Hydration Mechanism and Stability Regulation of Hemihydrate Calcium Sulfate Whiskers
GAO Chuan-HuiDONG Ya-JieREN XiuCHEN Ying WANG Hui-ZiHU Zun-FuWANG JingLIU Yue-Tao*,
(1State Key Laboratory Base for Eco-Chemical Engineering in College of Chemical Engineering,Qingdao University of Science and Technology,Qingdao,Shandong 266042,China)
(2College of Materials Science and Engineering,Linyi University,Linyi,Shandong 276000,China)
Abstract:Hydration mechanism of hemihydrate calcium sulfate whisker(HCSW)was studied by discussing the change of morphology and crystal form in the storage process in the air,and the regulation of stability by different treatment methods was analyzed.It is found that the hydration of HCSW was caused by-OH group,Ca2+active site at the surface and the internal channel inside of the whisker.Calcination and diol modification could improve the water resistance of calcium sulfate whisker.The experimental results indicate that after calcination,the hemihydrate calcium sulfate whisker are transformed into anhydrous soluble calcium sulfate whiskers and anhydrous dead calcium sulfate whiskers,the internal channels easy to hydration disappear,and the water resistance is enhanced.In the hydrothermal synthesis process,the addition of binary alcohol was beneficial to the adsorption of alcohol hydroxyl on the (200),(020)and (220)surfaces of HCSW,preventing the adsorption of hydroxyl in H2O molecules on the whisker surface,and further improving the water resistance of the whisker.When the additive was triethylene glycol(TEG)and the concentration was 18.8 mmol·L-1,the water resistance stability of HSCW in the air was not less than 7 days.
Keywords:hemihydrate calcium sulfate whisker;hydration;calcination;triethylene glycol
0 Introduction
In recentdecades,calcium sulfate whiskers(CSW,CaSO4)has been found to be one of the most promising reinforcing fillers in many inorganic fillers because of their unique properties such as excellent mechanical properties,chemical and thermal stability,high toughness and low cost[1-4].In general,there are three types of CSW:dehydration,hemihydrate and dihydrate[5].These three types of whiskers can be converted to each other under certain conditions.Compared with the dihydrate whisker and dehydration whisker,hemihydrate calcium sulfate whisker(HCSW)is a new type of needle-like fiber sub-nanomaterial grown in the form of single crystal,which has a uniform cross-section,a complete profile and a perfect internal structure[6-8].HCSW is a typical high strength,low cost,energy-saving,non-toxic and enviroμmentally friendly material widely used as the reinforcing agent in many fields,such as rubbers,plastics and adhesives[9-13].There is direct relationship between structure,morphology and size of HCSW with its properties and functionalities.The mechanical properties of 1D materials as HCSW are usually improved with an increase in the aspect ratios[14].However,we found that HCSW wasextremely sensitive to water in the air,and hydration was carried out at room temperature.After hydration,the length of whiskers was smaller,the diameter became larger,and the aspect ratio was significantly reduced,which seriously affected their performance[15].Therefore,it is urgentto study the hydration mechanism and stabilization method of HCSW.
In order to control the structure and morphology of HCSW,several chemical agents have been added to control the crystallization[16-17].Recently,the salt of the chloride is well developed,it could be MgCl2,KCl,CaCl2,NaCl,HCl and so on[18-19].For instance,Wang et al.[20]investigated the effects of CuCl2on crystal morphology, phase structure, aspect ratio and crystallization of hydrothermal products prepared via hydrothermal crystallization in H2SO4-H2O solutions.The results showed that CuCl2could influence the morphology,aspect ratio and crystallization of HCSW,but cannot affect their phase transformation.In order to eliminate the potential danger to the environment of Cl-,Jiang et al.[21]developed a newly nitrate system which provided an improved alternative for HCSW preparation with controlled process and product quality,and helped to push forward the industrial recycling of FGD gypsum.Furthermore,metal ions and organics have been reported to have effects on crystallization and aspect ratio of the hydrothermal products[3,22-24].The metal ions that influenced the morphology and aspect ratio of the whiskers included Na+,K+,Mg2+,Cu2+,Al3+,Fe3+and so on.And the organic additives including ethylene glycol were also proved to have relationship with morphology of the whisker.For instance,by simply tuning the volume ratio of ethylene glycol to water(G/W)in Na2EDTA-containing ethylene glycol aqueous solutions,the structure and morphology of hemihydrate calcium sulfate whiskers could be controlled[25].In addition,we have previously found that the adsorption of ethylene glycol could promote the 1D growth of HH whiskers,leading to the formation of whiskers with high aspect ratios[26].
