Systemic review and network meta-analysis: Prophylactic antibiotic therapy for spontaneous bacterial peritonitis
Nolan Faust, Akihiro Yamada, Haider Haider, Yuga Komaki, Fukiko Komaki, Dejan Micic, Atsushi Sakuraba
Nolan Faust, Department of Medicine, The University of Chicago, Chicago, IL 60637, United States
Akihiro Yamada, Haider Haider, Yuga Komaki, Fukiko Komaki, Dejan Micic, Atsushi Sakuraba,Section of Gastroenterology, Hepatology and Nutrition, Department of Medicine, The University of Chicago Medicine, Chicago, IL 60637, United States
Akihiro Yamada, Section of Gastroenterology, Department of Internal Medicine, Toho University Sakura Medical Center, Sakura 2850841, Japan
Yuga Komaki, Digestive and Lifestyle Diseases, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima 890-8544, Japan
Abstract BACKGROUND Spontaneous bacterial peritonitis (SBP) is an important prognostic factor for outcomes in patients with cirrhosis.Antibiotic prophylaxis is recommended in patients at high risk for developing SBP, but the choice of antibiotics remains unclear.AIM To evaluate the efficacy of various antibiotics for prophylaxis of SBP based on randomized control trials (RCTs).METHODS Electronic databases were searched through November 2018 for RCTs evaluating the efficacy of therapies for primary or secondary prophylaxis of SBP.The primary outcome was the development of SBP.Sensitivity analyses limited to studies of primary or secondary prophylaxis and studies reported after 2010 were performed.The secondary outcome was the risk of all-cause mortality or transplant.The outcomes were assessed by rank of therapies based on network meta-analyses.Individual meta-analyses were also performed.RESULTS Thirteen RCTs (1742 patients) including norfloxacin, ciprofloxacin, rifaximin,trimethoprim-sulfamethoxazole (TMP-SMX), or placebo/no comparator were identified.Individual meta-analyses showed superiority of rifaximin over norfloxacin as well as norfloxacin and TMP-SMX over placebo.Network metaanalysis demonstrated the rank of efficacy in reducing the risk of SBP as:Rifaximin, ciprofloxacin, TMP-SMX, norfloxacin, and placebo/no comparator.Rifaximin ranked highest in sensitivity analyses limited to studies of primary or secondary prophylaxis and studies reported after 2010.Similarly, rifaximin ranked highest in reducing the risk of death/transplant.CONCLUSION The present comprehensive network meta-analysis provides RCT based evidence for superior efficacy of rifaximin compared to other antibiotics for the prophylaxis of SBP and reducing risk of death/transplant.Further RCTs are warranted to confirm our findings.
Key words: Spontaneous bacterial peritonitis; Prophylaxis; Antibiotics; Network metaanalysis; Systemic review; Cirrhosis
INTRODUCTION
Spontaneous bacterial peritonitis (SBP) is the most common infection seen in patients with advanced liver cirrhosis and ascites[1,2].Development of SBP can lead to renal dysfunction, hepatic encephalopathy, and deterioration of hepatic function, which adversely affect survival.Despite advances in treatment, in-hospital mortality of patients with SBP remains as high as 25%-30%[3].Risk factors for the development of SBP include ascites protein levels < 1 g/dL, high serum bilirubin, prior episodes of SBP, and advanced liver disease[4,5].Recurrences are also common following a single episode of SBP and are seen in up to 69% of infected patients within one year[6].Thus,the first onset of SBP is an important prognosticator for health outcomes in patients with advanced liver disease.The use of antibiotics in patients with variceal bleeding and as secondary prophylaxis of SBP is recommended by the American Association for Study of Liver Diseases[7]and European Association for the Study of the Liver[8]guidelines[7,9,10].However, evidence for the role and choice of antibiotics in both primary and secondary prophylaxis in the absence of gastrointestinal (GI) bleeding remains unclear.
