Breast non-mass-like lesions on contrast-enhanced ultrasonography:Feature analysis,breast image reporting and data system classification assessment
Ping Xu,Min Yang,Yong Liu,Yan-Ping Li,Hong Zhang,Guang-Rui Shao
Ping Xu,School of Medicine,Shandong University,Jinan 250100,Shandong Province,China
Ping Xu,Min Yang,Yong Liu,Hong Zhang,Department of Ultrasound,Beijing Shijitan Hospital affiliated to Capital Medical University,Beijing 100038,China
Yan-Ping Li,Department of Breast Surgery,Beijing Shijitan Hospital affiliated to Capital Medical University,Beijing 100038,China
Guang-Rui Shao,Department of Radiology,The Second Hospital of Shandong University,Jinan 250100,Shandong Province,China
Abstract
Key words:Breast tumor;Ultrasonography;Contrast agents;Feature exploration;Diagnosis;Non-mass-like lesions
INTRODUCTION
Breast non-mass-like lesions(NMLs)[1]refer to lesions that do not have clear boundaries on ultrasonography(US),and that do not have spatial mass effects in two or more different scanning directions,accounting for 9.2% of all breast lesions[2].The specificity of the ultrasound diagnosis of NMLs is low[3,4].NMLs cannot be objectively classified according to the fifth edition of the Breast Imaging Reporting and Data System(BI-RADS)standard issued by the American College of Radiology(ACR).Contrast-enhanced ultrasound(CEUS)can indicate the distribution and morphological information of the blood vessels in these lesions,but there are few studies on the CEUS features of NMLs,and the effect of the CEUS features of NMLs on BI-RADS classification is still unclear.Therefore,this study aims to:(1)Explore the features of US,color Doppler flow imaging(CDFI)and CEUS of NMLs;(2)Explorethe efficacy of the combined diagnosis by US and CEUS;and(3)Investigate the effect of CEUS features on the initial BI-RADS classification of NMLs,and the effect of the combined application of CEUS and the BI-RADS classification system on the diagnosis and clinical strategy of NMLs by including CEUS in the BI-RADS classification system.
MATERIALS AND METHODS
Patients
A retrospective study was performed on 51 patients(all of whom were women and 29-years-old to 64-years-old,with an average age of 45.35 ± 10.42 years)with breast NMLs who underwent breast CEUS from January 2017 to April 2019.The maximum diameter of the masses was 0.8-3.5 cm,the masses were palpable in 15 cases,and 12 cases had the symptom of nipple discharge.The pathological results of all focuses were obtained by puncture or surgery.
Inclusion criteria were US features meeting the criteria for NMLs.Exclusion criteria were biopsy or surgery performed in the lesion area,pathology not available,or CEUS results being incomplete.This study was approved by the Ethics Committee of our hospital.All subjects were informed of the purpose and risks of the examination,and signed informed consent was obtained prior to undergoing CEUS and ultrasound-guided breast biopsy.
Ultrasonic examination method
US,CDFI,and CEUS were performed for each lesion.All examinations were performed by three sonographers with more than 10 years of experience in breast US.US,CDFI and CEUS were performed with a GE LOGIQ E9 equipped with 15 L(US examination)and 9 L(CEUS examination)linear transducers.The contrast agent used was SonoVue(Bracco Imaging B.V.,Geneva,Switzerland),and the consumption was 4.8 mL for each lesion.
Analysis of US
Analysis of conventional US:Location,maximum diameter,echo pattern,architectural distortion,ductal changes,microcalcifications(strong echo < 2 mm in diameter[5]),posterior features,and the tumefaction in axillary lymph nodes were observed and recorded.All lesions were initially classified according to the BI-RADSUS classification of breast NMLs published by Koet al[1]:Type I for ductal NML pattern(Ib with calcification,Ia without);type II for non-ductal NML pattern(IIb with calcification,IIa without);type III NMLs with architectural distortion;and type IV NMLs with posterior acoustic shadowing.Type IIa was correlated with BI-RADS categories 4a;types Ia,III and IV were correlated with 4b;and types Ib and IIb were correlated with 4c.The cutoff points of the benign and malignant groups were 4a and 4b.Type 4a was benign;types 4b and 4c were malignant.Biopsy is recommended for 4a and above.
