Pear pomace water extract reduces adiposity in vivo and in vitro by activating the AMPK-dependent pathway
Mi-Kyoung You, Gwang-Woong Go, Hwa-Jin Kim, Jin Rhyu, Hyeon-A Kim✉
1Department of Nutrition and Health Sciences, University of Nebraska, Lincoln, NE, USA
2Department of Food and Nutrition, Hanyang University, Seoul, Republic of Korea
3Department of Food and Nutrition, Mokpo National University, Jeollanam-do, Republic of Korea
ABSTRACT Objective: To explore the inhibitory effect of water extract from pear pomace on abdominal fat accumulation and its underlying mechanism in high fat diet-fed animals. Methods: Three groups of male C57BL/6J mice were fed with a 60% kcal fat diet for 8 weeks. Pear pomace water extract (200 or 400 mg/kg body weight) was administered once daily via oral gavage. To confirm the possibility of the water extract of pear pomace acting as an activator of adenosine 5’-monophosphateactivated protein kinase (AMPK), differentiation of 3T3-L1 preadipocytes was induced in the presence of the water extract of pear pomace with or without compound C. Body weight, food efficacy ratio, insulin resistance, and adipogenic protein expression were measured. Moreover, in the 3T3-L1 cells, lipid content and lipogenesis-related proteins were measured using Oil Red O staining and Western blotting analysis.Results: Body weight gain and total abdominal fat weight were reduced in mice treated with pear pomace water extract. Pear pomace water extract reduced fasting blood glucose and insulin, thereby reducing the homeostatic model assessment of insulin resistance. It also resulted in dose-dependent decreases in triglyceride, total cholesterol, and low-density lipoproteincholesterol. The protein expression of p-AMPK increased, while the expression of AMPK-downstream proteins including PPAR-γ, C/EBPα, SREBP-1c, ACC, and FAS decreased in the adipose tissue of mice treated with pear pomace water extract. Furthermore, the inhibition of AMPK by compound C blocked pear pomace water extract-induced reduction of lipid content and the expression of lipogenesis-related genes. Conclusions: Pear pomace water extract prevents fat accumulation both in vivo and in vitro by activating AMPK.
KEYWORDS: Pear pomace; AMPK; Compound C; Obesity; Insulin resistance
1. Introduction
Obesity is characterized as excessive body weight by hypertrophy and hyperplasia of adipocytes[1,2]. Obesity induces adipose and hepatic inflammation, thereby inducing insulin resistance and leading to hyperglycemic and hyperlipidemic conditions[3,4]. Adipocytes play important roles in the regulation of adipose mass and obesity, with respect not only to lipid homeostasis but to the secretion of transcription factors[5,6].
Adenosine 5’-monophosphate-activated protein kinase (AMPK) is a ubiquitously expressed serine/threonine-protein kinase. When activated by phosphorylation, AMPK promotes energy-producing processes such as fatty acid oxidation and glucose uptake[7,8], whereas it suppresses energy-consuming processes such as lipogenesis and triglyceride (TG) synthesis[9,10]. The evidence from AMPK-deficient models suggests AMPK is a latent nutraceutical target for obesity. It is well-established that AMPK is involved in the control of the central regulators of adipocyte differentiation[11]. Furthermore, AMPK plays a crucial role in the control of body fat stores[12,13]. Therefore, the identification of compounds that activate AMPK would substantially contribute to obesity and obesity-related health problems.
Different approaches, beyond diet therapy and exercise, have been recommended for treating obesity, including the use of drugs to promote weight loss. However, some anti-obesity drugs have been shown to cause serious adverse effects including pulmonary hypertension, oily stool, and increased heart rates[14]. Consequently, natural products that regulate the expression of proteins involved in the control of lipogenesis and TG accumulation have attracted much attention. It has been actively emerging that herbal medicine and functional food ingredients can exert beneficial effects in the treatment of obesity[5,15-17].
Pear pomace is a residual product produced during pear processing. Pears have been reported as a potential source for polyphenols, exhibiting excellent antioxidant capacity[18,19]. Among the polyphenols in pears, chlorogenic acid is predominant, followed by p-coumaric acid, ferulic acid, gallic acid, and vanillic acid[20]. Caffeic acid and its ester, chlorogenic acid, significantly reduced body weight and improved lipid metabolism in high-fat diet (HFD) induced obese mice[21].
