A Simple Synthetic Route for Coordinated Amidine Complexes:a Series of New Anti-tumor Drugs
LIAO Ai-ling ZHOU Xin LI Xiao-dong XIANG Jing
(1.School of Chemistry and Environmental Engineering, Yangtze University,Jingzhou 434020, China;2.Shenzhen Nanshan People's Hospital, Shenzhen 518052, China)
Abstract In this context, we have provided a facile synthetic route to synthesize the coordinated amidine compounds via the in-situ reactions of various amines with [Ru(salchda)(H2O)2](PF6) (1) in various nitrile solvents.All the compounds obtained have been well characterized by IR, UV/Vis, CV, ESI/MS, and satisfactary elemental analysis.The crystal structrues of a bis(amine) compound and a bis(amidine) ruthenium(III) compound have also determined by X-ray crystallography.These compounds are potential anti-tumor drugs.
Key words amidine complexes; in-situ synthesis; ruthenium complexes; anti-tumor drug
0 Introduction
Amidine is a fundamental unit in medicinal and synthetic chemistry that is widely applied in pharmaceutical science and metal complexation ligands[1-5].In the past years, great efforts have been focused on developing various new methods to prepare amidine compounds.Although the coordination chemistry of amidine and/or amidinate complexes have been well developed[6-10], ruthenium amidine complexes remain scarce.However, recent studies have revealed that some amidine (salen)Ru(III) complexes have potential anti-tumor properties[11].This series of complexes are cytotoxic against various cancer cell lines, and some complexes have remarkable cancer-cell selectivity.However, the different substituents on the ligand’s structures have significant effect on their anti-tumor properties[12].Thus, the search for a facile synthetic route to obtain various functionalized amidine complexes is necessary.Herein, we demonstrate that the coordinated nitrile ligands could be readily activated by the (salchda)Ru(III) moiety, which could further react with various amines to generate (salcha)Ru(III) amidine complexes with different substituents.
1 Experimental
1.1 Reagents and instrumentation
The compoundtrans-[RuIII(salchda)(H2O)2]PF6(1) was synthesized according to literature methods.[13][nBu4N]PF6(Aldrich) for electrochemistry was recrystallized three times from ethanol and dried under vacuum at 120 °C for 24 h.Acetonitrile (Aldrich) for electrochemistry was distilled from calcium hy dride.All other chemicals were of reagent grade and used without further purification.All manipulations were performed without the precaution of excluding air or moisture unless otherwise stated.IR spectra were recorded as KBr discs with a Nicolet 360 FTIR spectrophotometer.UV/Vis spectra were recorded with a Perkin-Elmer Lambda 19 spectrophotometer in 1 cm quartz cuvettes.Elemental analysis was performed with an Elementar Vario EL Analyzer.Electrospray ionization mass spectrometry (ESI-MS) was performed with a PE-SCIEX API 365 triple quadruple mass spectrometer.
1.2 X-ray Crystallography
The data collection for7and8were carried out with an Oxford CCD diffractometer using graphite-monochromated Mo-Kαradiation (λ=0.71073 Å).Details of the intensity data collection and crystal data are given in Tables 2 and 3.Absorption corrections were carried out by the multi-scan method.The structures were resolved by the heavy atom Patterson method or direct methods and refined by full-matrix least-squares methods by using SHELX-97[14]and expanded by using Fourier techniques.All non-hydrogen atoms were refined anisotropically.Hydrogen atoms except H1 were generated by the program SHELXL-97.The positions of the hydrogen atoms were calculated on the basis of a riding model with thermal parameters equal to 1.2 times those of the associated carbon atoms and used in the calculation of the final R indices.All calculations were performed by using the teXsan[15]crystallographic software.