In summary,although there were many reports on the prevention of hydration research by controlling the HCSW crystal form,almost all of the studies were centered on how to control the morphology of HCSW in crystal forming process,and the research on the hydration mechanism of HCSW instability was not reported.Research on the hydration mechanism and stability of HCSW was expected to provide an effective theoretical basis for the application of whiskers.
The purposes of this study was to verify the hydration mechanism ofHCSW to achieve its stabilization.Especially,the Diamond software was used to simulate the crystal structure to help us clearly understand the hydration process of HCSW.In addition,this experiment revealed the effects of crystal modifier on the structure,morphology and size of HCSW.In this context,triethylene glycol(TEG)was used as the crystal modifier to promote the onedimensional growth of HCSW and improve their crystallization.The effects of TEG on the morphology,phase structure,aspect ratio and crystallization of the hydrothermal products were investigated.
1 Experimental
1.1 Materials
Analytical grade CaSO4·2H2O was purchased from Sinopharm Chemical Reagent Co.Ltd.,Shanghai,China.The glycol-water was prepared by deionized water and ethylene glycol,diethylene glycol,triethylene glycol or polyethylene glycol 400 (reagent grade,Sinopharm Chemical Reagent Co.Ltd.,Shanghai,China).
1.2 Methods
The procedure was as follow:(1)Analytical grade CaSO4·2H2O was mixed with deionized water at room temperature to get the suspensions containing 1.0%~5.0%(w/w)calcium sulfate dihydrate.The slurries were then treated under hydrothermal condition(140℃,5.0 h),filtrated,and dried at 105,300 and 600℃for 3 h to obtain three kinds of whiskers.(2)Analytical grade CaSO4·2H2O was mixed with deionized water and minor amount of ethylene glycol,diethylene glycol or polyethylene glycol 400 with a purity of 98.0%at room temperature to get the suspensions containing 1.0%~5.0%(w/w)calcium sulfate dihydrate and 18.8 mmol·L-1glycol-water solution.The slurries were then treated under hydrothermal condition (140℃,5.0 h),filtrated,and dried at 90℃for 6 h.
1.3 Characterization
Scanning electron microscopy(SEM,JEOL JSM-6,Japan)was used to observe the morphology of HCSW and the test voltage was 12 kV,the magnification was 50~10000.The former was previously sputter coated with gold before observation and analysis and the latter was dispersed in alcohol via ultrasonic disperser and placed on a copper grid by a dropper before detected.Energy-dispersive spectrometry device(EDS,TEAM EDAX Co.,USA)was used to analysis the qualitative and quantitative of elements in microregion.The structures of the samples were identified by powder X-ray diffractometer(XRD,D-MAX 2500/PC,Rigaku,Japan)using Cu Kα radiation (λ=0.154 178 nm)with a scanning rate of 4°·min-1,a scanning 2θ range of 5°to 80°,a tube voltage of 40 kV and a tube current of 50 mA.Fourier transform infrared spectrometry(FT-IR)(Thermo-Nicolet Avatar-360)was conducted to characterize HCSW in a wavenumber range from 4 000 to 400 cm-1with a resolution of 4 cm-1using an transmission infrared method.