In our previous study, we showed that pear pomace water extract suppressed adipocyte differentiation, and induced apoptosis in 3T3-L1 cells[5]. However, the animal study and the detailed anti-obesity signaling pathway have not been clearly explored. In this study, we focused on evaluating the impact of pear pomace water extract on weight gain, lipid accumulation, and insulin resistance by using HFD-fed mice. Furthermore, we elucidate if AMPK was involved in pear pomace water extract-induced inhibition of lipid accumulation.
2. Materials and methods
2.1. Reagents
High-fat diet (60% kcal from fat) was purchased from Research Diets Inc (New Brunswick, NJ, USA). Detection kits of serum triglyceride (TG), total cholesterol (TC), and LDL-cholesterol (LDL-C) were purchased from Beckman Coulter Inc (Brea, Cal, USA). Kits of fasting blood glucose and insulin were supplied by Allmedicus (Gyeonggi-do, Republic of Korea) and Shibayagi Co. Ltd (Gumma Pref, Japan), respectively. Dulbecco’s modified eagle’s medium, newborn calf serum, and fetal bovine serum were purchased from Gibco (BRL, NY, USA). Oil Red O (ORO), compound C {6-[4-(2-piperidin-1-yl-etoxy)-phenyl]-3-pyridin-4-yl-pyrrazolo [1, 5-α]pyrimidine}, isobutylmethylxanthine, insulin, and dexamethasone were obtained from Sigma (St. Louis, MO, USA). Primary antibodies against AMPK, p-AMPKThr172, PPAR-γ, FAS, ACC, and p-ACCSer79were obtained from Cell Signaling Technology (Beverly, MA, USA). C/EBPα, SREBP-1c, and β-actin were also purchased from Santa Cruz Biotechnology Inc (Dallas, Tex, USA). Horseradish peroxidase-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Inc (West Grove, PA, USA). A Western blot enhanced chemiluminescence kit was purchased from IMGENEX (San Diego, CA, USA).
2.2. Preparation of pear pomace water extract
In brief, pears were washed, de-seeded, and then the juice was extracted using juice extractor (Duckwoo machinery, Jeollanam-do, Republic of Korea). Pear pomace, the juice extract cake from pears, was freeze-dried (Freeze Dry System, IlshinBioBase, Gyeonggi-do, Republic of Korea) and ground to powder. Powdered pear pomace was provided by Good F&B Inc. Pear pomace (100 g) was then soaked in 2 L of water for 24 h at room temperature. The extract was filtered under reduced pressure, evaporated and freeze-dried. The yield obtained was 32.6%. Pear pomace water extract was dissolved in distilled water and stored at -20 ℃ until further use.
2.3. Animal treatment
Male C57BL/6J mice (5 weeks old) were purchased from Central Lab. Animal Inc. (Seoul, Republic of Korea). All mice were housed in a climate-controlled room [(22±2) ℃, (50±10)% relative humidity]under a 12 h light/dark cycle. After acclimation for a week, mice were randomly divided into three groups (n=10) and fed with HFD (60% kcal from fat, AIN-93G) for 8 weeks. Based on previous study[19], the mice were administered with pear pomace water extract (200 or 400 mg/kg body weight dissolved in distilled water) by oral gavage once a day. Change in the body weight and food intake of all mice was monitored weekly. The food efficacy ratio was calculated as the weight gain/food intake during the experimental period.
2.4. Tissue preparation
After 8 weeks, the mice were fasted for 8 h and then euthanized for tissue collection. Their blood was collected through cardiac puncture. The blood was centrifuged at 3 000 rpm for 20 min at 4 ℃ to yield serum fraction, which was used for subsequent analyses. Abdominal adipose tissue and liver were also collected, weighted, and stored at -70 ℃ until further analyses.
2.5. Biochemical analysis of blood samples
Fasting blood glucose level was measured using a commercial kit. The serum insulin concentrations were also determined using a commercial kit according to the instructions. Serum TG, TC, and LDL-C were enzymatically analyzed using Automated Chemistry Analyze. The index of insulin resistance (IR), also called HOMAIR, was calculated based on the original model from Matthews et al.: fasting insulin (mU/L) × fasting glucose (mM)/22.5[22,23].
