, and the washing efficiencies were calculated according to the following formula: PQ concentration × fluid volume / the total PQ amount × 100%. As presented in Table 2, the PQ concentrations of the fi rst 10 L eluates were signifi cantly higher than those of the second 10 L eluates in both the H1 (31.81±7.82 μg/mL vs. 7.25±4.04 μg/mL, P<0.001) and H6 GL groups (22.21±9.46 μg/mL vs. 6.58±4.13 μg/mL, P<0.01). Nevertheless, there was no significant difference in the washing efficiencies of the first (17.79%±4.56% vs. 12.42%±4.91%) and second (3.99%±2.22% vs. 3.35%±1.97%) 10 L eluates for each GL group (Figure 3).
Animal model and effect of GL
After the administration of PQ, the vital signs of the pigs were altered within 24 hours. These alterations included body temperature elevation, heart and respiratory rate acceleration, and a decrease in the mean arterial pressure. Without the GL treatment, each vital sign significantly deteriorated at H24 in comparison with the original state (H0; P<0.01). Both the GL groups exhibited trends toward improvement in vital signs at H24 in comparison with the untreated group, and the H1 GL group exhibited more effective improvements (3 of 4 indexes were recovered; only the body temperature was signifi cantly abnormal at H24) than the H6 GL group (2 of 4 parameters were recovered; the respiratory rate and blood pressure at H24 were not significantly different from the measurements collected at H0) (Table 3).
In the laboratory test, the total white blood cells and the percentage of neutrophils were highly elevated at H24 in both the untreated group and the H6 GL group except for the H1 GL group; the liver and the kidney functions (including AST, ALT, BUN, Cr) were also seriously impaired in the untreated group and the H6 GL group compared with the H1 GL group (Table 4).
The PQ plasma concentration in the H1 GL group remained at a relatively stable level during observation as compared to the other two groups. At H6, plasma concentrations in H1 GL group was reduced signifi cantly in comparison to H6 GL group (P=0.001) and no treatment group (P=0.001), and the plasma concentration in delayed GL or no GL were obviously increased compared to H1 time point (P<0.01, respectively). At H24, the plasma concentrations in all the groups were reduced in comparison to H6 (only in delayed GL and no GL group there was signifi cantly difference). Moreover, the plasma concentrations were significantly decreased in both the GL groups as compared to the untreated group. However, there was no signifi cant difference between these two GL groups (Figure 2B). Most importantly, the trend of gastric juice PQ concentration in each group at H6 and H24 was consistent with the PQ plasma concentration.
DISCUSSION
In this study, a rapid and cost-saving quantitative method that utilizes a sodium dithionite test with a spectrophotometer was developed and validated for the determination of the PQ concentrations in gastric juice and eluate samples from an acute PQ poisoning swine model. The plasma PQ concentrations were detected using mass spectrometry, and the vital sign as well as the laboratory results were recorded. Thus, the effi ciency and utility of GL for PQ poisoning was evaluated. We suggest that this detection method can be performed in patients as an individualized GL treatment; early GL at H1 after PQ ingestion is valuable and will help to improve the signs of poisoning in animals. Furthermore, a regular 20 L volume of eluate may be excessive since the wash effi ciency of the post-10-L volume was relatively low in comparison to that of the fi rst 10 L volume.

Figure 3. Comparison of the PQ clearance efficiencies between the fi rst 10 L GL and the second 10 L GL. *P<0.001 using ANOVA for the difference in the PQ+H1 GL between the fi rst 10 L GL and the second 10 L GL; **P<0.01 using ANOVA for the difference in the PQ+H6 GL between the fi rst 10 L GL and the second 10 L GL.
