Microbiota-gut-brain axis and its affect inflammatory bowel disease:Pathophysiological concepts and insights for clinicians
Emanuele Sinagra,Erika Utzeri,Gaetano Cristian Morreale,Carlo Fabbri,Fabio Pace,Andrea Anderloni
Emanuele Sinagra,Gastroenterology and Endoscopy Unit,Fondazione Istituto Giuseppe Giglio,Contrada Pietra Pollastra Pisciotto,Cefalù 90015,Italy
Emanuele Sinagra,Euro-Mediterranean Institute of Science and Technology,Palermo 90100,Italy
Erika Utzeri,Nuova Casa di Cura di Decimomannu,Cagliari 09100,Italy
Gaetano Cristian Morreale,Section of Gastroenterology,S.Elia-Raimondi Hospital,Caltanissetta 93100,Italy
Carlo Fabbri,Gastroenterology and Digestive Endoscopy Unit,Forlì-Cesena,Azienda USL Romagna,Forlì 47121,Italy
Fabio Pace,Unit of Gastroenterology,Bolognini Hospital,Bergamo 24100,Italy
Andrea Anderloni,Digestive Endoscopy Unit,Division of Gastroenterology,Humanitas Research Hospital,Rozzano 20089,Italy
Abstract
Despite the bi-directional interaction between gut microbiota and the brain not being fully understood,there is increasing evidence arising from animal and human studies that show how this intricate relationship may facilitate inflammatory bowel disease(IBD) pathogenesis,with consequent important implications on the possibility to improve the clinical outcomes of the diseases themselves,by acting on the different components of this system,mainly by modifying the microbiota.With the emergence of precision medicine,strategies in which patients with IBD might be categorized other than for standard gut symptom complexes could offer the opportunity to tailor therapies to individual patients.The aim of this narrative review is to elaborate on the concept of the gutbrain-microbiota axis and its clinical significance regarding IBD on the basis of recent scientific literature,and finally to focus on pharmacological therapies that could allow us to favorably modify the function of this complex system.
Key words:Irritable bowel syndrome;Inflammatory bowel disease;Gut-brain axis;Therapy
INTRODUCTION
The complex interplay between gut microbiota and the brain,and vice versa,has recently become not only the focus of neuroscience,but also the start point for research regarding many diseases such as inflammatory bowel diseases(IBD) and irritable bowel syndrome(IBS)[1-4].
The bi-directional interaction between gut microbiota and the brain is not completely understood.Nonetheless,there is increasing evidence arising from animal and human studies that show how this intricate relationship may contribute to the pathogenesis of IBD,with consequent important implications for the possibility to improve the clinical outcomes of the diseases themselves by acting on the different components of this axis[5-7].
The aim of this narrative review is to elaborate on the concept of the-gut-brainmicrobiota axis and its clinical significance regarding IBD on the basis of recent scientific literature,and finally to focus on pharmacological therapies that could allow us to favorably modify the function of this complex system.
WHAT IS THE GUT-BRAIN-MICROBIOTA AXIS?
While the idea that the brain can alter intestinal functions has long been recognized and accepted,the concept that signals from the gut can have effects on mood,behavior and cognitive function is less widely accepted.
First of all,it should be highlighted that the digestive system is very complex.This is due to the fact that it represents not only a surface of about 300 square meters for absorption,but it also a very complex hormonal system that produces more than thirty hormones,and of a very complex immune system,since it contains 60%-70% of a person's immune cells.Furthermore,it is an extremely innervated system,with an extensive intrinsic nervous system,namely,the enteric nervous system(ENS),that controls bowel function even though it is completely separate from the central nervous system(CNS)[8,9].
Finally,our digestive system contains various microorganisms [about 10(14)],such as bacteria,fungi,parasites,and viruses,and more than 100 million bacteria reside in the human gastrointestinal tract,establishing a mutually beneficial symbiotic state with the human organism.The gut microbiota controls the development and homeostasis of the host by acting on human metabolism and immune function,and also by controlling xenobiotic and drug metabolism,maintaining the structural integrity of the gut mucosal barrier,regulating the motility of the gut and promoting protection against pathogens.