2.6. Cell culture study
3T3-L1 cells were purchased from American Type Culture Collection (ATCC). 3T3-L1 cells were cultured in Dulbecco’s modified eagle’s medium containing 10% newborn calf serum and differentiated to adipocytes using the protocol previously established[5]. Compound C was dissolved in dimethyl sulfoxide and was pretreated (defined as day 0) for 1 h. Besed on previous study[5], 100 or 250 µg/mL pear pomace water extract was administered to the culture medium from day 0 to day 8. According to the results of cell viability, up to 250 µg/mL showed no cytotoxicity (data not shown). Therefore, 250 µg/mL was chosen for evaluation of lipid content of 3T3-L1 cells. The effect of pear pomace water extract on the lipid content of 3T3-L1 cells was assessed using Oil Red O staining as reported[5].
2.7. Western blotting analysis
AMPK plays a critical role in cellular sensors, including fatty acid oxidation, ketogenesis, glucose uptake, cholesterol synthesis, and insulin secretion. In particular, AMPK is an inhibitor of adipocyte differentiation and TG accumulation. To investigate the effect of pear pomace water extract on the AMPK activation, we determined the protein expression of adipogenesis genes in abdominal adipose tissue and cell pellets using Western blotting. The sample was homogenized in RIPA buffer with protease and phosphatase inhibitor cocktail. Total protein was quantified using a Bradford protein assay (Sigma, St. Louis, MO, USA). The protein (15 µg) was separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking, the membranes were incubated with primary antibodies against AMPK, p-AMPKThr172, PPAR-γ, FAS, ACC, p-ACCSer79, C/EBPα, SREBP-1c, and β-actin at 4 ℃ overnight. The membranes were incubated with a horseradish peroxidase-conjugated secondary antibody. The membranes were washed, and proteins were detected using a chemiluminescence reagent. The protein expression was quantified using UVP (image system, Upland, CA, USA) and Vision Works TMLS (Analysis software, Upland, CA, USA)[5].
2.8. Statistical analysis
Statistical analysis of all data was conducted using SPSS (ver 23.0, SPSS Inc, Chicago, IL, USA). All in vitro studies were carried out in triplicate. Multiple comparisons between groups were performed by Duncan’s multiple range test in conjunction with one-way ANOVA. Data are expressed as mean ± SE, and statistical significance is defined as P-value < 0.05.
2.9. Ethical statement
Procedures involving animal care were conducted in conformity with the institutional guidelines of Mokpo National University, Republic of Korea (ethics committee in Mokpo National Univ.; 20 August 2014; MNU-IACUC-2014-002).
3. Results
3.1. Pear pomace water extract suppressed fat accumulation in HFD-fed mice
Pear pomace water extract significantly reduced body weight compared to the control group. There was a significant reduction in body weight after 4 weeks of pear pomace water extract (400 mg/kg) treatment in the HFD-fed mice in comparison to the control (Figure 1A). Moreover, body weight gain was markedly decreased in pear pomace water extract-fed mice (Figure 1B). As expected, abdominal adipose tissue weight was significantly lower in pear pomace water extract-fed mice than that in the control group (Figure 1C). In the sixth week of the experiment, the food intake of mice treated with pear pomace water extract was significantly reduced compared with that in the control group (Figure 1D). Additionally, pear pomace water extract also remarkably decreased food efficacy ratio (Figure 1E) and relative liver weight (Figure 1F). Taken together, these data indicated that pear pomace water extract decreased body weight and abdominal adipose tissue weight in HFD-fed mice.
3.2. Pear pomace water extract reduced insulin resistance and lipid level in HFD-fed mice
Pear pomace water extract at a dose of 200 mg/kg/day reduced the levels of fasting blood glucose and insulin (165.80 mg/dL, P<0.01; 32.11 µIU/mL, P<0.05) (Figure 2A and B). HOMA-IR was decreased at 200 and 400 mg/kg/day of pear pomace water extract (Figure 2C). In addition, serum TG, TC, and LDL-C concentrations in mice treated with pear pomace water extract were reduced in a dose-dependent manner (Figure 2D, E, and F).