2 Results and discussion
2.1 Surface property and structure of HCSW
The HCSW prepared by hydrothermal synthesis were dried and then exposed to the air.The ambient temperature was controlled at 25℃and the relative humidity of air was 40%.Fig.1 showed the morphological features observed after the HCSW had been exposed for different times in the air.The products mainly consisted of uniform fiber-like whiskers with the diameter ranging from 2 to 5 μm,as shown in Fig.1(a).As shown in Fig.1(b~d),the surface properties and morphology of HCSW were directly related to the duration of whiskers when it was exposed in the air.The appearance of the whiskers was still fibrous when exposed to the air for 12 h,and cracks were only present on the surface of small part of whiskers.The diameter of whiskers was further increased when the whiskers were exposed to the air for 24 h,and the aspect ratio was further reduced,while the whiskers were still keeping fibrous in the air.When exposed to the air for 48 h,there are few flake and granular products on the surface of whisker surface.Saha et al.[27]have shown that calcium sulfate can produce amorphous particles through hydration, cracks appeared on the surface of the other whiskers,and even the fracture occurred.Nearly half of the whiskers were hydrated into a plate-like products after they were exposed to the air for 7 days,while a small number of products had jagged crystal defects on the surfaces of products,with a small part of products having an aspect ratio of only 1~3.Thus the hemihydrate calcium sulfate whiskers had a clear hydration phenomenon in the air,and the longer the duration exposing to the air,the shorter the whiskers,the smallerthe aspectratio,and the more chaotic morphology uneven.

Fig.1 SEM images of HCSW hydration process in the air

Fig.2 Characterization of three crystal forms of calcium sulfate whisker:(a)XRD patterns;(b)Standard PDF for XRD;(c)FT-IR spectra
In order to know the hydration reasons of HCSW,the HCSW were calcined at 105,300 and 600℃for 3 h,respectively,which were marked as CaSO4-Ⅰ,CaSO4-Ⅱ and CaSO4-Ⅲ.Their surface properties and crystal structure were studied,respectively.Fig.2(a)was the XRD patterns of calcium sulfate whiskers under three calcination temperatures.It was not difficult to find that the major diffraction peaks of calcium sulphate whiskers at three calcination temperatures were compatible with the diffraction peaks of hemihydrate calcium sulfate whiskers(CaSO4·0.5H2O,or HCSW),anhydrous soluble and anhydrous dead calcium sulfate whiskers in the PDF(No.83-0439,CaSO4-Ⅰ ;No.26-0329,CaSO4-Ⅱ ;No.72-0503,CaSO4-Ⅲ)standard card shown in Fig.2(b).From Fig.2(a),it can be seen that the characteristic peaks of anhydrous soluble calcium sulfate whiskers disappeared at 29.80°and 14.80°with the increase of calcination temperature.While those of anhydrous calcined calcium sulfate whiskers appeared at 25.42°and 52.28°.Moreover,the atomic accumulation in the lattices of calcined calcium sulfate whiskers,anhydrous soluble calcium sulfate whiskers and anhydrous calcined calcium sulfate whiskers is different at different temperatures, and the characteristic diffraction peaks and surface spacing are different in XRD patterns.According to the Bragg diffraction equation[28],the crystal structure becomes dense with the increase of calcination temperature.This indicate that the whiskers obtained at calcination temperature of 105,300 and 600℃were semi-water,anhydrous soluble and anhydrous dead calcium sulfate whiskers,respectively.As shown in Fig.3,the calcined whiskers were fibrous,and the morphology of anhydrous soluble calcium sulfate whiskers still kept uniform and thin,while the length of anhydrous dead calcium sulfate whiskers became smaller.