The gut-brain-microbiota axis is defined as a two-way communication system that allows intestinal microbes to communicate with the brain and vice versa.This system,which has not been entirely explored,is based on neural,endocrine,immunological and metabolic pathways[10,11].This network contains several levels of communication and complex interplay,shown in Figure 1,namely[12]:(1) Gut microbiota,acting bidirectionally in this system through several mechanisms;(2) The ENS,which is made up of interneurons,sensory neurons,motor neurons,and neurotransmitters;(3) The autonomic nervous system(ANS),the pivotal modulator of the ENS;(4) The enteroendocrine and immune signaling agents;(5) The neuroendocrine signaling network mediated by the hypothalamic-pituitary-adrenal(HPA) axis,which is activated by the integrative reactions of specific centers in the CNS,and represents a central integrative system mandatory for the successful physiological adaptation of our organism to stress;and(6) The CNS,includes the hypothalamus,amygdala,and hippocampus and their interaction with emotional centers localized within the limbic system,which are mainly involved in the control of body reaction in response to stress.
In this system,in presence of a stressor or perturbating agent,inflammatory cytokines as IL1-beta,IL6 and TNF-α,several chemical substances,such as short chain fatty acids(SCFA),and microbial substances,can modify the ANS causing the secretion of cortisol and adrenaline from the adrenal glands through CRF and ACTHdependent pathways[13].
Gut microbiota and neuroactive products(released from the enteroendocrine and immune system) impair intestinal secretion and compromise the integrity of intestinal mucosa in response to perturbating agents[13].Therefore,several stressors render the intestinal mucosa more permeable to bacterial cytotoxins and neurotransmitters(norepinephrine),and cause the development of inflammation.In all these situations associated with stress,microbial agents translocate from the intestinal lumen into the systemic circulation,affecting the central and peripheral organs[13].
Efferent communication between the CNS and the gut mucosa include the vagal nerve and pelvic parasympathetic efferents and postganglionic sympathetic neurons[13,14].
Furthermore,the stress-induced activation of the HPA results in an enhanced production of corticosteroids from the adrenal glands[13,15,16].Furthermore,the sympathetic autonomic system also becomes activated,thus leading to the secretion of catecholamines such as epinephrine and norepinephrine[13,17].
Gut microbiota and its effect on the gut- brain axis
Gut microbiota is one of the densest,and quickly developing bacterial ecosystems and is characterised by great biodiversity.The intestinal microbiome,which is its collective genome,is an adaptive entity that varies in response to food intake,daily habits and surroundings,providing the host with an extra metabolic plasticity as well as functions that humans have not developed[18,19].
Several experimental approaches have been used to study the modulatory effect of gut microbiota on gut-brain interactions,including gut microbial manipulation with antibiotics[20,21],fecal microbial transplantation[21,22],and the use of germ-free animal models[20].
For a recent overview of the complex mechanism of the microbiota-gut-brain axis,the reader is referred to the work by Carabottiet al[23].In summary,microbiota modulation of the intestinal barrier is an important mechanism,with a net effect of strengthening tight junction integrity[23,24].Gut microbiota is also involved in hippocampal neurogenesis and expression in hypothalamic genes involved in synaptic plasticity[25].Furthermore,gut microbiota modulates pain perception and gut motility by acting on enteric sensory afferents[26]through the production of local neurotransmitters,such as gamma-aminobutyric acid,serotonin,nitric oxide,melatonin,catecholamine,histamine,acetylcholine and hydroxide sulfite[27-29],but also neurotrophic factors(brain-derived neurotrophic factor)[23].Finally,microbiota affects the immune regulation of the intestinal mucosa by means of several mechanisms,such as the increase of substance P in the ENS,and the down-regulation of proteases[30,31],and it produces small molecules,such as SCFA involved in many neural processes,such as the stimulation of sympathetic activity and the release of serotonin[23].
On the other hand,gut microbiota is affected by the brain through the secretion of signaling molecules(i.e.,catecholamines and gamma-aminobutyric acid) by neurons,immune and enterocromaffin cells,which may affect the microbiota itself[23].Furthermore,controlled by the brain,also intestinal motility and permeability,as well as the production of mucus,acid,bicarbonates and fluids,are modulated through direct action(host-enteric microbiota signaling) and indirectly(by the actions of the HPA axis or by variations of the composition,and therefore of the function,of microbiota)[23,32].