Antibiotic prophylaxis has been shown to reduce the incidence of SBP in patients who are at high risk[11,12].Overgrowth, translocation, and dissemination of intestinal bacteria are early steps in the pathogenesis of SBP and are more prevalent in cirrhotic patients compared to non-cirrhotic controls[13,14].The majority of SBP are caused byEscherichia colior other gram-negative bacteria, though gram-positive bacteria have been increasingly seen in the setting of antibiotic resistance[15,16].Antibiotic prophylaxis primarily works via decontamination of the gut, thus lowering the bacterial reserves available for translocation.Guidelines recommend ceftriaxone for patients with advanced cirrhosis and GI bleeding or norfloxacin twice daily for seven days with severe liver disease as these patients are at high-risk for developing SBP.Trimethoprim/sulfamethoxazole (TMP-SMX) and ciprofloxacin are also listed as effective alternatives[7,10].Additionally, two recent meta-analysis by Goelet al[17]and Sidhuet al[18]suggested a benefit for primary or secondary SBP prophylaxis in using rifaximin, a gut-selective antibiotic, compared to norfloxacin.
Several randomized control trials (RCTs) and cohort studies have demonstrated efficacy of various antibiotics, either in comparison to placebo or other antibiotics for prophylaxis of SBP[19].Yet the number of trials remains small, and comparisons between antibiotics remains sparse, thus limiting our ability to compare treatments which have been studied separately.A network meta-analysis can be used to study outcomes of multiple interventions within the same disease process[20,21].This study uses a network meta-analysis method to rank and provide a comprehensive evaluation of recommended options for primary and secondary antibiotic prophylaxis of SBP based on RCTs.
MATERIALS AND METHODS
Search strategy and study selection
We performed this study according to a previously defined protocol and in accordance with the PRISMA for Network Meta-Analyses (PRISMA-NMA)guidelines[22].The protocol of this meta-analysis has been registered to the International prospective register of systematic reviews (PROSPERO)[23].We conducted a systemic literature search of PubMed/MEDLINE, Google scholar,Scopus, EMBASE and Cochrane Central Register of Controlled Trials (inception to November 1, 2018) for studies assessing the efficacy of antibiotic prophylaxis for SBP.For Google scholar, only the first 1000 articles were reviewed at each search as results are not provided past this number.We also searched abstracts from medical conferences (Digestive Disease Week, American College of Gastroenterology, United European Gastroenterology Week, and AASLD) and bibliographies of identified articles for additional references.
Only RCTs evaluating the efficacy of one or more antibiotic interventions for prophylaxis (primary or secondary) of SBP or reported it as an outcome were eligible for inclusion.Studies of SBP prophylaxis in the setting of GI bleeding were excluded.Control arms were placebo, no treatment, or alternative treatments.For the purpose of this study, placebo and no treatment arms were combined and are aggregately referred to as placebo from this point forward.Inclusion was not restricted based on age, sex, or duration of study.No geographic restrictions were placed on eligible articles and articles in languages other than English were translated if necessary.Studies were searched with a combination of terms including “spontaneous bacterial peritonitis”, “prophylaxis”, “antibiotics” and “randomized”.Terms were searched as both medical subject headings and free text and were combined using the set operators.Two authors (Faust N and Yamada A) independently screened potential titles and abstracts in the primary search in order to identify articles addressing the question of interest.The full text of selected articles was then evaluated for eligibility and content areas of disagreement or uncertainty were resolved based on discussion and consensus between the two authors and principal investigator.
Data extraction and quality assessment
Data was abstracted using a standardized data abstraction form.Study characteristics including the authors, location, year of study, study period, sample size, mean age of patients, sex of patients, inclusion and exclusion criteria, antibiotics used, and endpoints were collected.Outcomes and adverse events were extracted for each study when reported.The Jadad scale, a validated method for assessing the methodological quality of a clinical trial, was used to assess the quality of each included study[24].Cochrane scores were also used as a qualitative measure for bias[25].
Outcome assessment
The primary outcome for this study was the proportion of patients who developed SBP in each intervention arm.Incidence of SBP was determined in each study by a combination of clinical characteristics (fever, abdominal pain), cytologic criteria, and ascitic fluid cultures.The secondary outcome was the risk of death/transplant as assessed by the proportion of patients who died or were transplanted in each intervention arm due to any cause.Data was extracted as intention-to-treat whenever allowed by individual RCT reporting.Outcomes were assessed by risk difference between the two treatment arms.