In order to verify the chemical composition of the surface of three products,EDS energy spectra was used to analyze the elemental atomic fraction.The results of EDS were shown in Table 1.According to Table 1,the relative contents of Ca element and S element in CaSO4-Ⅰwere 15.36%and 15.28%,respectively,which were both less than their theoretical value (15.38%).While the relative content was 69.36%for O element,which was more than the theoretical value (69.24%).As same as in CaSO4-Ⅰ,the relative contents of Ca element and S element in CaSO4-Ⅱ and CaSO4-Ⅲ were also less than the theoretical value (16.67%)and the relative content of O was more than the theoretical value(66.66%).This might be due to the adsorption of H2O or CO2in the air by calcium sulfate whiskers.However,the elemental content in CaSO4-Ⅲwas basically consistent with the theoretical values,meaning that CaSO4-Ⅲis difficult to hydration.Therefore,it could be concluded that the crystal shape had a great influence on the stability of calcium sulfate whiskers.CaSO4-Ⅰand CaSO4-Ⅱwere easily hydrated in the air,and CaSO4-Ⅲwas not easy to hydrate,which could be stable in the air.

Table 1 EDS analysis of three crystal forms of calcium sulfate whiskers
In combination with related literature[28],the binding energy of CaSO4-Ⅰ,CaSO4-Ⅱ and CaSO4-Ⅲin XPS was 347.5,347.45 and 347.85 eV,respectively.Compared with the Ca2p3/2binding energy(348 eV)of CaSO4,the binding energies of CaSO4-Ⅰ ,CaSO4-Ⅱand CaSO4-Ⅲall shifted to lower energy,with offset of 0.50,0.55 and 0.15 eV,respectively,which indicates that Ca2+might be reacted with moisture.The binding energy of CaSO4-Ⅱwas closest to the Ca2p3/2binding energy of Ca(OH)2(346.7 eV),which shows that the Ca2+on the surface of CaSO4-Ⅱwas easiest to take place hydroxylation reaction.

Fig.3 SEM images of three crystal forms of calcium sulfate whiskers
The FT-IR spectra obtained for CaSO4-Ⅰ,CaSO4-Ⅱand CaSO4-Ⅲwere shown in Fig.2(c).The calcium sulfate whiskers displayed characteristic bands at 3 618,3 560 and 3 440 cm-1(-OH),1 620 cm-1(Ca2+),599,657 and 1 152 cm-1(SO42-).The results show that-OH,SO42-and Ca2+are the main groups in surface of calcium sulfate whiskers.The absorption peaks(3 618 and 3 560 cm-1)did not exist in the spectrum of CaSO4-Ⅲ,which means that the Ca2+ions on the surface of CaSO4-Ⅲ don′t hydrate with the water molecules in the air,and the water molecules cannot enter the internal channels inside of the whiskers.Compared with the strength of-OH vibration peaks of calcium sulfate whisker, the strength of -OH absorption band produced by the whisker and water molecules is the strongest in hemihydrate calcium sulfate whisker,followed by anhydrous soluble calcium sulfate whisker,and anhydrous dead burned calcium sulfate whisker is the weakest.This order shows that the hemihydrate calcium sulfate whisker is the most likely to react with water molecules in the air,while the anhydrous dead burned calcium sulfate whisker is the most difficult to react with water molecules in the air.

Fig.4 Cell structure(a)and hydration diagram(b)of HCSW

Fig.5 Crystal structures of CaSO4-Ⅱ (a),CaSO4-Ⅲ (b),CaSO4-Ⅰ (c)and CaSO4·2H2O(d)
In order to further study the hydration mechanism of HCSW,the cell structure diagram of HCSW,the hydration schematic of HCSW and the crystal structure simulation diagram of CaSO4are shown in Fig.4(a),Fig.4(b)and Fig.5,respectively.The cell structure diagram of HCSW and the hydration diagram of the synthesized HCSW shows that there are many thermodynamically unstable active sites(Ca2+3.5×10-4nm-2)on the whisker surface distribution in Fig.4(a)and Fig.4(b).When the whiskers are placed in water or exposed to the air containing water molecules,the water molecules will combine with the active sites on the surface of the whiskers.After that,the water molecules reach the whisker surface and pass through the internal pores of the whiskers.The hydration occurs inside the whisker.Meanwhile,the water molecules continuously enter the whiskers interior,and the hemihydrate calcium sulfateis converted into the dihydrate calcium sulfate.The system is in a metastable state,which makes the crystallattice defective calcium sulfate whisker dissolve or even break,and Ca2+and SO42-on the dihydrate calcium sulfate whiskers surface diffuse and precipitate continuously,and the whiskers become shorter and thicker until the hydration is complete[28-29].