1.3 Synthesis and characterization
[RuIII(Salchda)(NHC(NHCH2CH3)CH3)2](PF6) (2).1(100 mg, 0.16 mmol) was dissolved in about 25 mL CH3CN and the solution was refluxed for about 0.5 hour and then ethylamine (0.23 mL, 3.52 mmol) was added into this solution for further refluxed overnight.The solution was concentrated toca. 1 mL.Slow addition of diethyl ether gave a green precipitate which was recrystallized from CH2Cl2/diethyl ether.Yield: 56%.IR (KBr, cm-1):ν(N-H) 3361, 3283;ν(CN) 1633, 1600;ν(P-F) 839.Anal.Calcd.for C28H40N6O2PF6Ru: C, 45.53; H, 5.46; N, 11.38.Found: C, 45.37, H, 5.37, N, 11.19.UV/Vis (CH3CN):λmax[nm] (ε[mol-1dm3cm-1]) 214(58598), 350(18076), 385(21084), 3613(4067).ESI-MS:m/z=594 (M+).μeff=1.949 μB.
[RuIII(Salchda)(NHC(NHCH(CH3)2)CH3)2](PF6) (3).1(100 mg, 0.16 mmol) was dissolved in about 25 mL CH3CN and the solution was refluxed for about 0.5 hour and then isopropylamine (0.30 mL, 3.52 mmol) was added into this solution for further refluxed overnight.The solution was concentrated toca. 1 mL.Slow addition of diethyl ether gave a green precipitate which was recrystallized from CH2Cl2/diethyl ether.Yield: 68%.IR (KBr, cm-1):ν(N-H) 3364;ν(CN) 1632, 1596;ν(P-F) 838.Anal.Calcd.for C30H44N6O2PF6Ru: C, 46.99; H, 5.78; N, 10.96.Found: C, 46.95; H, 5.72; N, 10.80.UV/Vis (CH3CN):λmax [nm] (ε[mol-1dm3cm-1]) 214(57846), 349(19249), 383(21102), 614(3577).ESI-MS:m/z=622 (M+).μeff=1.905 μB.
[RuIII(Salchda)(NHC(NHC6H11)CH3)2](PF6) (4).1(100 mg, 0.16 mmol) was dissolved in about 25 mL CH3CN and the solution was refluxed for about 0.5 h and then cyclohexylamine (0.41 mL, 3.52 mmol) was added into this solution for further refluxed overnight.The solution was concentrated toca.1 mL.Slow addition of diethyl ether gave a green precipitate which was recrystallized from acetone/diethyl ether.Yield: 72%.IR (KBr, cm-1):ν(N-H) 3363, 3272;ν(CN) 1631, 1613;ν(P-F) 840.Anal.Calcd.for C36H52N6O2PF6Ru: C, 51.06; H, 6.19; N, 9.92.Found: C, 51.06; H, 6.03; N, 10.13.UV/Vis (CH3CN):λmax [nm] (ε[mol-1dm3cm-1]) 214(62497), 348(22006), 384(23698), 611(4027).ESI-MS:m/z=702 (M+).μeff=1.926 μB.
[RuIII(Salchda)(NHC(NHCH2Ph)CH3)2](PF6) (5).1(100 mg, 0.16 mmol) was dissolved in about 25 mL CH3CN and the solution was refluxed for about 0.5 hour and then benzylamine (0.39 mL, 3.52 mmol) was added into this solution for further refluxed under argon overnight.The solution was concentrated toca.1 mL.Slow addition of diethyl ether gave a green precipitate which was recrystallized from CH2Cl2/diethyl ether.Yield: 68%.IR (KBr, cm-1):ν(N-H) 3358;ν(CN) 1630, 1603;ν(P-F) 837.Anal.Calcd.for C38H44N6O2PF6Ru: C, 52.90; H, 5.14; N, 9.74.Found: C, 53.25; H, 4.96; N, 9.44.UV/Vis (CH3CN):λmax [nm] (ε[mol-1dm3cm-1]) 349(21608), 384(22248), 619(3604).ESI-MS:m/z=718 (M+).μeff=1.909 μB.