Analysis of CDFI:Referring to Adler’s grade,grades 0-I were determined to reflect scarce blood supply,and grades II- III were determined to reflect an abundant blood supply.
Analysis of CEUS:Enhancement time(earlier,synchronous,later),enhancement intensity(hypo-,iso- or hyper-enhanced),enhancement mode(homogeneous or heterogeneous),enhancement direction(centripetal,centrifugal or diffuse),lesion scope(expansion,without expansion),peripheral blood vessels:Radial perfusion or penetration perfusion(with or without),and regression time(earlier,later,synchronous)were recorded.The enhancement time,regression time and enhancement degree of focus were all based on the surrounding normal breast tissues.
Pathological grouping
Pathology was divided into two groups,namely group A(benign lesion group)and group B(malignant lesion group).Group A was divided into group A2(precancerous lesion group)and group A1(another benign lesion group).Precancerous lesions include sclerosing adenosis,atypical ductal or lobular hyperplasia,ductal epithelial florid hyperplasia(high ductal epithelial hyperplasia)[6],and epithelial columnar cell lesions[7].
Statistical analysis
All data were entered into the database in Excel,and statistical analysis was performed with SPSS 21.0 statistical software.The experimental data were allenumeration data expressed by frequency or percentage,and the chi-square test was used to analyze the differences in the features of US,CDFI and CEUS in the benign and malignant groups.Binary logistic regression was used to determine the independent risk factors for malignant NMLs.With independent risk factors as independent variables,and pathological results as dependent variables,a logistic regression equation was built to calculate diagnostic efficiency.Based on the regression equation,the combined diagnostic efficiency of US + CEUS was obtained.The initial BI-RADS classification for NMLs was adjusted according to independent risk factors from CEUS to obtain the CEUS + BI-RADS diagnostic efficacy.Finally,ROC curves were drawn to compare the sensitivity,specificity,positive predictive value(PPV),negative predictive value(NPV),accuracy and area under the curve of the three methods.P< 0.05 suggested that the difference is statistically significant.
RESULTS
Of the 51 cases included in this study,28 focuses(54.90%)were benign,of which 3(5.89%)were combined with precancerous lesions;additionally,23 focuses(45.10%)were malignant.The pathology of the lesions is shown in Table 1.
Clinical information and ultrasound imaging between benign and malignant NMLs
Comparisons of clinical information and US,CDFI and CEUS imaging between benign and malignant NMLs are shown in Tables 2 and 3.
The Table shows no significant difference in age and clinical symptoms in the two groups;that is,these factors have no effect on pathology.
In the US features of NMLs,microcalcifications were different between benign and malignant NMLs(P= 0.001),indicating that microcalcifications were associated with malignant NMLs.
Regarding CEUS features,enhancement time(P= 0.006),enhancement intensity(P= 0.002),lesion scope(P= 0.000),and peripheral blood vessels(P= 0.046)were different between benign and malignant NMLs.Earlier enhancement,higher enhancement,range expansion,and lesions of peripheral blood vessels in contrastenhanced US suggested malignant lesions.
Independent risk factors and logistic regression formula
Binary logistic regression analysis was used to identify the independent risk factors for malignant NMLs.The selected risk factors included microcalcifications,enhancement time,enhancement intensity,lesion scope,and peripheral blood vessels.Binary logistic regression analysis was conducted for all of these factors,and microcalcifications(P= 0.035),higher enhancement(P= 0.041),and lesion scope(P=0.008)were identified as independent risk factors.The regression equation is as follows:
Logit(P)= -27.266 +(4.008 × combined microcalcification)+(5.386 × peripheral blood vessels)+(4.885 × higher enhancement)
The area under the ROC curve obtained by this equation was 0.907.When the cutoff value was 0.5,the sensitivity was 95.7% and the specificity was 85.7%.