The crystal structures of CaSO4-Ⅱ ,CaSO4-Ⅲ ,CaSO4-Ⅰ (CaSO4·0.5H2O)and CaSO4·2H2O were simulated by Diamond software.The models are shown in Fig.5 and the lattice parameters of four crystal forms of calcium sulfate whiskers are shown in Table 2.As shown in Fig.5a and b,the common character of CaSO4-Ⅱ and CaSO4-Ⅲ is a structural unit which consists of one Ca2+attached to six adjacent SO42-.Ca2+and SO42-are linked to each other to form layered structures on (100)and (010)planes,while Ca2+and SO42-are linked into a chain instead of layered structures on(001)plane.The difference between CaSO4-Ⅱand CaSO4-Ⅲis that there is a large circular tunnel with a large internal surface in the perpendicular to the caxis direction on the crystal structure of CaSO4-Ⅱ.So,in the presence of water,CaSO4-Ⅱcan be converted to CaSO4-Ⅰ,because the water can be absorbed inside of it by the channel gravity.While CaSO4-Ⅲhas no circular tunnel,hence the crystal structures of CaSO4-Ⅲis more solid,less prone to hydration formation of CaSO4-Ⅰ.As shown in Fig.5c,Ca2+in CaSO4-Ⅰ is connected to six SO42-tetrahedra surrounding,and the H2O molecule is linked to SO42-by hydrogen bonding.The Ca2+and SO42-tetrahedron in the c-axis direction alternately become chain-like to form channels along the c-axis,in which crystal water loosely distributes.As shown in Fig.5d,apart from four SO42-tetrahedron,the Ca2+in CaSO4·2H2O is connected to two H2O molecules.The Ca2+with four SO42-tetrahedron forms a two-layer structure layer,which parallels to(001)direction.And the H2O molecules distribute between the two-layer structure layers.
Through the above analysis,the reasons of the hydration of HCSW is that the HCSW has inner channels and its surface has-OH,SO42-groups and active Ca2+.The active Ca2+can react with the water through a hydrocarbylation mechanism,the -OH groups on the surface can also bind to the water molecules through the hydrogen bond,and the internal pore of the whisker also attracts the water molecules through capillary attraction.All of these factors come together to help the water molecules (dwater=0.400 0 nm)to reach the HCSW crystal surface and then move into its inner channels until the hydration process completed.Furthermore,the hydration process of CaSO4-Ⅱ was the same with CaSO4-Ⅰ (CaSO4·0.5H2O,or HCSW),except for it had more active Ca2+.
Based on the above reasons,when the whisker is exposed to the air,it is easy to hydrate,making the whisker shorter and thicker,and losing its mechanical properties.By changing the crystalstructure to eliminate the internalchannels,the hemihydrate calcium sulfate whisker is transformed into anhydrous soluble and anhydrous dead calcium sulfate whiskers,which not only maintain the fibrous mechanical properties of hemihydrate calcium sulfate whisker,but also enhance the water resistance.However,in order not to change the structure of whisker,the hydrationcapacity of HCSW can also be reduced by adding additives such as diol and-OH on the whisker surface.