We performed the following subgroup analyses: (1) Excluding studies with low quality as assessed with the Jadad scale ≤ 2; (2) Analysis of primary prophylaxis,including only patients without a history of SBP; (3) Analysis of secondary prophylaxis including only patients with a history of SBP; and (4) Analysis of studies performed after 2010 (after rifaximin was approved by United States Food and Drug Administration to reduce the risk of hepatic encephalopathy).
Statistical analysis
The network meta-analysi s is a technical method which allows readers to visualize and interpret data for the relative merits of multiple interventions in a given condition.This synthesis of data allows preservation of the randomization within each trial[26].Two assumptions necessary for the validity of the network meta-analysis’mixed comparisons are that the data across sets is transitively related and consistent[27].
In the framework of this study, transitivity is a measure of methodological homogeneity and can be assumed when the data sets for two direct comparison studies are similar in their distributions.Such is the case when subject demographics for the included studies are similar in distribution, and subjects for any given study eligible for any of the interventions based on eligibility and exclusion criteria across all studies.Still, some clinical and methodological heterogeneity is expected across studies.The Bayesian Markov chain Monte Carlo method was used account for this[28].Our model contained parameters describing the relative treatment effect of each treatment compared with each other and a common comparator (placebo).Other treatment comparisons were derived by analyzing differences between model parameters.
Consistency refers to statistical heterogeneity, or the degree to which disagreements in study specific treatment effects exist beyond what can be explained by chance[29].RCT consistency in this study was measured using the node-splitting method.The results were presented as median effect sizes along with 95% confidence intervals(CIs).No significant inconsistency was present when 95%CIs of inconsistency factors included zero or when a largePvalue (> 0.05) for the comparison between direct and indirect effects in the node splitting analysis was found.
Each Bayesian Markov chain Monte Carlo cycle provided a ranking of the treatments according to the estimated effect size and the full set of simulations.We calculated the surface under the cumulative ranking (SUCRA) probabilities[20].SUCRAs expressed as percentages compare each intervention to an imaginary intervention that is always the best without uncertainty.The ranking probability for each drug,i.e., the most efficacious, the second-best, the third-best, and so on, was calculated and the overall ranks were interpreted by SUCRA technique.The larger SUCRAs denote more effective interventions.
For direct meta-analysis, we evaluated the presence of heterogeneity across trials of each therapy by using theI2statistic.AnI2value of < 25% indicates low heterogeneity,25%-75% moderate heterogeneity, and > 75% high heterogeneity, respectively[30].We also evaluated the presence of heterogeneity across trials of each therapy by using the statistic Q and used aPvalue of < 0.10 as evidence of statistically significant heterogeneity[31].All analysis was performed with ADDIS 1.× (drugis.org)[32].We followed the Cochrane Handbook for Systematic Reviews of Interventions in the report of this meta-analysis[25].
RESULTS
Study characteristics
Literature review identified 171 citations through the initial search.We excluded 154 titles and abstracts after initial screening and assessed 18 articles for eligibility (Figure 1).Ultimately, 13 RCTs, including a total of 1757 patients, were included in the evaluation of 5 interventions for SBP prophylaxis: Norfloxacin, ciprofloxacin,rifaximin, trimethoprim-sulfamethoxazole (TMP-SMX), and placebo.All studies were parallel studies and 5 were placebo-controlled trials.Inclusion criteria for participants among each study included diagnosis of cirrhosis by clinical diagnosis, imaging, liver biopsy, laboratory values and/or presence of ascites.Exclusion criteria included documented anaphylaxis to one of the study interventions, hepatocellular carcinoma or other neoplasias that could shorten life expectancy, bacterial infection at admission,HIV infection or hepatic encephalopathy, and pregnant and lactating women.All trials included ascitic fluid PMN count in the diagnosis of SBP.The majority of trials diagnosed SBP with PMN ≥ 250, with one study using diagnostic criteria of polymorphonuclear cells ≥ 350.The majority of the studies included advanced cirrhotic patients (Child-Pugh class B or C) with alcoholic or viral hepatitis as its cause.The study by Assemet al[33]included a treatment group that alternated norfloxacin and rifaximin, but it was excluded from our analysis.Five studies used antibiotics for primary prophylaxis (excluded patients with a history SBP and the remainder contained a mixed cohort of patients with or without a history of SBP.Seven and 6 studies were published before and after 2010, respectively.All 3 studies that included rifaximin were published after 2015 and compared its efficacy to norfloxacin in a non-double-blinded manner[33-35].A summary of individual study characteristics and outcome data for the included studies are summarized in Table 1.The median JADAD for all included studies was 3, with individual scores for each study ranging from 1 to 4.JADAD scores and Cochrane meta-analysis bias scores are shown in Table 2.
