Crebanine N-oxide, a natural aporphine alkaloid isolated from Stephania hainanensis, induces apoptosis and autophagy in human gastric cancer SGC-7901 cells
Zheng-Wen Wang, Hao Liu, Geng-Tai Ye, Zhi-Yong Sheng, Yan-Feng Hu, Yin-Feng Tan, Guo-Xin Li✉
1Department of General Surgery, Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong, China
2Department of Hepatopancreatobiliary Surgery and General Surgery, Hainan Cancer Hospital, Affiliated Cancer Hospital of Hainan Medical University, Haikou 570312, Hainan, China
3Hainan Provincial Key Laboratory of R&D on Tropical Herbs, Hainan Medical University, Haikou 571199, Hainan, China
ABSTRACT Objective: To investigate the cytotoxic effects and the potential mechanisms of crebanine N-oxide in SGC-7901 gastric adenocarcinoma cells. Methods: The cytotoxicity of crebanine N-oxide was evaluated by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide assay and cellular morphology was observed under a microscope. Cell apoptosis was determined by flow cytometry using propidium iodide staining. The expression levels of apoptotic-related proteins, cleaved caspase-3, cytochrome C, p53 and Bax, and autophagyrelated proteins p62, beclin1 and LC3 were detected by Western blotting assays. Results: Crebanine N-oxide treatment significantly inhibited the proliferation of SGC-7901 cells in a dose-dependent and timedependent manner via induction of G2-phase cell cycle arrest, apoptosis, and autophagy in SGC-7901 cells.Conclusions: Crebanine N-oxide could inhibit the growth of gastric cancer cells by promoting apoptosis and autophagy and could be used as a potential agent for treating gastric cancer.
KEYWORDS: Crebanine N-oxide; Gastric cancer; SGC-7901 cells; Apoptosis; Autophagy
1. Introduction
Gastric cancer is a commonly diagnosed cancer worldwide and posed a serious threat to the public health[1]. In addition, it is also the leading cause of cancer deaths in China and ranks the third most common malignancy[2]. Currently, surgical resection and chemotherapy are the main treatments for gastric cancer. Although surgical resection is quite effective for patients with early-stage gastric cancers, most of the patients were diagnosed at the advanced stage and were not suitable for surgery[3]. So, chemotherapy has become the main treatment for patients with advanced-stage cancers or with recurrence. However, most chemotherapy drugs have side effects. Therefore, it is necessary to find more effective agents with lower toxicity[4,5]. Herbal medicine has been emerging as a promising approach for treating gastric cancer.
Crebanine N-oxide (Supplementary Figure 1) is a natural aporphine alkaloid isolated from Stephania hainanensis, which is mainly distributed in South China and is traditionally used to treat toothache, neuralgia, and stomach ache. Previous studies have proved that many herbal aporphine alkaloids have possessed potent anticancer activities[6,7]. They inhibited the growth of cancer cells via induction of cell cycle arrest or stimulation of apoptosis. Although several studies have demonstrated the cytotoxic effects of crebanine in cervical cancer cells[8], little is known about the effect and action mechanism of crebanine N-oxide in gastric cancer cells.
Apoptosis is a programmed cell death and is regarded as a major approach to eradicate tumors. Caspase-3, cytochrome C, p53 and Bax are the four key proteins involved in apoptosis. Many antitumor agents such as astragalin exert their cytotoxic effects through upregulating cleaved caspase-3, cytochrome C, p53 and Bax, and then stimulating cell apoptosis[9]. Besides apoptosis, autophagy, a highly conserved cellular self-digestion process, is also a novel action mechanism of anti-cancer including promoting autophagic cell death and inhibiting cancer growth[10,11]. Microtubule-associated protein light chain 3 (LC3), p62 and beclin 1 are the key regulating proteins and are broadly used as autophagy markers[12].
In the present study, we determined the cytotoxic activity of crebanine N-oxide in human gastric cancer SGC-7901 cells and explored its action mechanism.