Table 2 Lattice parameters of four crystal forms of calcium sulfate whiskers
2.2 Effect of diols on the stability of HCSW
To investigate the effect of diol additives on the stability of HCSW,ethylene glycol,diethylene glycol,triethylene glycol and polyethylene glycol 400 with the same concentrations (3.76 mmol·L-1)were added to prepare the HCSW.Fig.6 shows the morphology of the HCSW prepared with different kinds of diols after being exposed 7 days in the air.It can be seen in Fig.6a that the whiskers are almost completely broken,which indicating that the whiskers prepared with no additive have a serious hydration phenomenon in the air.When ethylene glycol was selected as an additive(Fig.6b),some of the whiskers were fractured and others were fibrous.As shown in Fig.6c,when the additive was diethylene glycol,the whiskers were more complete than those in Fig.6b,while the length of whiskers is still not too long.This indicates that the whiskers still have hydration phenomenon.Nevertheless,when the triethylene glycol was added(Fig.6d),the whiskers were relatively intact and the surface became smooth because of the adsorption of triethylene glycol.It could be seen that the diol can significantly improve the long diameterratio of HCSW,and with the growth of molecular chains of diols,more elongated whiskers appear.But when polyethylene glycol 400 was used as an additive in Fig.6e,the whiskers were not better but a little worse than those added triethylene glycol,which may be the result of the mutual repulsion between the polyethylene glycol 400 carbon chain molecules.
The IR spectroscopy analysis is widely regarded as an effective technique for estimating the modification effect and determining the absorption behavior of additives.Fig.7(a)shows the spectra of HCSW formed in deionized water and glycol-water solution.The FTIR spectrum of blank sample(curve of no additive)showed the characteristic bands of HCSW at 1 620 cm-1(ν3(Ca2+)),1 152 cm-1(ν3(SO42-)),660 cm-1(ν3(SO42-))and 3 560~3 660 cm-1(crystal water O-H)[30-31],and the absorption bands of ν3(SO42-)in curve of ethylene glycol,diethylene glycol,triethylene glycol and polyethylene glycol 400 appeared at 1 149 and 657 cm-1,which exhibited small red shift in comparison with the blank samples.These changes suggested thatethylene glycol,diethylene glycol,triethylene glycol and polyethylene glycol 400 interact strongly with the SO42-group,and probably form hydrogen bonds.The stretching vibration absorption peaks at 1 450 and 3 400 cm-1are the stretching vibration absorption peaks of alcohols(-OH)in glycol molecules.The new absorption peak of methylene(-CH2)in curves of ethylene glycol,diethylene glycol,triethylene glycol and polyethylene glycol 400 appeared at 2 900 cm-1,which became sharpest in curve of triethylene glycol.There were three absorption peaks appeared at 1 450,3 400 and 2 900 cm-1in curves of ethylene glycol,diethylene glycol,triethylene glycol and polyethylene glycol 400 compared with curve of no additive,which indicates that the alcoholis adsorbed on the surface of HCSW.

Fig.6 Morphology of HCSW prepared with diols as additive after 7 days in the air:(a)no additive;(b)ethylene glycol;(c)diethylene glycol;(d)triethylene glycol;(e)polyethylene glycol 400
Fig.7(b)shows the XRD patterns of HCSW after being exposed 7 days in the air with no additive,ethylene glycol,diethylene glycol,triethylene glycol and polyethylene glycol 400, respectively.Interestingly, the additives of ethylene glycol,diethylene glycol,triethylene glycol and polyethylene glycol 400 did not lead to any changes in crystal morphology of the CaSO4·0.5H2O to CaSO4·2H2O after being exposed 7 days in the air.However,with no additive,the disappearance of fine needle shaped crystals could be seen in Fig.5a indicating partial phase transformation of CaSO4·0.5H2O to CaSO4·2H2O,which was also detected by XRD(Fig.7b).