[RuIII(Salchda)(NHC(NHCH2CH3)Ph)2](PF6) (6).Benzylnitrile (0.36 mL, 3.52 mmol) was added to a solution of1(100 mg, 0.16 mmol) in tetrahydrofuran (15 mL) and the solution was refluxed for about 0.5 h and then ethylamine (0.23 mL, 3.52 mmol) was added into this solution for further refluxed overnight.The solution was concentrated toca.1 mL.Slow addition of diethyl ether gave a green precipitate which was recrystallized from CH2Cl2/diethyl ether.Yield: 46%.IR (KBr, cm-1):ν(N-H) 3363, 3272;ν(CN) 1631, 1613;ν(P-F) 840.Anal.Calcd.for C38H44N6O2PF6Ru: C, 52.90; H, 5.14; N, 9.74.Found: 52.88; H, 5.21; N, 9.70.UV/Vis (CH3CN):λmax [nm] (ε[mol-1dm3cm-1]) 218(43742), 231(40575), 352(15352), 385(17636), 633(3806).ESI-MS:m/z=718 (M+).μeff=2.048 μB.
[RuIII(Salchda)(NHC(NHCH(CH3)2)Ph)2](PF6) (7).Benzylnitrile (0.36 mL, 3.52 mmol) was added to a solution of1(100 mg, 0.16 mmol) in tetrahydrofuran (15 mL) and the solution was refluxed for about 0.5 hour and then isopropylamine (0.30 mL, 3.52 mmol) was added into this solution for further refluxed overnight.The solution was concentrated toca.1 mL.Slow addition of diethyl ether gave a green precipitate which was recrystallized from CH2Cl2/diethyl ether.Yield: 63%.IR (KBr, cm-1):ν(N-H) 3341, 3248;ν(CN) 1618, 1601;ν(P-F) 841.Anal.Calcd.for C40H48N6O2PF6Ru: C, 53.93; H, 5.43; N, 9.43.Found: C, 54.77; H, 5.81; N, 9.60.UV/Vis (CH3CN):λmax [nm] (ε[mol-1dm3cm-1]) 217(68320), 348(23320), 384(23686), 625(3995).ESI-MS:m/z=746(M+).μeff=2.057μB.
[RuIII(Salchda)(NH2CH2Ph)2](PF6) (8).Benzylamine (0.39 mL, 3.52 mmol) was added to a solution of1(100 mg, 0.16 mmol) in acetone (15 mL).The green solution was refluxed for 1.5 h under argon and then concentrated toca.1 mL.Slow addition of diethyl ether into the solution gave a green precipitate, which was recrystallized from CH2Cl2/diethyl ether.Yield: 72%.IR (KBr, cm-1):ν(N-H) 3312, 3255;ν(CN) 1596; ν(P-F) 839.Anal.Calcd.for C34H38N4O2PF6Ru: C, 52.31; H, 4.91; N, 7.18.Found: C, 52.01; H, 4.92; N, 7.03.UV/Vis (CH3CN):λmax[nm] (ε[mol-1dm3cm-1]) 210(56000), 234(38400), 360(14700), 385(18800), 643(4110).ESI-MS:m/z=636 (M+).μeff=2.03μB.
2 Result and discussion
The diaqua compound,trans-[RuIII(salchda)(H2O)2]PF6(1), was synthesized as the previously reported procedure.Reactions of1with various excess amines in ethanol or acetone under Ar afforded varioustrans-[RuIII(salchda)(amine)2]+compounds in high yields, which were formed by direct substitution of the aqua ligands by amines.However, when CH3CN were used as solvent instead of ethanol/acetone, the reactions of1with amines gave various amidine compounds2-5with moderate yields.When benzonitrile was used instead of acetonitrile as the nitrile source, the reactions of1with ethylamine or isopropylamine gave the coupling product [RuIII(salchda)(NH=C(NHCH2CH3)2)Ph)2](PF6)(6),[RuIII(salchda)(NH=C(NHCH(CH3)2)Ph)2](PF6)(7), respectively.As shown in Scheme 1, the synthesis of these compounds involves two successive steps.The first step is the substitution of aqua ligands by the solvent nitriles and the second step is nucleophilic addition of amines towards the coordinated organonitrile that was activated by the Ru(III) center.
Obviously, when nitrile is used as solvent,1first reacts with the nitrile to formtrans-[RuIII(salchda)(NCR)2]+, which could be validated by ESI/MS in the different reaction time intervals.The coordinated nitriles in the ruthenium(III) salchda complex are found to be electrophilic.The amines which act as nucleophiles then attack the electron deficientα-carbon atoms of the nitrile ligands with the formation of C-N bonds.These bis(amidine)ruthenium(III) compounds are all air-stable.No ligand exchange reaction between the solvent molecules (such as the CH3CN, benzonitrile and THF) and the amidine ligands was observed for at least 7 days.