3.3. Pear pomace water extract inhibited the protein expressions of adipogenesis gene in abdominal adipose tissue
As shown in Figure 3, pear pomace water extract increased the level of p-AMPK at Thr172, which is the activated form, in a dose-dependent manner. In accordance with the activation of AMPK by pear pomace water extract, pear pomace water extract led to decreased expressions of FAS, ACC, SREBP-1c, PPAR-γ, and C/EBPα (Figure 3).
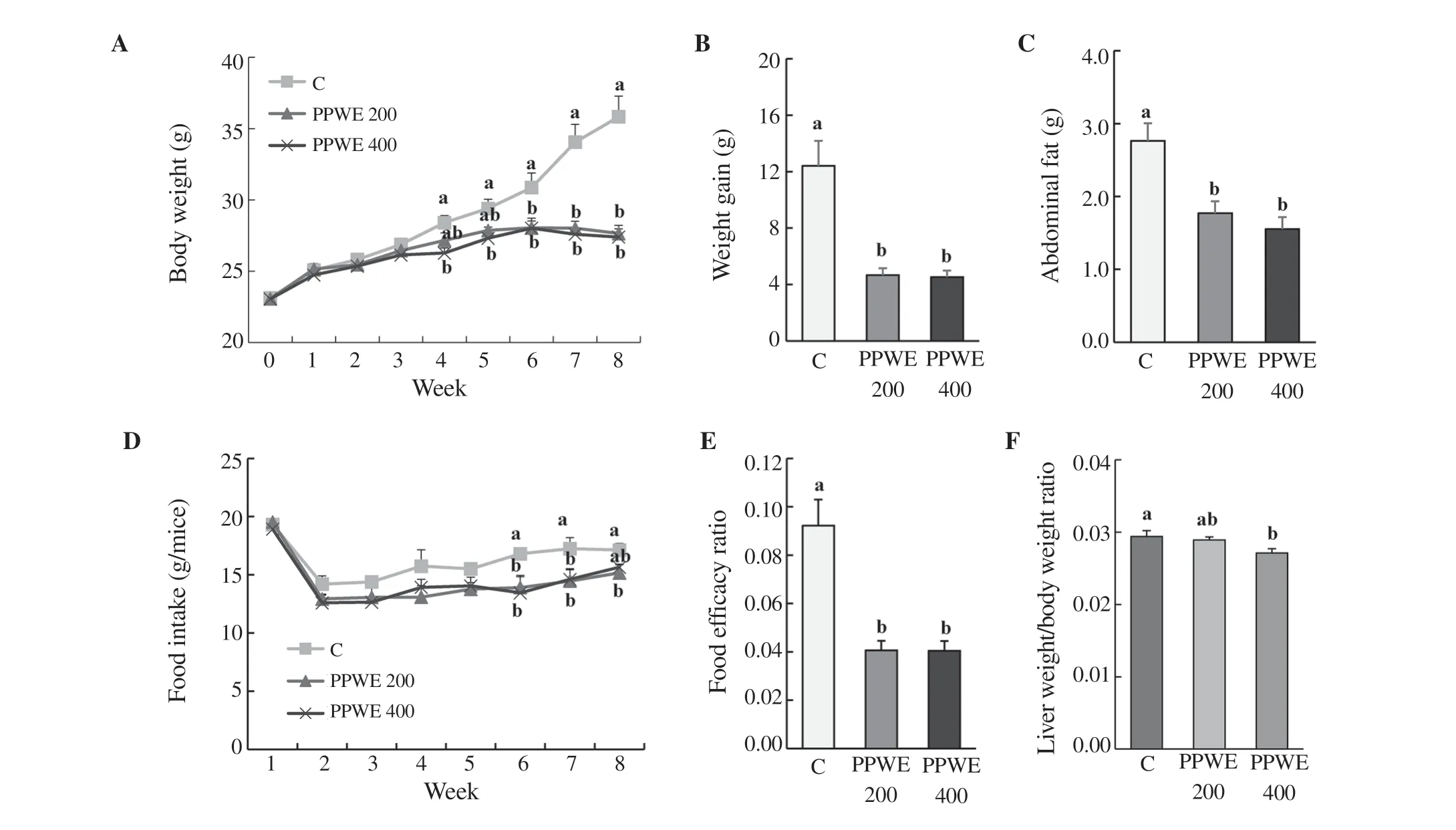
Figure 1. Effect of pear pomace water extract (PPWE) on the body and abdominal fat weight, food intake, food efficacy ratio, and relative liver weight in HFDfed mice. Mice were administered with distilled water, PPWE 200 or 400 mg/kg body weight for 8 weeks. Abdominal adipose tissue and liver were collected and weighted. (A) Body weight, (B) weight gain, (C) abdominal adipose tissue weight, (D) food intake, (E) food efficacy ratio, (F) relative liver weight. Means with different letters are significantly different by Duncan’s multiple range test (P<0.05).