Combined diagnosis based on the regression equation
Among the three risk factors(microcalcifications,higher enhancement,and lesion scope),lesions with no or only one risk factor were defined as possibly benign,and lesions with two or three risk factors were defined as potentially malignant.The sensitivity,specificity,PPV,NPV and accuracy of the diagnosis by this method were 87.0%,92.9%,90.9%,89.7% and 90.2%,respectively.
Adjustment of the initial BI-RADS classification of 51 NMLs according to CEUS features
The independent risk factors identified by CEUS for malignant NMLs were higher enhancement and lesion scope.The adjustment method is shown in Table 4.According to the number of contrast-enhanced US features that were independent risk factors for NMLs,the classifications included decreased(Figure 1),remained unchanged(Figure 2)or increased(Figure 3).
The BI-RADS classifications for 51 cases of NMLs before and after adjustment are shown in Table 5.
As shown in Table 5,after adjustment,classification of the 51 cases changed,showing a decrease in the number of type 4 cases(4a decreased by 12 cases,4b decreased by 4 cases,and 4c decreased by 5 cases)and an increase in the number of type 3 and type 5 cases(type 2 increased by 12 cases and type 5 increased by 9 cases).After adjusting the classification of NMLs according to the independent risk factorsidentified by contrast-enhanced US,the sensitivity,specificity,PPV,NPV and accuracy of NML diagnosis were 91.3%,89.2%,87.5%,92.6% and 90.2%,respectively.

Table 1 Pathology of 51 non-mass-like lesions
ROC curves
The ROC curves of the US,US + CEUS,and CEUS + BI-RADS methods are shown in Figure 4,and their diagnostic efficiency is shown in Table 6.Areas under the curves for the US,US + CEUS,CEUS + BI-RADS were 0.752,0.877,and 0.903,respectively.The Table shows that the diagnostic sensitivity and specificity of US + CEUS and CEUS + BI-RADS methods were significantly improved compared with that of US,and the area under the curve between US and US + CEUS(Z= 2.050,P= 0.040)and between US and CEUS + BI-RADS(Z= 3.280,P= 0.001)were significantly different.There was no significant difference in the areas under the curves between US + CEUS and CEUS + BI-RADS(Z= 0.545,P= 0.586).
DISCUSSION
Breast NMLs are very common in the clinic,accounting for 9.2% of all breast lesions[4].The pathology of benign NMLs is mostly adenosis,and malignant NMLs are mostly intraductal carcinomas and invasive ductal carcinomas.ACR BI-RADS-US cannot classify breast NMLs,and has low diagnostic specificity[1,3].Contrast-enhanced US can provide information on the blood supply of the lesions,but there are few studies on the contrast-enhanced US features of NMLs.Our study aimed to investigate the features of US,CDFI and CEUS in benign and malignant NMLs,and to explore the efficacy of a combined diagnosis.Additionally,the initial BI-RADS classification and CEUS were combined to facilitate the management of NMLs and clinical communication,providing a basis for clinical treatment.
Compared with breast mass-like lesions,the grayscale US of NMLs is usually hypoechoic without clear boundaries,and the pattern is usually irregular and mainly characterized by a striped or flaky hypoecho(27/51,52.94%),duct ectasia(22/51,43.14%),microcalcification(17/51,33.33%),structural distortion(27/51,19.61%),rear echo attenuation(7/51,13.73%),etc.Of the above US features,only microcalcifications were different in benign and malignant NMLs,and microcalcifications were identified as an independent risk factor for malignant NMLs,suggesting that microcalcification was associated with malignant NMLs.This finding is consistent with previous studies[3,8].The PPV of combined microcalcification in the hypoecho zone can reach 78.26%[3].For this reason,when Koet al[1]conducted BI-RADS-US classification for NMLs,they viewed microcalcification as an important risk sign.