In order to validate metal-activated amine-nitrile coupling in this process.Reaction of1with ethylamine, isopropylamine and cyclohexylamine in refluxing ethanol solution gave the bis(amine) complexes, respectively.For example, the reaction of1with benzylamine in acetone under argon also generated the bis(benzylamine)ruthenium(III) complex8.
The bis(amidine) compounds2-7and bis(amine) compound 8 are paramagnetic and they have room temperature magnetic momentsμeff(solid sample, Gouy method) in the range of 1.91-2.07μB, which are consistent with their formulation as low-spind5ruthenium(III) complexes.Infrared spectra (IR) of these bis(amidine) complexes2-7show mediumν(N-H) stretches at 3200-3400 cm-1and they also show two sharp peaks around 1630 cm-1and 1600 cm-1, which are partially overlapped.The former peak is assigned to theν(CN) stretch of the amidine ligand and the latter one is assigned toν(CN) stretch of the salchda ligand.The IR spectrum of the bis(amine)ruthenium(III) complex8displays a sharpν(CN) stretch around 1600 cm-1and a ν(N-H) stretch around 3300 cm-1.All compounds obtained have a strong broad stretching band at around 840 cm-1, which is attributed to theν(P-F) stretches of the counter-ion.The ESI-MS of7shows a predominant peak atm/z=746, which is assigned to the parent ion [RuIII(salchda)(NH=C(NHCH(CH3)2)Ph)2]+.As shown in Figure 1, the simulated isotopic distribution pattern form/z746 is in well agreement with the experimental one.Moreover, there is also a minor peak atm/z=584 due to [RuIII(salchda)(NHC(NHCH(CH3)2)Ph)]+, which arises from the loss of a benzamidine ligand from the parent ion.The ESI/MS of the other amidine complexes2-6have also been investigated in acetone (+ve mode), which are similar to that of7and are summarized in the corresponding experimental section.The ESI-MS of bis(amine)Ru(III) compound8in acetone exhibit a dominant peak atm/z636, due to the parent ion.
The UV/Vis spectra of2-7in CH3CN (Table 1) show the strong higher energy absorption bands (ɛ~ 2-6 × 104M-1·cm-1)with wavelengths less than 400 nm, which are assigned to the ligand-centered π-π* transitions of Schiff bases and amidine ligands.These compounds also show the moderate strong absorption bands in the range of 610-633 nm, which are tentatively assigned to the ligand-to-metal charge transfer (LMCT), possibly mixing with metal-centered (d-d transitions).Compared with these bis(amidine) compounds, the corresponding LMCT absorption bands of 8 is red-shifted and appears at around 640 nm[16].
The cyclic voltammograms (CV) of2-8in CH3CN show two reversible waves, which are assigned to RuIV/IIIand RuIII/IIcouples, respectively.As shown in Figure 2(a), the CV of7shows two reversible couples centered atE1/2=0.38 V and -1.08 VvsCp2Fe+/0, respectively.Obviously, the redox potentials for these bis(amidine) complexes are similar, but their couples are also sensitive to the axial amidine ligands.When PhCN is used instead of MeCN, both redox couples are shifted to higher potentials, which are in well agreement with that the well π-accepting ligand could stabilize effectively the compounds with the lower oxidation states.For these salchda Ru(III) compounds with neutral axial ligands, the RuIII/IIpotential increases with the π-accepting ability of the axial ligands.These amino complexes have very closest RuIV/IIIand RuIII/IIpotentials around +0.553 V and -0.950 VvsCp2Fe+/0,Figure 2(b).Compared with the corresponding redox potentials observed in2-7, both couples are shifted to higher potentials. both couples are shifted to higher potentials, indicating that the axial amidine ligands are better electron-donating ligands than amines.