Fig.7 FT-IR spectra(a)and XRD patterns(b)of HCSW formed in deionized water(no additive)and glycol-water solution
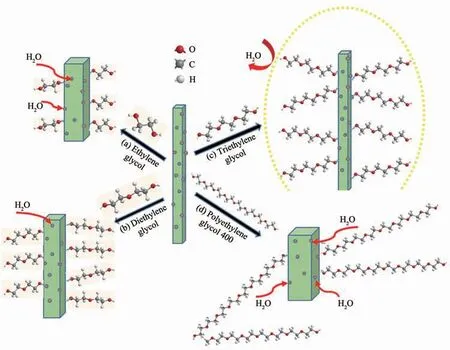
Fig.8 Schematic of preparation of water-resistant HCSW
The schematic diagram of preparation of waterresistant HCSW using glycols as additive is shown in Fig.8.In order to improve the water resistance of whisker,the hydroxyl group on the glycols is adsorbed on the surface of HCSW,thereby preventing the adsorption of the hydroxyl group of water molecule.When the additive is ethylene glycol or diethylene glycol,the water molecule can still“pass through” the carbon chain of glycol to bind the active site on the surface of whisker owing to that the carbon chain length is too short.Therefore,the prepared whiskers have poor water resistance.When the additive is triethylene glycol,it will forms a “shield” on the surface of the whisker,which hinders the direct contact between the water molecules and the whisker′s surface,so the obtained whisker has better water resistance.Additionally,if the additive is polyethylene glycol 400,its carbon chain length is too long.The adsorption capacity between the non-polar group and the whisker is reduced due to the repulsion between the molecules,so the active site on the surface of whisker is exposed in the middle of water molecule.In a word,the too long carbon chain length results in a decrease in the water resistance of resulting whiskers when polyethylene glycol 400 is used as an additive.
In summary,when triethylene glycol is used as an additive,HCSW morphology can remain the most complete and uniform.However,the amount of triethylene glycol as additive also has an effect on the stability of HCSW,so the effect the concentration of triethylene glycol to the HCSW has been investigated.
2.3 Effect of triethylene glycol on stability of HCSW
The influence of triethylene glycol on morphology of HCSW formed at 140℃is shown in Fig.9.When the triethylene glycol concentration was 3.76 mmol·L-1,the HCSW with an average width of 1.95 μm and an average aspect ratio of about 27.1 was synthesized(Fig.9a).When the triethylene glycol concentration increased from 3.76 mmol·L-1to 18.8 and 22.56 mmol·L-1,the average diameter first decreased from 1.95 to 0.82 μm and then increased to 2.16 μm as shown in Fig.9.However,when the glycol concentration was 7.52,11.28 and 15.04 mmol·L-1,the diameter and length of HCSW were not uniform.The low or high concentration of triethylene glycol did not favor the preservation of morphology of HCSW.When the concentration was too low,the triethylene glycol molecules have little contribution to the stabilization of HCSW.However,when the concentration of triethylene glycolwas too high,the molecular polymerization of triethylene glycol appeared at 140℃,resulting in the decrease of corresponding surface adsorption barrier,which does not have a greater role on the stabilization of HCSW.The results explain that the concentration of triethylene glycol has a significant effect on the crystal morphology of HCSW.With the increase of triethylene glycol concentration,the average width of HCSW decreases first and then increases,while the average aspect ratio of HCSW increases first and then decreases.When the concentration of triethylene glycol is 18.8 mmol·L-1,the average aspect of HCSW is the smallest and the average aspect ratio is the largest,which is the optimal concentration.