2. Materials and methods
2.1. Cells, chemicals, and reagents
Human gastric cancer SGC-7901 cells and human gastric epithelial cells (GES-1) were stored by our lab. Crebanine N-oxide was obtained from the plant Stephania hainanensis by our lab. Its purity (>95%) was determined by HPLC method. Other reagents used in this research were purchased from suppliers indicated in the brackets: MTT (Sigma, M2128), 3-MA (MCE, HY-13259). Antibodies used in this research were also purchased from suppliers indicated in the brackets: β-actin (Santa Cruz, sc-1616), caspase 3 (Millipore, MAB4703), LC3B (MBL, PM036), beclin1 (Santa Cruz, sc-11427).
2.2. Cell viability assay
Cell viability was measured using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay as previously described[13]. The cultured SGC-7901 cells and GES-1 cells at the exponential growth phase were harvested from the culture flasks by trypsin and then suspended in fresh medium. The cell suspensions were dispensed into a 96-well microplate with about 10 000 cells/well. After 24 h, 100 µL fresh medium with various concentrations (0, 10, 20, 40, 80, 160, 320 µmol/L) of crebanine N-oxide was added into the GES-1 cells microplate and then incubated for 72 h. MTT assay was used to determine the cell proliferation after incubation. A total of 100 µL of MTT (5 mg/mL) was added into each, incubated for 4 h, and then the medium was removed from each well carefully. DMSO (150 µL) was added to each well. The absorbance was measured by a microplate readerg (MDC, Sunnyrale) at 490 nm. The rate of cell growth inhibition (I%) was calculated using the following equation: I% = (Acontrol- Atreated)/Acontrol×100. The IC50value that caused 50% inhibition of cell viabilities was calculated by the Logit method[14]. According to the results of MTT assay in GES-1 cells, various concentrations (0, 8, 16, 32, 64, 128 µmol/L) of crebanine N-oxide were added into the SGC-7901 cells microplate and then incubated for 24, 48 and 72 h. MTT assay was used to determine the cell proliferation as previously described.
2.3. Observation of morphological changes
SGC-7901 cells were equally seeded into 6-well plates (3.0×104cells/well) and incubated in RPMI 1640 media supplemented with 10% FBS at 37 ℃ in a humidified atmosphere under 5% CO2and 95% air for 24 h, then were treated with crebanine N-oxide at 0, 12.4, 24.8 and 49.6 µmol/L. After 24 h, 48 h, and 72 h, the morphology of SGC-7901 cells was observed under an inverted phase-contrast microscope. Photographs were then taken at a magnification of 400 times.
2.4. Cell cycle and apoptosis analysis by flow cytometry
The DNA content during cell cycle phases was determined by flow cytometry. SGC-7901 cells were treated with crebanine N-oxide at 0, 12.4, 24.8 and 49.6 µmol/L for 72 h, respectively. At the end of the treatment, both floating and attached cells were collected and centrifuged before, and then were washed twice with cold phosphatebuffered saline (PBS). The cells were then fixed in 70% cold ethanol overnight at 4 ℃. The fixed cells were washed twice with PBS to remove the traces of ethanol, resuspended in fluorochrome solution containing propidium iodide (PI, 50 µg/mL) and then incubated in the dark at room temperature for 30 min. Finally, the samples were subjected to flow cytometry (Dako Cytomation, Fort Collins, CO, USA) for cell cycle and apoptosis analyses. All experiments were performed in triplicate. At least 10 000 cells were used for each analysis, and the results were displayed as histograms. Cell cycle and apoptosis distribution were analyzed by multi-cycle Software[13].
2.5. Acidic vesicular organelles analysis by flow cytometry
SGC-7901 cells were treated with crebanine N-oxide at 0, 12.4, 24.8 and 49.6 µmol/L for 72 h, respectively. At the end of the treatment, the cells were collected, centrifuged, and then washed twice with cold PBS. SGC-7901 cells were resuspended in fluorochrome solution containing acridine orange (0.1 mg/mL) and then incubated in the dark at room temperature for 20 min[15]. Finally, the samples were subjected to flow cytometry (Dako Cytomation, Fort Collins, CO, USA). All experiments were performed in triplicate.