Individual meta-analyses of SBP risk
Individual meta-analyses were performed to compare the efficacy between each antibiotic.It should be noted that the number of studies in each meta-analysis was small ranging from 1-5.Superiority of norfloxacin and TMP-SMX over placebo were demonstrated in meta-analyses including 5 and 1 study, respectively(Supplementary Figure 1B and C).One study comparing ciprofloxacin to placebo demonstrated a non-significant superiority of ciprofloxacin over placebo (Supplementary Figure 1A).Three studies compared rifaximin to norfloxacin, and the metaanalysis showed superiority of rifaximin over norfloxacin with no heterogeneity(Supplementary Figure 1D).Two studies and one study compared TMP-SMX to norfloxacin and ciprofloxacin to norfloxacin, respectively, and the meta-analyses showed no difference between the two agents (Supplementary Figure 1E and F).
Network meta-analysis of SBP risk
There were 5 studies comparing norfloxacin to placebo, 3 studies comparing norfloxacin to rifaximin, and two studies comparing norfloxacin to TMP-SMX.The remainder of comparisons (ciprofloxacinvsplacebo, norfloxacinvsciprofloxacin,TMP-SMXvsno treatment) included only one study each.The network of all intervention comparisons analyzed for efficacy of SBP prophylaxis is shown in Figure 2A.The network meta-analysis for the relative effects of each treatment for SBP prophylaxis is shown in Figure 2B.SUCRA interpretations of the rank probability for efficacy is shown in Figure 2C, with larger SUCRA scores indicating higher efficacy.In ascending order, the treatments ranked as (1) rifaximin; (2) ciprofloxacin; (3) TMPSMX; (4) norfloxacin; and (5) placebo.Most of the 95%CIs of SUCRA for active treatments overlapped with each other, but none of those overlapped with the one of placebo.Similar results were found when we excluded studies with low quality(Jadad scale ≤ 2) (Supplementary Figure 2).
The results were shown to meet criteria for consistency based on the inconsistency model analyses and node-splitting analyses.The median inconsistency factors for norfloxacin/placebo/TMP-SMX and ciprofloxacin/norfloxacin/placebo were -0.26[95%CI: (-2.85, 1.36)] and 0.06 [95%CI: -1.92, 2.41].Comparison data from the node split model did not show significant differences between the direct and indirect effects(ciprofloxacinvsnorfloxacin,P= 0.72; ciprofloxacinvsplacebo,P= 0.91; norfloxacinvsplacebo,P= 0.64; norfloxacinvsTMP-SMX,P= 0.35; placebovsTMP-SMX,P=0.35) supporting the consistency of the network meta-analysis.
As part of the subgroup analysis, we performed a network meta-analysis among the 5 studies that used antibiotics for primary prophylaxis of SBP (Supplementary Figure 3).In ascending order, the treatments ranked as (1) rifaximin; (2) norfloxacin;(3) ciprofloxacin; and (4) placebo.There was no study that used TMP-SMX for primary prophylaxis, thus, it was not included in this particular network metaanalysis.Network meta-analysis undertaken among the 8 studies that included patients who used antibiotics for secondary prophylaxis of SBP (Supplementary Figure 4), demonstrated that the treatments ranked as (1) rifaximin; (2) ciprofloxacin;(3) TMP-SMX; (4) norfloxacin; and (5) placebo.When network meta-analysis was performed among the 6 studies that were published after 2010 (Supplementary Figure 5), the treatments ranked in ascending order as (1) rifaximin; (2) TMP-SMX; (3)ciprofloxacin; (4) norfloxacin; and (5) placebo.