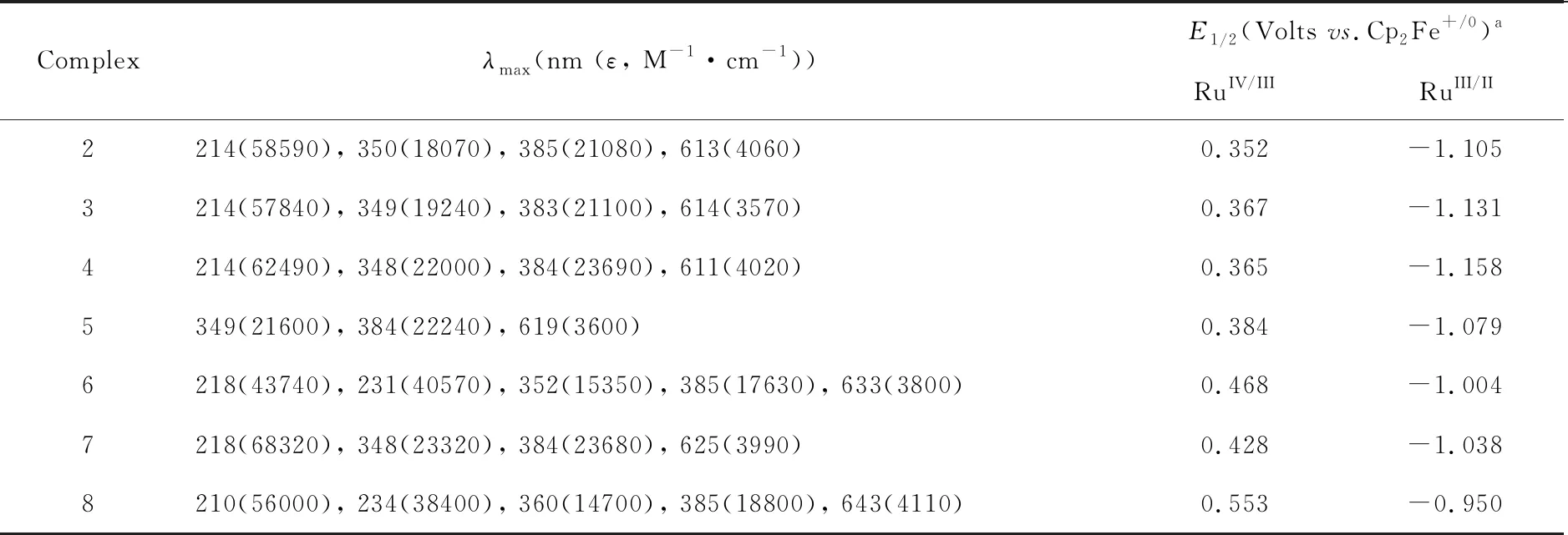
Table 1 UV-Vis (CH3CN) and electrochemical data for complexes 2-8
Note:a:Glassy carbon working electrode, Pt counter electrode, Ag/AgNO3reference electrode, 0.1 M [NnBu4]PF6in CH3CN as supporting electrode.Ferrocene was added as internal standard.
The crystal structures of7and8have been determined by X-ray crystallography.Figure 3a shows the ORTEP diagram of the cation of7.Selected bond lengths (Å) and bond angles (°) are listed in Table 2 and 3.The compound crystallizes in a monoclinicC2/cspace group and features a distorted octahedral geometry.The equatorial bond lengths of Ru-N (1.984(7) Å) and Ru-O (2.029(5) Å) are similar to that of1.The axial positions are occupied by twoN-isopropylbenzamidine ligandsviaits imino nitrogen in atransmode.The N-Ru-N bond angle is 178.8(2)°, which is virtually linear.The bond parameters on the amidine ligand are N3-C11=1.303(6) Å, N2-C11=1.323(5) Å and N1-C17-N2=121.12°.These data indicate substantial delocalization of CN2group inN-isopropylbenzamidine.Intra-molecular H-bonding is observed between N-H of amidine and phenolic oxygen with a distance of 2.852(8) Å.