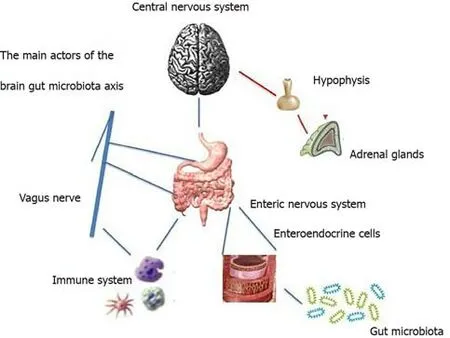
Figure 1 The main actors in the gut-brain-microbiota axis.
WHAT IS THE CLINICAL SIGNIFICANCE OF THE CHANGES TO THE GUT-BRAIN-AXIS MICROBIOTA IN INFLAMMATORY BOWEL DISEASE?
IBD pathogenesis is only partly understood,and some studies highlight a link with intestinal microbiota.In particular,the bidirectional relationship of the gut-brain axis,seems to be central also in the onset and development of IBD.
How is dysbiosis involved in the pathogenesis of IBD?
Similar to IBS,there is an alteration of the balance between microbiota and the gastrointestinal tract,with the onset of dysbiosis[33,34].The dysbiosis present in patients with IBD is characterized by reduced bacterial diversity,reduction of Bacteroidetes and Firmicutes,and an increase in Proteobacteria[35],in particularEscherichia coli[36].Other species such as Faecalibacterium prausnitzii and Roseburia hominis are selectively reduced in patients with IBD[37,38].Patients with active IBD have a lower abundance of intestinal flora than patients in remission[39].An alteration of intestinal bacterial flora in genetically susceptible individuals can lead to abnormal intestinal immune responses and intestinal imbalance[40].Interestingly,antibiotics may have some effect on IBD symptoms[41],and it is known that the predisposing genes involved in IBD pathogenesis are those with an important role in recognition of pathogen microbes[42]or in tolerance of commensals.
Furthermore,as discussed below,a confirmation of the influence of the microbiota on IBD pathogenesis is given by the benefits of using probiotics or prebiotics for these patients.The use of selected probiotics is effective in inducing and maintaining remission in ulcerative colitis(UC)[43]and in the treatment of pouchitis[44],whereas the use of prebiotics has been shown to be successful in reducing inflammation and bringing about remission in UC[45].Data are far less impressive in Crohn's disease(CD)[46,47].Faecal transplantation also appears to produce a modest increase in remission rates in patients with IBD[48].
How inflammation could modify the function of the gut-brain microbiota axis?Lessons from animal and human studies
IBD results in an inflammatory reaction in the brain,the activation of the hypothalamic-pituitary-adrenal axis and parts of the brain involved in behavioralalteration,an alteration of the blood-brain barrier and an intestinal-microbiota imbalance[49].Pro-inflammatory cytokines play a crucial role in the IBD pathogenesis and interact with the CNS directly by means of the blood-brain barrier orviathe vagus nerve[50].Such inflammatory pathwayviacytokines works by dysregulating HPA by over-activating microglia,altering neuroplasticity,and inducing structural and functional changes in the brain[49].Several mouse studies showed that cytokines determine the activation of astrocytes,thus affecting neural functions during the processes of inflammation during the period between behavioral effects such as depression[51].These in fact can affect the HPA axis,activating it and consequently causing an increase in glucocorticoids involved in pathogenesis of depression[52-54].
Other animal models have been used to investigate the relationship between gastrointestinal disorders and psychological manifestations.For example,maternal separation is a stressor induced in the early stages of life[55].Other examples of chronic stressors are housing problems[56,57]and overcrowding[58],such stressors can induce gastrointestinal dysfunction or increase susceptibility to chemically induced colitis.These studies may explain the link between stress and gastrointestinal disorders and therefore IBS-like symptoms in patients with IBD,although not entirely specific to IBD.
In further animal studies,the mechanism of immune activation in the gut and its interaction with the central nervous system were explained[59,60].
There are several murine models of IBD,which include,for example,the use of dextran sodium sulfate(DSS),the colorectal instillation of dinitrobenzenic sulfonic acid and 2,4,6-trinitrobenzenesulfonic acid(TNBS)[61-67],that show an increase in hippocampal,cerebral and hypothalamic pro-inflammatory cytokines.Indeed,the disruption of the blood-brain barrier and the leukocyte infiltration of the brain following colic inflammation leaves the CNS vulnerable to inflammation mediators and to substances of bacterial and viral derivation[67].