2.6. Western blotting assay
To determine the effect of crebanine N-oxide on the expression of apoptosis and autophagy-related proteins, SGC-7901 cells were incubated with crebanine N-oxide (0, 12.4, 24.8 and 49.6 µmol/L) and hydroxycamptothecin (HCPT, 4.96 µmol/L), an anticancer drug, for 72 h. After treatment, the whole protein was extracted to detect levels of cleaved caspase-3, p53, Bax, p62, beclin1, and LC3 and the cytoplasmic protein was extracted for Cyt-C. ECL plus kits (Amersham, Piscataway, NJ, USA) were used to analyze the protein bands. GAPDH was used as an internal reference for relative quantification. The immune reactive bands were quantified using ImageJ 4.1 software (NIH, Bethesda, MD, USA).
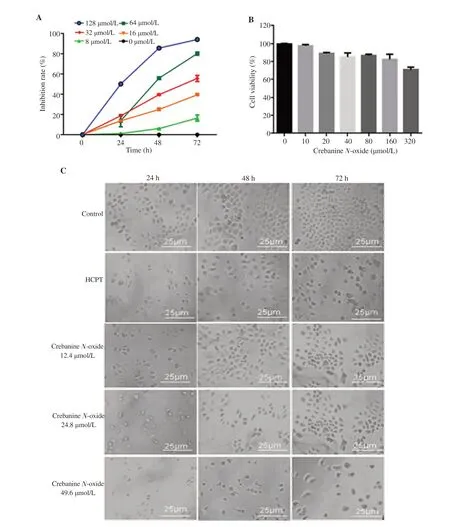
Figure 1. Effect of crebanine N-oxide on cell growth of human gastric cancer SGC-7901 cells and human gastric epithelial cells (GES-1). (A) Inhibition rate of SGC-7901 cells; (B) Cell viability of GES-1. HCPT: hydroxycamptothecin. All experiments were performed in triplicate; (C) SGC-7901 morphology under light microscopy at 400×; Scale bar: 25 μm.
2.7. Statistical analysis
The data are expressed as mean ± SD. Statistical analysis was performed using SPSS16.0. One-way analysis of variance (ANOVA) was used to analyze statistical differences between groups under different conditions. P<0.05 was considered to be statistically significant.
3. Results
3.1. Crebanine N-oxide inhibits viability of human SGC-7901 cells
Treatment with crebanine N-oxide for 24, 48, and 72 h decreased the cell viability in a dose-dependent manner (Figure 1A). The IC50value of crebanine N-oxide (170.41 µmol/L) was much lower than the anticancer drug HCPT (2 497.59 µmol/L) at 24 h but was similar with HCPT (crebanine N-oxide, 45.70 µmol/L; HCPT, 40.27 µmol/L) at 48 h, and higher than HCPT at 72 h. As the IC50value of crebanine N-oxide at 72 h was 24.8 µmol/L while that of HCPT was 4.96 µmol/L, 24.8 µmol/L was chosen as the main (middle) active dose for crebanine N-oxide with 12.4 and 49.6 µmol/L as the lower and higher doses in the subsequent experiments. Also, 4.96 µmol/L of HCPT was used as a positive control. All the three doses of crebanine N-oxide inhibited SGC-7901 cell proliferation at 24, 48, and 72 h (Figure 1C). The inhibitory effects of crebanine N-oxide at 24.8 µmol/L and above concentration were more potent than HCPT at 4.96 µmol/L. In contrast, 0-160 µmol/L crebanine N-oxide showed no obvious cytotoxicity in GES-1 cells ( Figure 1B).