CEUS can objectively and noninvasively evaluate the blood supply of NMLs,thus helping identify benign and malignant NMLs[9,10].Many studies have reported the contrast-enhanced US features of breast cancer[11-13].In a study by Wanget al[12],the independent risk factors for breast cancer were enhancement intensity,enhancement sequence,enhancement boundary,peripheral blood vessels and increased diameter.Xiaoet al[13]viewed lesion scope,boundary and shape as independent risk factors forbreast cancer.Since the number of breast NMLs is lower in breast lesions,previous studies[12,13]tended to explore the features,boundaries and forms of mass-like lesions,which had important diagnostic significance.

Table 2 Clinical information and ultrasonography,and color Doppler flow imaging between benign and malignant non-mass-like lesions
Our study found that higher enhancement and lesion scope were independent risk factors for malignant NMLs.The finding of higher enhancement as an independent risk factor for malignant NMLs is consistent with the studies of Zhanget al[11]and Wanget al[12].Previous studies[14]have shown that breast focuses were associated with increased expression of vascular endothelial growth factor and increased tumor microvessel density in the development of atypical hyperplasia,carcinomain situ,and invasive carcinoma.Microvessel density is considered to be an independent prognostic factor for breast cancer,and is associated with histological grade and proliferative activity to some extent[15,16].The enhancement intensity reflects the abundance of the blood supply of the focus,and has a good correlation with the microvessel density of the focus[17],suggesting that enhancement intensity can reflect the proliferative activity of the focus and may be related to the prognosis of NMLs.In this study,16 cases(16/23,69.57%)in the malignant group and only 6 cases(6/28,21.43%)in the benign group showed higher enhancement,of which 2 cases had precancerous lesions,suggesting that the higher enhancement in NMLs suggests the active proliferation of the focus,and that microvascular changes may precede pathological changes during the progression from benign lesions to malignant lesions.
In addition to enhancement intensity,lesion range was also identified as an independent risk factor for malignant NMLs,similar to the findings of previous studies[12,13].van Esseret al[18]found that grayscale US often underestimated the size of breast cancer foci,especially those of intraductal carcinoma[19],while the maximum value measured by contrast-enhanced US was closer to the measured value of surgical specimen pathology.This suggested that the size and shape of the lesion identified by contrast-enhanced US were closer to the true state of the lesion.Since most NMLs are mixed with normal glands,only lesions with low echo on grayscale US are identified.In the contrast-enhanced state,the baseline echo of the gland is masked,only the blood supply information is displayed,and the overall shape of the malignant NMLs is shown,showing a wide range after contrast-enhanced US(15/23,65.23%).Some studies have shown that there were more neoplastic microvessels around themalignant breast lesions than in the center[20],and contrast-enhanced US showed higher peripheral enhancement intensity[21].This study did not observe this finding,which may be due to the small number of cases in our study,the large proportion of intraductal cancers in the malignant cases,and their weak invasiveness.

Table 3 Contrast-enhanced ultrasound imaging between benign and malignant non-mass-like lesions
In addition to higher enhancement and lesion range,peripheral blood vessels and enhancement time were significantly different between benign and malignant NMLs.The lesions with earlier enhancement(20/51,39.23%)and peripheral lesions around the lesion(19/51,37.25%)tended to be malignant.Earlier enhancement and the appearance of peripheral blood vessels reflected the formation of abnormal blood vessels.Although these factors were not identified as independent risk factors,they provided some help for diagnosis.
In our study,there were several indicators that differed from previous studies:Age,accessibility of the mass,and lesion blood supply identified by color Doppler US[22].These differences may be related to several factors,such as fewer enrolled cases,differences among operators,and instrument performance and settings.Nonetheless,these three indicators can also provide a reference for diagnosis.