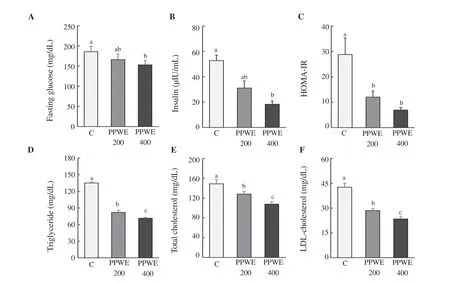
Figure 2. Effect of pear pomace water extract (PPWE) on blood biomarker in HFD-fed mice. For blood biomarker, blood was collected and measured using kit. (A) Fasting glucose, (B) insulin, (C) HOMA-IR, (D) triglyceride, (E) total cholesterol, (F) LDL-C. Means with different letters are significantly different by Duncan’s multiple range test (P<0.05).
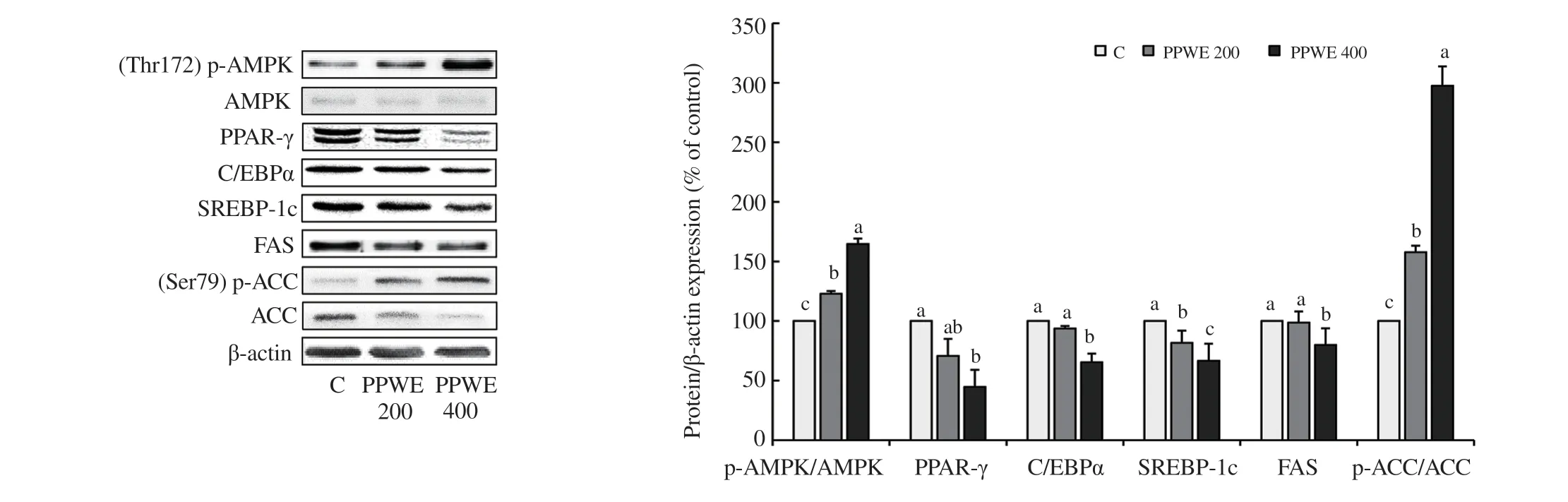
Figure 3. Effect of pear pomace water extract (PPWE) on protein expression of p-AMPK/AMPK, C/EBPα, PPAR-γ, SREBP-1c, FAS, and p-ACC/ACC in the adipose tissues of HFD-fed mice. Adipose tissues were lysed to measure the expression of AMPK pathway using Western blotting. The protein expression levels of AMPK, p-AMPK, PPAR-γ, C/EBPα, SREBP-1c, FAS, p-ACC, and ACC were examined by immunoblotting. Immunoblotting for β-actin verified equal loading of proteins. Means with different letters are significantly different by Duncan’s multiple range test (P<0.05).