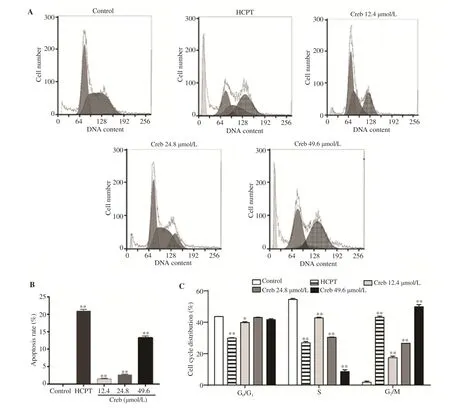
Figure 2. Effect of crebanine N-oxide on cell cycle arrest at G2/M phase in human gastric cancer SGC-7901 cells. (A) Flow cytometry assay after propidium iodide staining; (B) Apoptosis rate; (C) Percentage of cells at different phases. Creb: Crebanine N-oxide; HCPT: hydroxycamptothecin. All experiments were performed in triplicate. *P<0.05,**P<0.01 vs. vehicle control group.
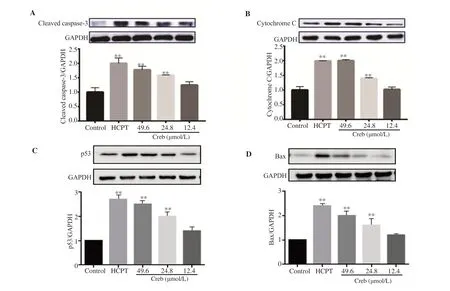
Figure 3. Effect of crebanine N-oxide on levels of cleaved caspase-3 (A), cytochrome C (B), p53 (C) and Bax (D) detected by Western boltting assay. Creb: Crebanine N-oxide; HCPT: hydroxycamptothecin. All experiments were performed in triplicate. **P<0.01 vs. vehicle control group.
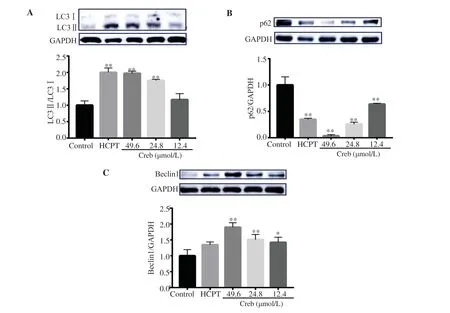
Figure 4. Effect of crebanine N-oxide on levels of autophagical marker proteins in human gastric cancer SGC-7901 cells detected by Western blotting assay. (A): LC3; (B): p62; (C): Beclin1. Creb: Crebanine N-oxide; HCPT: hydroxycamptothecin. All experiments were performed in triplicate. *P<0.05,**P<0.01 vs. vehicle control group.
3.2. Crebanine N-oxide induces cell cycle arrest at G2/M phase
As shown in Figure 2, crebanine N-oxide treatment increased the cell number at the G2/M phase and decreased the cell number at S phase in a dose-dependent manner, which was similar to that the effect of HCPT. However, crebanine N-oxide at 24.8 and 49.6 µmol/L showed no significant changes in the cell number at the G0/G1phase.
3.3. Crebanine N-oxide induces apoptosis in SGC-7901 cells
To verify whether crebanine N-oxide inhibits growth of SGC-7901 cells, flow cytometry with PI staining was performed. Figure 2B showed that crebanine N-oxide significantly induced apoptosis. The apoptotic rate of SGC-7901 cells treated with 12.4, 24.8 and 49.6 µmol/L crebanine N-oxide was (1.47±0.16)%, (2.59±0.22)% and (13.21±0.62)%, respectively. In addition, result of Western blotting assay displayed that cleaved caspase-3, cytochrome C, p53 and Bax were increased by crebanine N-oxide in a dose-dependent manner (Figure 3).