Considering the microcalcification and blood supply information of NMLs,combining the risk factors identified by grayscale US and contrast-enhanced US,lesions with one risk factor were classified as “possibly benign”,and lesions with two or three risk factors were classified as “possibly malignant”.The diagnostic efficiency of this method improved compared with that of grayscale US,in which the sensitivity,specificity,PPV,NPV and accuracy were 87.0%,92.9%,90.9%,89.7% and 90.2%,respectively.The method is simple and easy,but its result cannot grade the risks of breast NMLs,which is not conducive to the management of NMLs.
In clinical work,the BI-RADS-US classification issued by ACR is very effective in the diagnosis and management of breast nodules.Unfortunately,the fifth edition of the ACR BI-RADS-US standard does not objectively classify NMLs.There is no unified method for the classification of NMLs.Different scholars have also explained it differently,but their diagnostic performance was similar[1,3,5,8].A study showed that[23]the malignancy rate of NMLs may be greater than 2%,so NMLs should at least be classified as type 4a lesions.Therefore,the initial classification of NMLs in this study used the classification method of BI-RADS-US for NMLs developed by Koet al[1],and the lesions were classified into types 4a,4b,and 4c according to grayscale US features.Contrast-enhanced US provides blood supply information for breast lesions,so the diagnostic efficiency can be effectively improved if its results are included inthe BI-RADS-US classification system[11-13].Different studies have used different methods,which can be divided into counting methods[11],integral methods[13]and pattern methods[12].Since the BI-RADS-US classification predicts the malignant probability of NMLs based on the number of two-dimensional ultrasound risk signs,this study used the counting method,that is,the method in which the initial classification is adjusted according to the number of independent risk factors identified by contrast-enhanced US features.The adjustment method is shown in Table 4.After adjusting the classification,the diagnostic efficiency was significantly higher than that of US(P= 0.001),which was similar to the diagnostic efficiency of US+ CEUS(P= 0.586).However,the adjustment of BI-RADS classification was more convenient to combine with the grayscale US results,and facilitated the management of NML lesions and clinician communication.
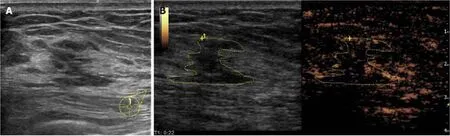
Figure 1 Fifty-three-year-old woman with a non-mass lesion in her left breast.
In this study,before and after the classification adjustment of 51 cases of NMLs,the distribution of cases changed.Before adjusting the classification,all NMLs were type 4.After adjusting the classification,the numbers of type 3 and 5 lesions increased(type 3 increased by 19,type 5 by 12),and only 20 cases were type 4 focuses.According to the recommendation of BI-RADS-US classification,type 3 lesions can be subject to follow-up observation.After adjusting the classification,19 patients could be converted from the recommended biopsy to follow-up observation,which effectively reduced unnecessary biopsy.The decrease in type 4 lesions and the increase in type 3 and 5 lesions enable clinicians to have a more objective understanding of the risk classification of focuses,and can more effectively guide clinical decision-making[24].
It is worth noting that of the three benign NMLs misdiagnosed by the adjusted BIRADS classification,the lesion pathology of two cases showed precancerous lesions,namely sclerosing adenosis and atypical ductal epithelial dysplasia.Some of the precancerous lesions of the breast can develop into cancer;the highest cancer rate in 25 years was 46.0%[25],and its imaging diagnosis revealed certain difficulties[26].Contrast-enhanced US can detect neovascularization of the lesion early,suggesting the potential diagnostic value for precancerous lesions.Another misdiagnosed case was plasma cell mastitis.Inflammatory lesions are rich in blood supply,and easily spread to surrounding perilobular and interlobular tissues[27].It is difficult to identify malignant breast lesions using contrast-enhanced US.However,inflammatory lesions have clinical symptoms,and should be comprehensively diagnosed.