What is the role of the stress,considered to be a perturbating agent of brain-gutmicrobiota axis,in IBD?
Stress may have a deleterious effect on IBD,through several pathways,as reviewed by Bonazet al[68],including the activation of mast cells and the CNS and the inhibition of the vagus nerve on inflammatory pathways,decreasing its anti-inflammatory effects and increasing sympathetic tone[69];this may lead to the inhibition of immune defenses and development/increase of intestinal inflammation.It has been shown that stress induces an imbalance in the ANS in patients with IBD and a vagal dysfunction in patients with UC[68].For patients with IBD there is a correlation between ANS imbalance,psychological disorders and pro-inflammatory profiles[70,71].
Important factors are also the effect of early childhood stressful events on colitis(the HPA axis is determined by early childhood events,and neonatal inflammatory stimuli exert long-term changes on HPA activity) and the impact of depression on exacerbation of colitis possibly through proinflammatory cytokines.Last,but not least,stress and CRF increase intestinal permeability as observed in mouse models,with passage of intestinal bacteria through the epithelial barrier[72,73].In patients with IBD,changes in stress-mediated intestinal microbiota may create susceptibility toinfection and alter neural activity in stress-sensitive areas of the brain[74].
Dysbiosis can directly affect mental health in patients with IBD[75].Behavioral disorders such as stress,anxiety and depression may change the composition of the intestinal flora and may influence the activity and recurrence of CD as demonstrated by numerous studies[76,77].Recent studies have highlighted that the most involved mechanisms in stress signaling,on the cellular level,are endoplasmic reticulum stress,oxidative stress and hypoxia[78].The subsequent host cellular response to these mechanisms interact with gut microbiota,thus modifying the microbiological microenvironment of the gastrointestinal tract[78].
Indeed,exposing mice to stressful stimuli resulted in the alteration of gut microbiota by reducing anti-inflammatory bacteria,in particularLactobacillus[79-81]andLachnospiraceae[79,82].Furthermore,psychological stress reduces the biosynthesis and metabolism of short chain fatty acids,which may increase susceptibility toward intestinal inflammation and further IBD[79,83].
It is now known that IBD is associated with psychological symptoms such as anxiety and depression,prevalent during active disease states and,as also observed in animal models of IBD,there are no differences in occurrence of CD and UC.Mood disorders may also influence the course of IBD because it is hypothesized that stress may be a risk factor for recurrence for patients of this type[84].Probably,the depressive behavior observed in patients with IBD constitutes a comorbidity[49],which worsens the state of intestinal disease[85].
In the murine models of DSS colitis,induction of depression with olfactory bulbectomy or intracerebroventricular injection of reserpine was associated with thereactivation of inflammation in mice with quiescent colitis,with effects mediated by the increase of pro-inflammatory cytokines[86].In contrast,the administration of tricyclic antidepressants prevented the reactivation of colitis for depressed mice but not in mice without depression[87].As previously mentioned,in many studies conducted on adults and children it is clear that both UC and CD are associated with a higher incidence of psychological symptoms[88],with an association between disease and mood.These data are confirmed by a recent systematic review,with equal rates in both sexes,but slightly higher for CD than for UC[89].Depression and anxiety are 2 to 3 times higher in patients with IBD than in the general population,affecting respectively 25% and 30% of people with IBD[90].Patients with IBD who suffer from psychiatric illness have a reduced chance of remission,and the condition worsens over a longer period of time[91].An increased risk of psychiatric disorders is also observed in adolescents and children with IBD[92].Adolescent patients with IBD are more likely to have mild behavioral and cognitive disturbances,particularly verbal memory loss[93].Active IBD correlates significantly with increased psychological disorders[94,95]and the highest pain scores are strong predictors of depression in UC and CD[96].In patients with UC,depression is usually diagnosed in the year before the onset of disease symptoms,while for patients with CD depression follows the diagnosis of the disease[97].Being female is also a predictor of anxiety and depression with IBD[98,99],with IBD having a bigger effect on health-related quality of life[100]and greater concomitance of symptoms similar to IBS[101].The manifestation of mood disorders by IBD patients correlates with a greater risk of requiring surgery and of incurring secondary FGID development[102].