PQ has been marketed in more than 130 countries as a contact herbicide since 1962.[5,7]However, PQ is also well known as a highly toxic compound. The acute ingestion of 7-8 mL of PQ solution (20% concentration) without treatment can lead to death within a few days.[4,19]Consistent with the clinical manifestations,[20]a large animal lavage model involving doses of PQ above 100 mg/kg can lead to severe poisoning and rapidly affects vital signs within 24 hours.[21]In clinical practice, rapid and accurate detection of PQ levels in plasma and urine samples helps physicians to predict prognosis and choose treatment strategies.[22]Conventional approaches include semi-quantitative dithionite reduction tests[10,11,13]and quantitative methods, such as HPLC[12]and LC-MS/MS,[17]but these approaches are rarely applied to gastric juice samples due to their strongly acidic properties and high local concentrations. Here, we developed a quantitative method that utilizes the sodium dithionite reaction principle and spectrophotometry. We optimized the detection solution, increased the quality of NaOH, and passed the dilution recovery test. We found that the gastric juice and eluate samples with PQ could both be detected and thus meet the needs of this experimental design.
Although a GL position paper published by the American Academy of Clinical Toxicology and the European Association of Poison Centres and Clinical Toxicologists has already reached the conclusion that GL should not be routinely performed for gastrointestinal decontamination and suggested initiation within an hour of ingestion,[23]evidence also suggests that situations in which GL would benefit patients suffering from lethal ingestion of agents such as PQ exist.[24]Chinese expert consensus documents on acute PQ poisoning diagnoses and treatment recommend that GL should be performed within 4-6 hours of PQ ingestion.[9]There is very little experimental information on the use of gastric lavage solely for the treatment of paraquat poisoning. Previous studies were only based on smaller sized animal models such as rats,[25]cats[26]and dogs.[27]Here we used swine model that was more similar to the human body in terms of body weight and stomach capacity for simulating a GL therapy for more valuable data. Because the peak PQ plasma concentration reached 1-4 hours after poisoning, we designed two time points for observing the efficiency of GL (i.e., 1 hour before the peak and 6 hours after the peak). We found that although the gastric concentration was still high at 6 hours post PQ ingestion, theoretically there was still a need for GL, however there was no improvement in physiological indicators in this delayed GL group. The reason may be associated with the high plasma PQ concentration and the relevant target organ injury at 6 hours if no early GL performed. We recommend that it is necessary to combine the toxicokinetic characteristic and the toxicological effect of the poison itself, with the toxic concentration of gastric contents, which may provide a better practice for GL method. In PQ poisoning, only GL at H1 gave concrete evidence about the benefits of GL in comparison to no treatment and delayed GL group. This suggests that in clinical practice, intra-corporeal elimination such as blood purification treatment[28]may be more crucial for those PQ poisoned patients whose time of ingestion exceeds one hour.

Table 1. Precision and accuracy of QC solutions

Table 2. Comparisons of PQ concentration in GL eluate between each group (μg/mL)
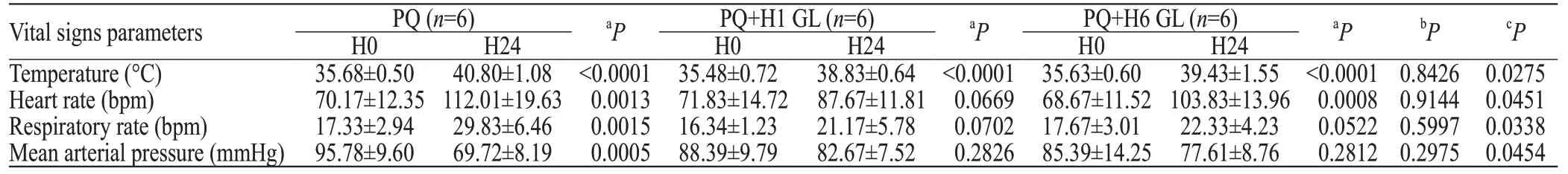
Table 3. Vital signs parameters between each group

Table 4. Laboratory examination at H24 between each group
Currently, the recommended GL fluid volume is not less than 5 L. However, in clinical practice, the commonly used GL fl uid volume is approximately 20 L, which is considered to be suffi cient to achieve colorless and odorless eluates. We designed a study to observe the differences in the PQ concentrations in the first 10 L of eluate and the second 10- L of eluate during GL. We found that the PQ concentration in the first 10 L of eluate in the delayed GL group was slightly lower than that in the early GL group, but this difference was not signifi cant. More importantly, regardless of whether early or late GL was applied, the PQ concentration and the wash effi ciency of the fi rst 10 L eluate were signifi cantly higher than those of the second 10 L eluate. These results strongly suggest that 10 L of GL fluid is sufficient to meet the needs of GL for PQ poisoning. Abandoning the second half of the GL process would not only save time for more important elimination therapies (such as hemoperfusion[28]), but also reduce the complications associated with excessive GL as we observed in this study and in others reports, such as electrolyte imbalances,[29]stomach bleeding,[24]oesophageal perforation[30], etc.