In this study,two cases of breast cancer were missed,and one case was mucinous carcinoma.Mucinous carcinoma forms a mucus lake due to abundant mucin,which is characterized by heterogeneity of enhancement or peripheral enhancement only[28].Moreover,because it was invasive carcinoma,there were no obvious changes in the enhanced range,and peripheral blood vessels were lacking,leading to a missed diagnosis of the lesions.Another case was intraductal carcinoma with a maximum diameter of approximately 1 cm.The focus was small,and the body’s immune mechanism formed an immune response zone around the focus,inhibiting the formation of peripheral microvessels.The lack of deformed blood vessels led to misdiagnosis.
The limitations of this study were that the sample size was small,and the sample size of precancerous lesions was only 3 cases,so large samples from multiple centers should be studied in the future.
In conclusion,compared with mass-like breast lesions,NMLs have unique contrastenhanced features.Malignant NMLs usually show earlier enhancement,higher enhancement,increased range,and the appearance of peripheral blood vessels.Among NMLs,higher enhancement and lesion range are independent risk factors for malignant NMLs.Grayscale US combined with contrast-enhanced US can improve diagnostic efficiency.Adjustment of the initial BI-RADS classification according to independent risk factors identified by contrast-enhanced US significantly improved the diagnostic efficiency compared with that of type B US.The diagnostic results of this method are objective and direct.This method is convenient for the management of NMLs and clinician communication,effectively reduces unnecessary biopsy,and provides the basis for providing patients with more personalized clinical strategies.

Table 4 Protocol for readjusting the Breast lmaging Reporting and Data System category with contrast-enhanced ultrasound
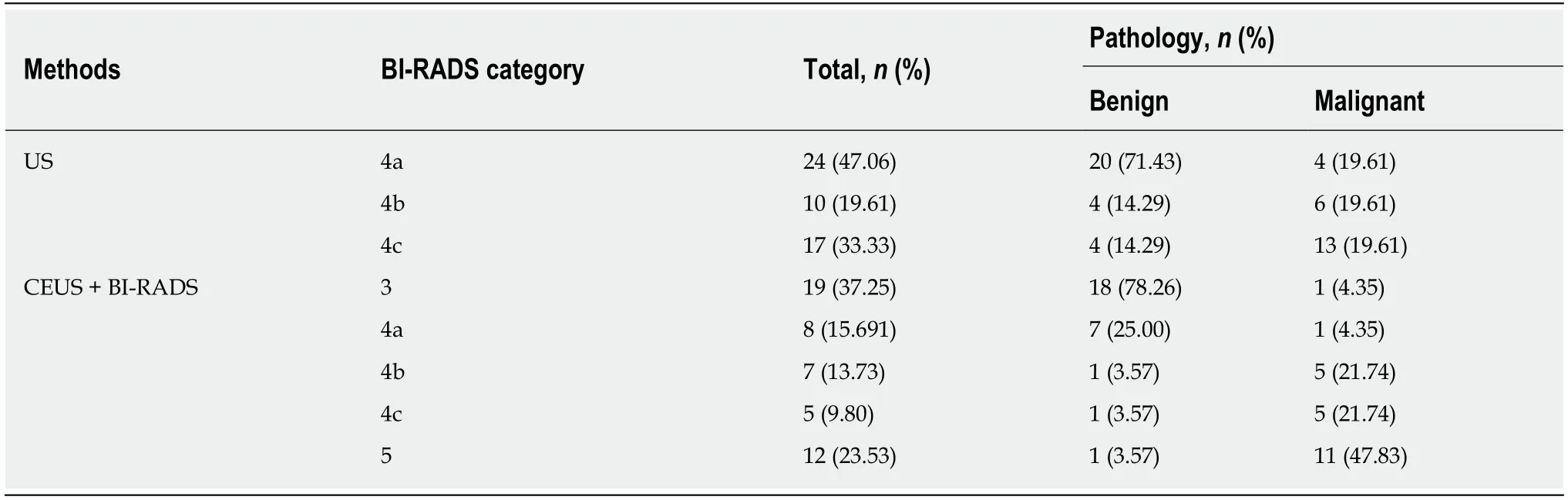
Table 5 Comparison of initial Breast lmaging Reporting and Data System category,and readjusted Breast lmaging Reporting and Data System category with pathology results
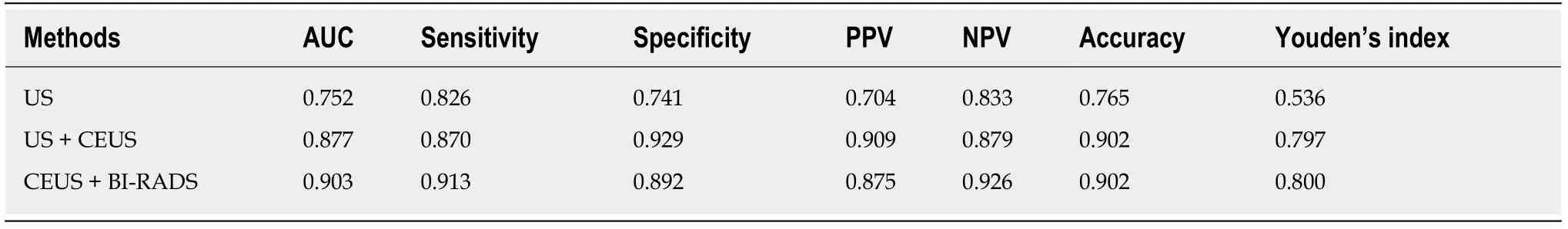
Table 6 Diagnostic performance of ultrasonography,ultrasonography + contrast-enhanced ultrasound and contrast-enhanced ultrasound + Breast lmaging Reporting and Data System

Figure 3 Fifty-two-year-old woman with a non-mass lesion in her right breast.