In the Manitoba IBD study,a cohort of patients with IBD monitored every 6 mo,and with annual interviews over a period of 12 years,psychological disorders were highlighted as a major factor in health perception for the IBD cohort[103].In another study involving 600 subjects with IBD,using health problem surveys conducted quarterly for 1 year,about 50% of patients showed a certain type of stress,most frequently family stress,followed by work or school and finance related stress[104].It was observed that psychological factors,important life events,high anxiety and highly negative feelings during the previous 3 mo were closely associated with the occurrence of a flare up.Targowniket al[105]observed that perceived stress correlated closely with the symptoms of active disease,but without any correlation between the symptom scores and the degree of inflammation associated with CD,and only a weak correlation associated with UC,concluding that there may be an association between the perceived stress and the symptoms of IBD regardless of inflammatory activity.
Furthermore,IBD also influence the volume of gray matter and the size of the brain according to an MRI study,which observed that patients with CD displayed a decrease in the volume of gray matter in the frontal cortex and in the cortex of the anterior cingulate[106].
Interestingly,Gracieet al[107]recently found evidence in a 24 mo study of CD or UC patients of a reciprocal relationship between IBD occurence or severity and psychological illness,thus concluding that IBD patients' psychological health should be monitored.
WHAT THERAPEUTIC INTERVENTIONS ARE THERE THAT TARGET THE GUT-BRAIN-MICROBIOTA AXIS WHEN IBD OCCURS?
Manipulation of gut microbiota
Since gut microflora may trigger changes leading to IBD,the manipulation of the microbiota through administration of probiotics,prebiotics,synbiotics,dietary modifications and faecal transplantation are potentially promising approaches to gastrointestinal diseases,including IBD[108-111].
The literature concerning the use of probiotics for the bringing about and preservation of CD remission is diverse in content and hard to interpret.The reasons for such heterogeneity are several: The different probiotics(strain and doses) used,the differences in study duration,the features of the included patients,and the measured endpoints[112].Furthermore,two meta-analyses[112,113]on the effects of probiotics as a group indicated that their impact was no different from placebo.
With regard to UC,it was shown that the use ofEscherichia coliNissle 1917 strain for patients with UC was effective in maintaining remission as mesalazine therapy alone[113,114].
Successively,Shadnoushet al[115]showed that consuming yogurt containing Bifidobacterium andLactobacillusmay contribute to the maintenance of the homeostasis in gastrointestinal tract and regulate pro- and anti-inflammatoryresponses by the intestinal immune cells[113]and may therefore be advised for patients with active IBD[113,115].
Similarly,the consumption ofBifidobacteria-fermented milk was observed to exert a possible preventive effect on the recurrence of UC and helped to maintain its remission[113,116],while the use of combined treatment withLactobacillusGG and mesalazine was found to be more effective in prolonging the relapse-free period than treatment withLactobacillusGG and mesalazine alone for UC patients[113,116].
With regard to pouchitis,VSL3,a probiotic preparation containing 8 strains,when used for patients with pouchitis in small controlled trials and it was found to be beneficial for the primary and secondary prevention of pouchitis[113,117-120].
In contrast to the many studies on probiotics,there have been few studies(with conflicting results) that have addressed the role of prebiotics in encouraging the increase in number and/or activity of one or a limited class of bacteria in the gastrointestinal tract with a resulting improvement in the host's IBD[113,119-121].
In these studies it was reported that increasing the SCFA concentration in the gut(as a result of the consumption of prebiotics) enhances growth of protective bacteria(symbionts),while limiting the growth of pathobionts[113,122-125].
With regards to synbiotics,they influence the development of beneficial intestinal microflora through the use of probiotics,whereas prebiotics inhibit the growth of pathogenic bacteria[113,126,127].
Finally,we move on to psychobiotics,which can be defined as live bacteria(probiotics) that “confer mental health benefits through interactions with commensal gut bacteria”[128].Such probiotics have an effect on emotional,cognitive,systemic,and neural variables that determine psychological well-being[128].Both rodents and human studies[129],as reviewed by Sarkaret al[129],showed the complex interactions between the modulation of gut microbiota with the gut-brain axis.