3.4. Crebanine N-oxide induces autophagy in SGC-7901 cells
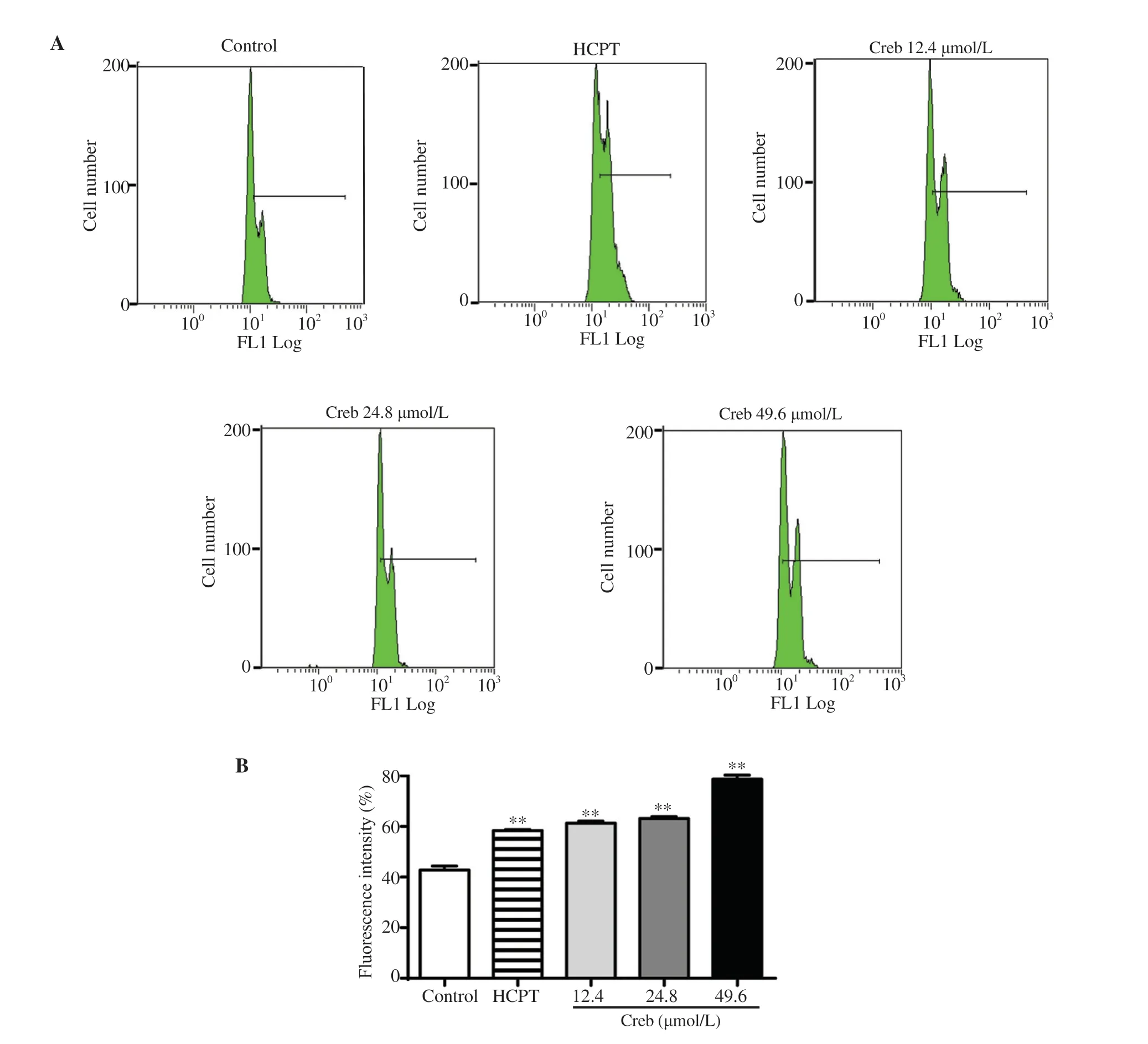
Figure 5. Effect of crebanine N-oxide on acidic vesicular organelles detection in human gastric cancer SGC-7901 cells. (A) Flow cytometry analysis after acridine orange staining. (B) Fluorescence intensity. Creb: Crebanine N-oxide; HCPT: hydroxycamptothecin. All experiments were performed in triplicate. **P<0.01 vs. vehicle control group.
To figure out if crebanine N-oxide resulted in autophagy, LC3Ⅱ/LC3Ⅰratio, p62, and beclin1 level were detected by Western blotting assay. The results showed that the LC3Ⅱ/LC3Ⅰratio, which is a classic marker for autophagy, was significantly increased after crebanine N-oxide treatment (Figure 4A). At the same time, the expression of p62 showed a dose-dependent decrease (Figure 4B), while beclin1 was increased (Figure 4C). Furthermore, the acidic vesicular organelles were measured by flow cytometry with acridine orange staining. Treatment with crebanine N-oxide significantly and dose-dependently increased the acidic vesicular organelles in SGC-7901 cells (Figure 5).