Fig.9 Influence of concertration of triethylene glycol on morphology of HCSW
Fig.10 shows the effects of triethylene glycol on the peak shifts in the XRD patterns of HCSW,and the relevant data are listed in Table 3.All of the XRD peaks,regardless of the concentration of triethylene glycol,are attributed to the sole existence of HCSW.Most of the occurred planes as(200),(020)and(220)are parallel to the c axis,reconfirming the preferential growth of HCSW along c axis.Fig.10 showed that the diffraction angles of(200),(020)and (220)planes were slightly shifted to the right when the concentration of triethylene glycol increased from 0 to 18.8 mmol·L-1.These are due to the adsorption of triethylene glycol on the(200),(020)and(220)crystal faces,which would decrease the surface free energy of corresponding crystal faces,thereby suppressing the radial growth of HCSW as well as promoting the growth along the c axis direction.When the concen-tration of triethylene glycol continued increasing,the diffraction angles of three crystal planes were smaller than the diffraction angles of HCSW with 18.8 mmol·L-1triethylene glycol, which indicates that the adsorption of triethylene glycol is weakened.Furthermore,MDI Jade X-ray diffraction analysis software could match the XRD data with the standard card of HCSW.FOM value is the reciprocal of matching rate,and the smaller the value,the higher the matching rate.Fig.11 shows the influences of triethylene glycol on the FOM of HCSW.The results explain that when the concentration of glycol was 18.8 mmol·L-1,the image had the lowest point,indicating that the matching rate is the largest.

Fig.10 Influence of concertration of triethylene glycol on XRD patterns of HCSW

Table 3 Effects of triethylene glycol on peak shifts of XRD patterns of HCSW

Fig.11 Effects of triethylene glycol on FOM of HCSW
The interaction energy (ΔE)represents the stability of molecule adsorbed on the crystal plane.The interaction energy is generally negative,and the larger the absolute value is,the higher the stability of the system is.The adsorption energy of triethylene glycol on the main crystal plane((200),(220)and(400))of HCSW was calculated by Materials Studio 7.0.It is found in Table 4 that the order of absolute adsorption energy is as follows: (400)>(200)>(220),which indicates that the interaction energy between triethylene glycol and (400)plane is the largest,and the triethylene glycol molecule is easier to adsorb on(400)surface.
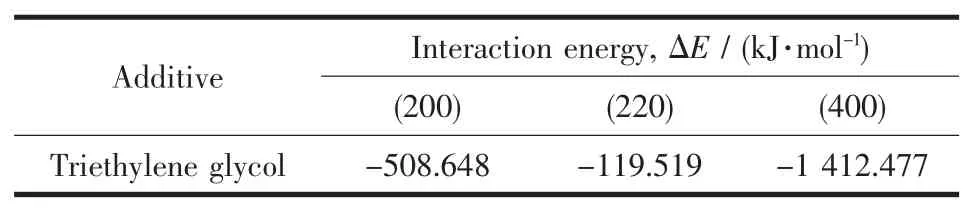
Table 4 Interaction energy of triethylene glycol on different faces of HCSW
The equilibrium adsorption configuration of triethylene glycol on HCSW(400)surface is shown in Fig.12.The hydroxyl alcohols on triethylene glycol combine with SO42+on the surface of HCSW to form hydrogen binding,resultingin the adsorption of triethylene glycol on the surface of HCSW[26].The carbon chain on the surface of HCSW increases its water resistance and reduces the surface free energy of corresponding crystal plane,which inhibits the radial growth of HCSW and promotes growth along the c-axis.

Fig.12 Equilibrium absorption configurations of triethylene glycol on the(400)face of HCSW
3 Conclusions
The presentstudy described the hydration mechanism of HCSW.HCSW has inner channels which could attract the water molecules through capillary attraction,and its surfaces have-OH,SO42-groups which could bind to the water molecules through the hydrogen bond and has active Ca2+that could react with the water through a hydroxylation mechanism.Thus,the key of the stabilization of calcium sulfate whiskers lies in covering their surface active site and eliminating their inner channels.To achieve the stabilization of HCSW,calcination was used to change the crystal structure to eliminate the internal channels.And diols could be used as additives to combine with the-OH of whiskers surface to reduce the hydration capacity.The presence of 18.8 mmol·L-1triethylene glycolleads to prepare hemihydrate calcium sulfate whiskers with high stability.Further increased concentration of triethylene glycolor increased molecular weight of the diol would lower the aspect ratio.