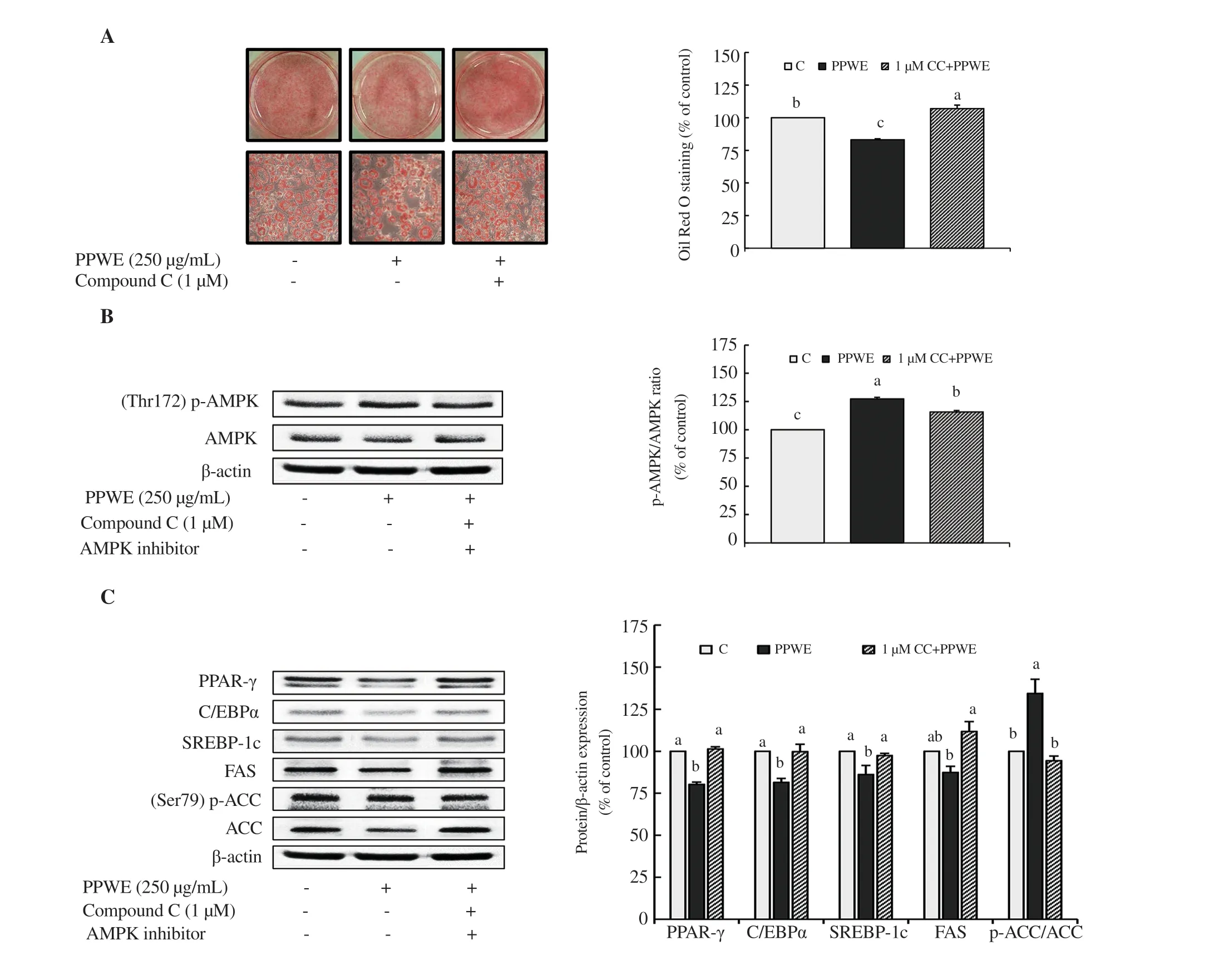
Figure 4. AMPK-dependent modulation by pear pomace water extract (PPWE) in 3T3-L1 cells. 3T3-L1 cells were pretreated with compound C for l h, and then differentiated with PBS or PPWE 250 µg/mL for 8 d. (A) Lipid content, (B) Protein expression of p-AMPK/AMPK, (C) Protein expression of C/EBPα, PPAR-γ, SREBP-1c, FAS, and p-ACC/ACC. Means with different letters are significantly different by Duncan’s multiple range test (P<0.05). C: PBS, PPWE: pear pomace water extract (250 µg/mL), 1 µM CC + PPWE: pear pomace water extract 250 µg/mL with 1 µM compound C.