4. Discussion
Herbal active ingredients are a rich source of novel therapeutics for various diseases. It is stated that since 1990 more than half of the newly developed drugs are from natural compounds or their analogs. Massive studies have proved that many herbs and their active products possessed excellent anti-cancer properties[16]. For instance, researchers have confirmed anti-cancer effects of alkaloids that are widely distributed in Chinese traditional herbal medicines[17]. In the present study, we studied the anti-cancer effect of crebanine N-oxide, which is a natural aporphine alkaloid and isolated from Stephania hainanensis (H.S.Lo et Y.Tsoong). Previous study reported that various aporphine alkaloids exert anticancer effects by inhibiting cell proliferation and stimulating apoptosis[18,19]. However, the anti-cancer effect of crebanine N-oxide on gastric cancer cells has not been reported to date. Our study demonstrated that crebanine N-oxide has significant inhibitory effects on the growth of human gastric cancer SGC-7901 cells by inducing G2/M phase arrest, apoptotic cell death, and autophagic cell death.
Apoptotic cell death is the main anti-cancer mechanism and caspase-3 and cytochrome C play key roles[20]. Treatment with crebanine N-oxide significantly and dose-dependently increased the expression of cleaved caspase-3 and cytochrome C. The efficacy of crebanine N-oxide at 24.8 µmol/L and above concentration was comparable to that of HCPT at 4.96 µmol/L. Although apoptosis takes place independently of caspase-3 and cytochrome C in some cells, some studies still proved that activation of caspase-3 and cytochrome C is a prerequisite for tumor cell apoptosis[21]. The stimulating effect of crebanine N-oxide on caspase-3 and cytochrome C suggested that crebanine N-oxide might induce apoptosis in SGC-7901 cells through a mitochondrial-dependent intrinsic signaling pathway.
Many investigations have found close correlation between autophagy and apoptosis[22]. The Nomenclature Committee on Cell Death uses the term “autophagic cell death” to define cell death that is accompanied by substantial autophagic vacuolization in the cytoplasm and can be suppressed by inhibiting the autophagic pathway[23,24]. In the present study, we treated the cells with different concentrations of crebanine N-oxide, then detected the expression of intracellular autophagy marker proteins LC3, p62 and beclin1. Crebanine N-oxide suppressed the expression of p62 and increased the protein levels of beclin1 as well as LC3Ⅱ/LC3Ⅰ ratio, eventually stimulating the autophagy of SGC-7901 cells.
In conclusion, crebanine N-oxide decreases the cell viability in human gastric cancer SGC-7901 cells via the induction of G2-phage cell cycle arrest, apoptotic cell death, and autophagic cell death in concentration-dependent manners. Our findings unveil the molecular mechanism underlying anticancer activity of crebanine N-oxide and indicate that a therapy with crebanine N-oxide is a potential alternative for the treatment of gastric cancer.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This research was supported by the National Natural Science Foundation of China (NO.81760628).
Authors’contributions
WZW and LGX conceived and designed the work, analyzed data, wrote and revised the article, and finally approved of the version to be published. LH, YGT, SZY, HYF, and TYF collected and analyzed data.
 Asian Pacific Journal of Tropical Biomedicine2020年5期
Asian Pacific Journal of Tropical Biomedicine2020年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Emerging mosquito-borne arboviral infection Zika - An epidemiological review
- Methanolic extract of Clausena excavata promotes wound healing via antiinflammatory and anti-apoptotic activities
- Piperlongumine inhibits cell growth and enhances TRAIL-induced apoptosis in prostate cancer cells
- Pear pomace water extract reduces adiposity in vivo and in vitro by activating the AMPK-dependent pathway
- Diversity of Phlebotomine sand flies (Diptera: Psychodidae) in mountainous and plain areas of an endemic focus of anthroponotic cutaneous leishmaniasis in Iran