The ORTEP drawing with partial atom numbering of complex8is shown in Figure 3b.The coordination geometry at ruthenium is best described as distorted octahedral structure with two benzylamine molecules occupying the axial positiontransto each other.One of phenyl group of benzylamine is disordered and six carbon atoms in this phenyl rings are refined with constrained bond parameters.The equatorial position of Ru is taken up by N2O2chromophore.The bond lengths of Ru-N(1.982(4) and 1.990(4)Å) and Ru-O(2.003(3) and 2.011(3)Å) are compared with the known complexes[17].The bond lengths of axial Ru-N(2.110(4) and 2.127(4)Å) are obviously longer than the equatorial Ru-N bond lengths.But these bond lengths are slightly shorter than that of the ruthenium(II) species [RuII(NH2Ph){PhNC(H)NPh}(Me2SO)2Cl] (2.1907(19)Å).[18]The metrical disparity reflects thesp3andsp2nature of the respective coordinated nitrogen.
Table 2 Selected bond parameter (Å, °) for 7
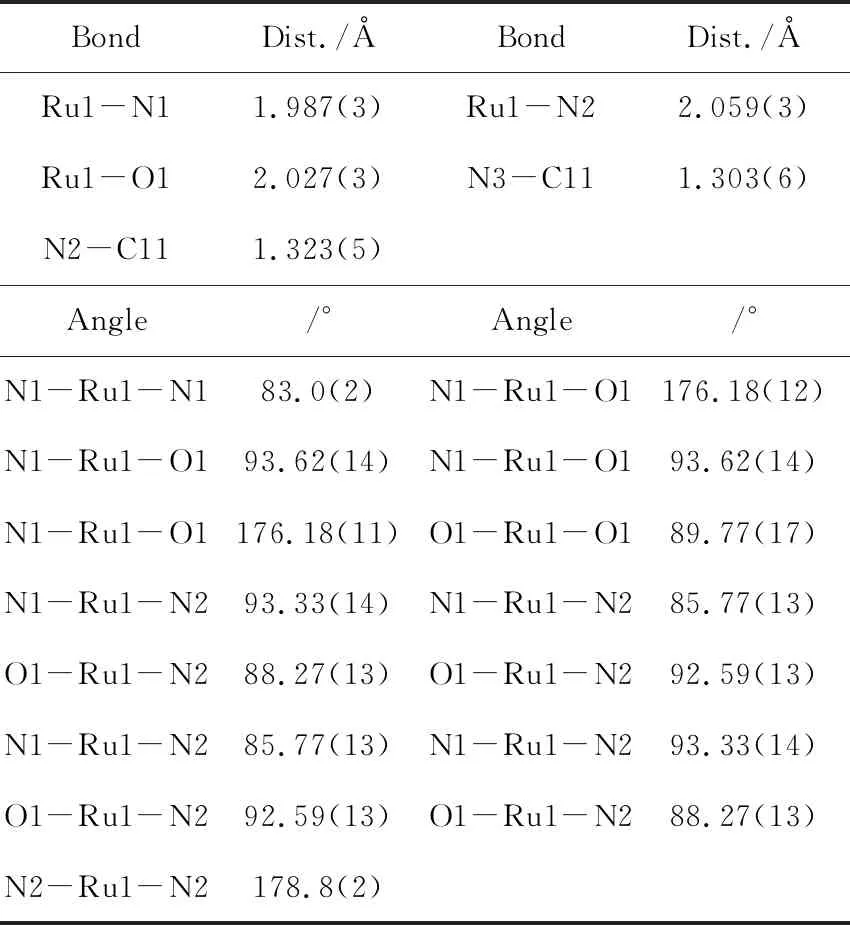
BondDist./ÅBondDist./ÅRu1-N11.987(3)Ru1-N22.059(3)Ru1-O12.027(3)N3-C111.303(6)N2-C111.323(5)Angle/°Angle/°N1-Ru1-N183.0(2)N1-Ru1-O1 176.18(12)N1-Ru1-O1 93.62(14)N1-Ru1-O1 93.62(14)N1-Ru1-O1 176.18(11) O1-Ru1-O1 89.77(17)N1-Ru1-N2 93.33(14)N1-Ru1-N2 85.77(13)O1-Ru1-N2 88.27(13)O1-Ru1-N2 92.59(13)N1-Ru1-N2 85.77(13)N1-Ru1-N2 93.33(14)O1-Ru1-N2 92.59(13)O1-Ru1-N288.27(13)N2-Ru1-N2 178.8(2)
Table 3 Selected bond length(Å) and bond angle(°) of complex 8
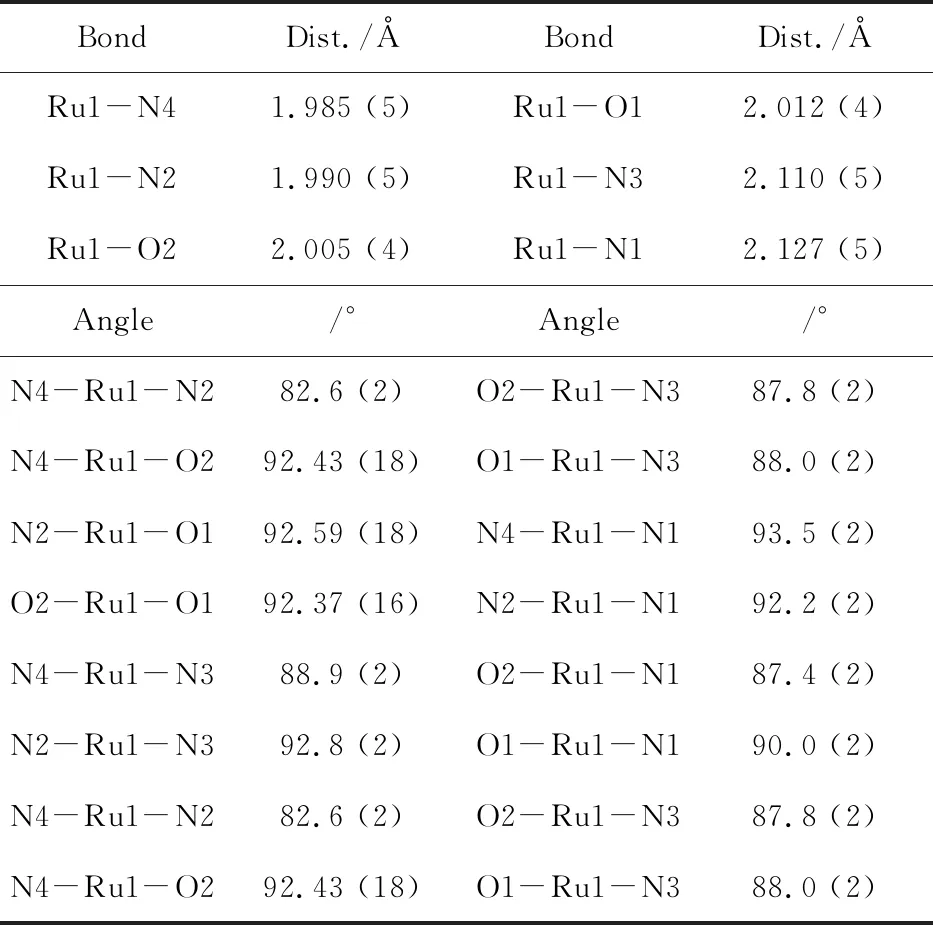
BondDist./ÅBondDist./ÅRu1-N41.985 (5)Ru1-O12.012 (4)Ru1-N21.990 (5)Ru1-N32.110 (5)Ru1-O22.005 (4)Ru1-N12.127 (5)Angle/°Angle/°N4-Ru1-N282.6 (2)O2-Ru1-N387.8 (2)N4-Ru1-O292.43 (18)O1-Ru1-N388.0 (2)N2-Ru1-O192.59 (18)N4-Ru1-N193.5 (2)O2-Ru1-O192.37 (16)N2-Ru1-N192.2 (2)N4-Ru1-N388.9 (2)O2-Ru1-N187.4 (2)N2-Ru1-N392.8 (2)O1-Ru1-N190.0 (2)N4-Ru1-N282.6 (2)O2-Ru1-N387.8 (2)N4-Ru1-O292.43 (18)O1-Ru1-N388.0 (2)
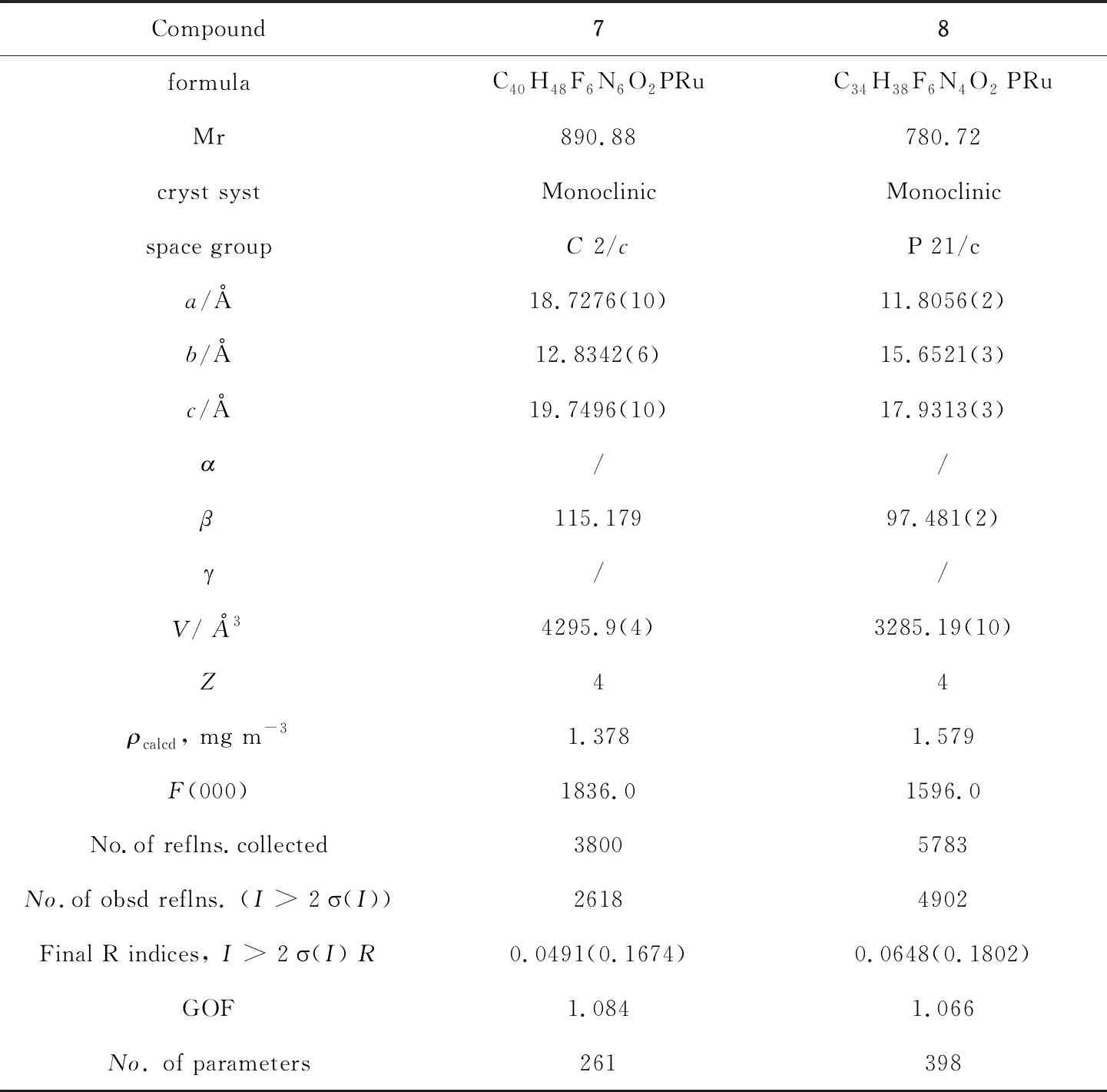
Table 4 Crystal data and structure refinement details for 7 and 8
3 Conclusion
We have provided a facile synthetic route to obtain various (salchda)Ru(III) bis(amidine) complexesviaone-pot synthesis with moderate to high yield.The various substituents on the amidine functional group could be tuned by using different nitrile solvent and different amines.These amidine Ru(III) complexes are potential anti-tumor drugs and further works on their application in cell are now in progress.