3.4. Pear pomace water extract-induced modulation of adipogenesis was blocked by compound C in 3T3-L1 cells
Based on observations that pear pomace water extract increased AMPK expression (Figure 3), we hypothesized that pear pomace water extract inhibits lipid accumulation via activation of the AMPKdependent pathway. We tested 3T3-L1 cells with compound C, a usually used cell-permeable AMPK inhibitor. We applied compound C in vitro to recover a metabolic pathway regulated by pear pomace water extract at 250 µg/mL. Oil Red O staining data revealed that compound C reversed pear pomace water extract-induced downregulation in lipid accumulation (Figure 4A). Consistent with the increase of lipid accumulation, compound C abolished the upregulation of p-AMPK expression by pear pomace water extract in 3T3-L1 cells (Figure 4B). Figure 4C shows compound C reversed the changes on PPAR-γ, C/EBPα, SREBP-1c, FAS, and ACC induced by pear pomace water extract. Taken together, these results suggested that pear pomace water extract regulates adipogenesis by activating the AMPK-dependent pathway.
4. Discussion
Obesity is associated with hyperplasia and/or hypertrophy of triglyceride-rich adipocytes and increased ectopic fat deposition. Prevalence of obesity and metabolic syndrome including dyslipidemia and type Ⅱ diabetes is rapidly rising. We hypothesized pear pomace water extract attenuates abdominal fat accumulation in HFD-fed animals, and we explored its mechanism of action in the current study.
We observed that pear pomace water extract decreased body weight gain and abdominal adipose tissue weight. Pear pomace water extract reduced food intake and food efficacy ratio, indicating mice treated with pear pomace water extract showed less weight gain with the same amount of food intake compared to the control. In the animal model, HFD feeding leads to an elevation of fasting blood glucose and insulin accompanied by an increased HOMA-IR index[24]. Obesity is generally accompanied by insulin resistance, which plays a key role in the regulation of lipid homeostasis[25,26]. In the present study, pear pomace water extract showed hyperinsulinemiaimproving effect in HFD-fed animals, which was supported by the decreased HOMA-IR. We demonstrated that 50% ethanol extract of pear pomace improved insulin resistance through enhancement of insulin signaling pathway[27]. Moreover, pear pomace water extract showed a decrease in serum lipids including TG, TC, and LDL-C. Similarly, a recent study demonstrated pear peel extract with mixed methanol/water (6:4) significantly reduced serum TC, TG, and LDL-C, but increased serum HDL-C in STZ-induced diabetic mice[20].