Limitations
This study has some limitations. First, large animal experiments cannot completely reproduce the processes involved in human oral ingestion due to physiological differences. Second, the animals were anesthetized during the whole experiment, which may have affected gastrointestinal peristalsis and prolonged the gastric emptying time. Third, since the post-10-L gastric lavage effi ciency was signifi cantly reduced, further observations are needed to reduce the gastric lavage dose and to evaluate the effect of different gastric lavage dose on the prognosis of poisoned animals.
CONCLUSIONS
Our rapid quantitative method provides evidence of situations in which early GL benefits PQ poisoning in an animal model. The currently used 20 L GL volume is excessive and may need reduced. The rapid quantitative method used in this study can be used for sample analysis of patients' gastric juice and has a certain value for clinical GL practices.ACKNOWLEDGEMENTS
This paper is proofread by Chen-Jiang Wu, a staff of Department of Radiology, the First Affiliated Hospital with Nanjing Medical University.
Funding:This work was supported in part by Natural Science Found of Jiangsu Province (BK20171500, 16KJB320003), Program for Key disease of Jiangsu Province Science and Technology Department (BL2014088), Program for Innovative Medical Research Team of Jiangsu Province (CXTDA2017007), Jiangsu Province's key provincial talents program (QNRC2016597).
Ethical approval:All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of University (Permit Number: 2016-SR-237).
Conf icts of interest:Dr. Sun report an issued Chinese patent (ZL 201721303215.6) for the First Affiliated Hospital with Nanjing Medical University related to this manuscript.
Contributors:YFJ and JK shared co-first authorship on this work. JSZ and HS conceived the study, brought together the collaboration, and drafted the manuscript. PPH conceived the paraquat poisoning studies and directed the research staff for this effort. YFJ developed the assay, performed the animal study, helped with manuscript drafting and finalization. JW performed the statistical analysis. LJ, LQ and BLZ helped to perform the animal study. JXY and ZHW helped to develop and optimize the rapid quantitative method. JK oversaw the assay development and contributed to troubleshooting the assay. All authors have reviewed, contributed to writing the manuscript and approved the fi nal product.
 World journal of emergency medicine2020年3期
World journal of emergency medicine2020年3期
- World journal of emergency medicine的其它文章
- Availability of basic life support courses for the general populations in India, Nigeria and the United Kingdom: An internet-based analysis
- The importance of visualization of appendix on abdominal ultrasound for the diagnosis of appendicitis in children: A quality assessment review
- Clinical characteristics and prognosis of communityacquired pneumonia in autoimmune diseaseinduced immunocompromised host: A retrospective observational study
- The life-saving emergency thoracic endovascular aorta repair management on suspected aortoesophageal foreign body injury
- Effects of intracoronary injection of nicorandil and tirof ban on myocardial perfusion and short-term prognosis in elderly patients with acute ST-segment elevation myocardial infarction after emergency PCI
- Morbidity and mortality risk factors in emergency department patients with Acinetobacter baumannii bacteremia