For IBD,data about the use of psychobiotics are limited to animal models,where they affected the gut-brain axis by modifying the immune system[129].
Other therapeutic interventions targeting the gut-brain axis in cases of inflammatory bowel diseases
Several factors interplay including triggering of the brain's inflammation response system,the hypothalamic-pituitary-adrenal axis,and areas of the brain are associated with altered behaviour,changes in the integrity of the blood brain barrier,and an emerging role of gut microbiota and the response to probiotics in IBD cases.It is advised that IBD patients be monitored for psychological problems and treated appropriately as intervening can better quality of life and could diminish rates of relapse[130].Several evidence-based treatments are available for most causes of psychological distress in IBD patients,the most widely accepted being rooted in cognitive behavioral theory[131].
Mindfulness interventions do not seem to impact disease activity and other diseaserelated factors in patients with UC or CD[132].These findings are consistent with data from other psychosocial interventions[132-134]which did not demonstrate a positive impact on IBD clinical course.There is limited preliminary evidence that mindfulness interventions may have a positive impact on some inflammatory markers.
Patients want their gastroenterologist to discuss psychological issues during routine visits,and many are open to or desire referral to qualified mental health providers for concurrent treatment[131].
An alternative therapy to conventional anti-TNF-α treatment,based on gut-brain interactions,is stimulation of the cholinergic anti-inflammatory pathway,either pharmacologically or through vagal nerve stimulation or nutrition,as reviewed by Bonazet al[70].For example,galantamine is a centrally acting acetylcholinesterase inhibitor and a positive allosteric modulator of nicotinic receptors,including alpha7nAChR,which stimulates efferent VN activity,suppresses serum TNF-α and IL-6 levels[135].In contrast,other drugs evaluated in this setting.i.e.,CNI-1493,which is a tetravalent guanylhydrazone that inhibits production of proinflammatory cytokines in macrophages[136],and GTS-21,an alpha7nAChR agonist,were both associated with markedly reduced TNF-α,IL-6,and IL-1ra plasma concentrations[137].Finally,another alpha7nAChR agonist,AR-R17779,has been used with success in a mouse model of postoperative ileus[138].
CONCLUSION
In conclusion,lines of communication between the brain and gut have a crucial role in the biology,clinical manifestations and clinical outcomes of gut diseases such as IBD.The central pathways through which this interplay is mediated are neural andimmune networks,including interplay between these systems.The bidirectional trafficking of these signals opens the possibility of some gut diseases being instigated by aberrant brain function,and conversely,gut homeostasis disorder being responsible for instigating brain pathology and,particularly,mood disorders in other patients.Overall,the mucosal immune system is potentially the key gatekeeper of these pathways as it specifically interacts with nerves,the luminal microbiota and it is an important target of the stress response.What effect these insights will have on clinical practice remains to be clarified,although it is conceptually appealing to envisage the emergence of new diagnostic criteria,biomarkers and psychological scoring tools in patients with gut diseases.With the emergence of precision medicine,strategies in which patients with IBD might be classified beyond standard gut symptom complexes might offer the opportunity to tailor therapies to individual patients.In particular,anti-cytokine therapy or efforts to manipulate the intestinal microbiota could hold the key to effectively uncoupling pathological gut-brain interplay,with the potential to disrupt the proximal drivers of these important diseases.It seems to be with good reason that experts have said “as we enter a new era of patient-centered health care,treating the “brain” is as important as the “gut” for comprehensive,whole-person IBD management”[139].
 World Journal of Clinical Cases2020年5期
World Journal of Clinical Cases2020年5期
- World Journal of Clinical Cases的其它文章
- Gut microbiota and nutrient interactions with skin in psoriasis: A comprehensive review of animal and human studies
- Distal esophageal spasm: Update on diagnosis and management in the era of high-resolution manometry
- Clinical course of percutaneous cholecystostomies: A crosssectional study
- Clinical characteristics and 28-d outcomes of bacterial infections in patients with hepatitis B virus-related acute-on-chronic liver failure
- Application of hybrid operating rooms for treating spinal dural arteriovenous fistula
- Ruxolitinib add-on in corticosteroid-refractory graft-vs-host disease after allogeneic stem cell transplantation: Results from a retrospective study on 38 Chinese patients