To elucidate the action mechanism for pear pomace water extractinduced anti-lipogenic effect, we examined the effect of pear pomace water extract on the expression and the phosphorylation of AMPK. AMPK is a “fuel gauge” used to maintain intracellular and body energy balance. In the present study, pear pomace water extract showed an increase in activated AMPK, thereby, increasing the expression ratio of p-AMPK/AMPK in the adipose tissue. Pear pomace water extract also downregulated the expression of PPAR-γ and C/EBPα, the regulators of adipocyte differentiation and lipogenic gene expression such as FAS[28]. AMPK is reported to downregulate the expression of PPAR-γ and C/EBPα[11,29]. Meanwhile, Foretz et al.[30]revealed that activation of AMPK mediates the suppression of lipogenic gene expressions such as FAS and ACC via the decreased action of the transcription factor SREBP-1c. SREBP-1c is a critical transcription factor that stimulates several lipogenic enzymes involved in de novo lipogenesis, fatty acid modification, and triglyceride synthesis[31]. Furthermore, ACC is also one of the first proteins identified as a target of AMPK. Qian et al.[31]reported that increased gene expression and the phosphorylation of AMPK by the phenolic acid fraction from Astragali rhadix suppressed gene expression of ACC and FAS without downregulation of SREBP-1c in 3T3-L1 cells. The results shown here clearly indicate that pear pomace water extract downregulates the expression of SREBP-1c as well as FAS and ACC in the adipose tissue of HFD-fed mice. Therefore, the results of our animal study show that pear pomace water extract inhibits body weight gain and fat accumulation in adipose tissue accompanied by improving insulin resistance and hyperlipidemia, and its anti-obesity effect may be associated with activation of the AMPK signaling pathway.
To confirm the involvement of AMPK signaling pathway in the anti-obesity effect of pear pomace water extract, ORO staining, and metabolic pathway analysis were conducted by differentiating 3T3-L1 cells in the presence of pear pomace water extract with or without compound C. Compound C is a potent and highly selective inhibitor of AMPK, however, it does not display significant inhibition of several structurally-related kinases such as PKA and JAK3[32]. The ORO staining showed the inhibition of AMPK activity by compound C blocked the pear pomace water extract-induced reduction in lipid accumulation. Compound C abolished the reduction of adipocyte differentiation markers induced by pear pomace water extract, including PPAR-γ, C/EBPα, and SREBP-1c. Likewise, diminished levels of lipogenic enzymes including FAS and ACC were recovered by compound C treatment.
The beneficial effects of pear pomace water extract can be largely ascribed to phenolic compounds. The phenolic compounds in pear pomace water extract totaled 3.9 mg/g according to our preliminary studies. Polyphenols including chlorogenic acid, coumaric acid, and caffeic acid have been known to stimulate AMPK phosphorylation, followed by inhibiting ACC[33-35]. Interestingly, pear pomace water extract showed a more potent inhibitory effect on lipid accumulation in 3T3-Ll cells than 50% ethanol extract of pear pomace[27], even though the contents of caffeic and chlorogenic acid in pear pomace water extract were lower than those in pear pomace ethanol extract[36]. Therefore, the bioactive compounds in pear pomace water extract which show anti-obesity effects need to be further examined. In conclusion, pear pomace water extract reduced body weight gain, body fat accumulation, and lipogenic enzymes by enhancing the AMPK pathway in HFD-fed mice. These in vivo data were further confirmed by in vitro observation. Compound C abolished anti-adipogenic effects of pear pomace water extract in the 3T3-L1 cells. Consequently, both in vivo and in vitro data suggest that pear pomace water extract reduces the adiposity by activation of the AMPK signaling pathway. This study provides new insight into the possibility of pear pomace water extract being applied as an antiobesity agent.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This research was supported by the Bio-Industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (313019-09-3-HD030).
Authors’contributions
HAK developed theoretical formalism, contributed to the original, revision, and final version of the manuscript, as well as supervised the project. MKY performed the analytic calculation and formal analysis, and contributed to the original, revision, and final version of the manuscript. GWG contributed to the original version of the manuscript. Both HJK and JR performed the analytic calculation and formal analysis.
 Asian Pacific Journal of Tropical Biomedicine2020年5期
Asian Pacific Journal of Tropical Biomedicine2020年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Emerging mosquito-borne arboviral infection Zika - An epidemiological review
- Methanolic extract of Clausena excavata promotes wound healing via antiinflammatory and anti-apoptotic activities
- Crebanine N-oxide, a natural aporphine alkaloid isolated from Stephania hainanensis, induces apoptosis and autophagy in human gastric cancer SGC-7901 cells
- Piperlongumine inhibits cell growth and enhances TRAIL-induced apoptosis in prostate cancer cells
- Diversity of Phlebotomine sand flies (Diptera: Psychodidae) in mountainous and plain areas of an endemic focus of anthroponotic cutaneous leishmaniasis in Iran