Figure 4 Receiver-operating characteristic curves for ultrasonography,ultrasonography + contrast-enhanced ultrasound,and contrast-enhanced ultrasound + Breast lmaging Reporting and Data System.
ARTICLE HIGHLIGHTS
Research background
The diagnosis of breast non-mass-like lesions(NMLs)by ultrasonography(US)is not satisfactory.Contrast-enhanced ultrasound(CEUS)can provide additional information for the differentiation of breast lesions.However,there are few studies about the characteristics and application of CEUS in breast NMLs.
Research motivation
Improve the diagnostic efficiency of breast NMLs by combining conventional ultrasound with CEUS.
Research objectives
To explore the CEUS features of breast NMLs,and to improve the diagnostic efficiency combined with the Breast Imaging Reporting and Data System(BI-RADS).
Research methods
A retrospective study was carried out in 51 breast NMLs.The independent risk factors of malignant NMLs were identified by binary logistics regression,with pathology as the gold standard,and the independent risk factors of CEUS were used to modify the initial BI-RADS classification of NMLs.
Research results
Malignant breast NMLs show high enhancement and enlarged range on CEUS.The diagnostic efficiency can be improved by conventional ultrasound combined with CEUS.
Research conclusions
US combined with CEUS can improve diagnostic efficiency for NMLs.CEUS combined with the BI-RADS system enables the diagnostic results to be simple and intuitive,and effectively reduces the incidence of unnecessary biopsy.
Research perspectives
Combined diagnosis of breast NMLs,and fusion with the existing BI-RADS classification system.
World Journal of Clinical Cases2020年4期
- World Journal of Clinical Cases的其它文章
- Must pilots permanently quit flying career after treatment for colorectal cancer? - Medical waiver for Air Force pilots with colorectal cancer:Three case reports
- Prevalence and associated factors of suicide among hospitalized schizophrenic patients
- Lymphoepithelioma-like carcinoma of the upper urinary tract:A systematic review of case reports
- Extrapleural solitary fibrous tumor of the thyroid gland:A case report and review of literature
- Delayed right coronary ostial obstruction after J-valve deployment in transcatheter aortic valve implantation:A case report
- Diverticulum of the buccal mucosa:A case report