Individual and network meta-analyses of the risk of death/transplant
Individual meta-analyses assessing the risk of death/transplant are shown in Supplementary Figure 6.One study compared ciprofloxacin to placebo and 3 studies compared rifaximin to norfloxacin, and the meta-analyses showed superiority of ciprofloxacin and rifaximin over their comparators in reducing the risk of death,respectively (Supplementary Figure 6A and D).The remainder of the individual metaanalyses demonstrated no significant superiority between each treatment arm.It should be noted that the number of studies in each meta-analysis was small ranging from 1-5.Faust Net al.Meta-analysis of antibiotic prophylaxis for SBP
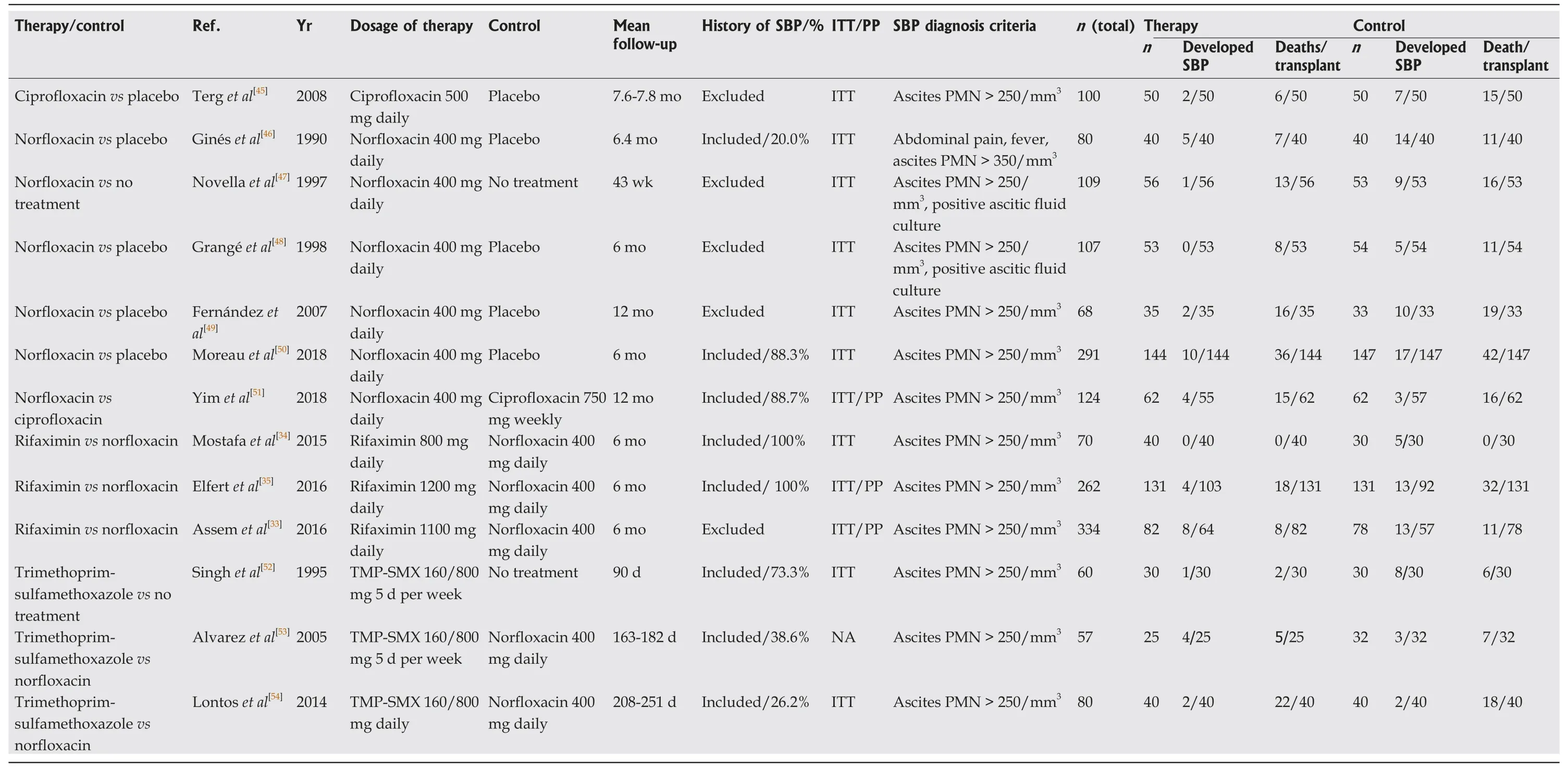
Table 1 Characteristics of the studies included in the network meta-analysis
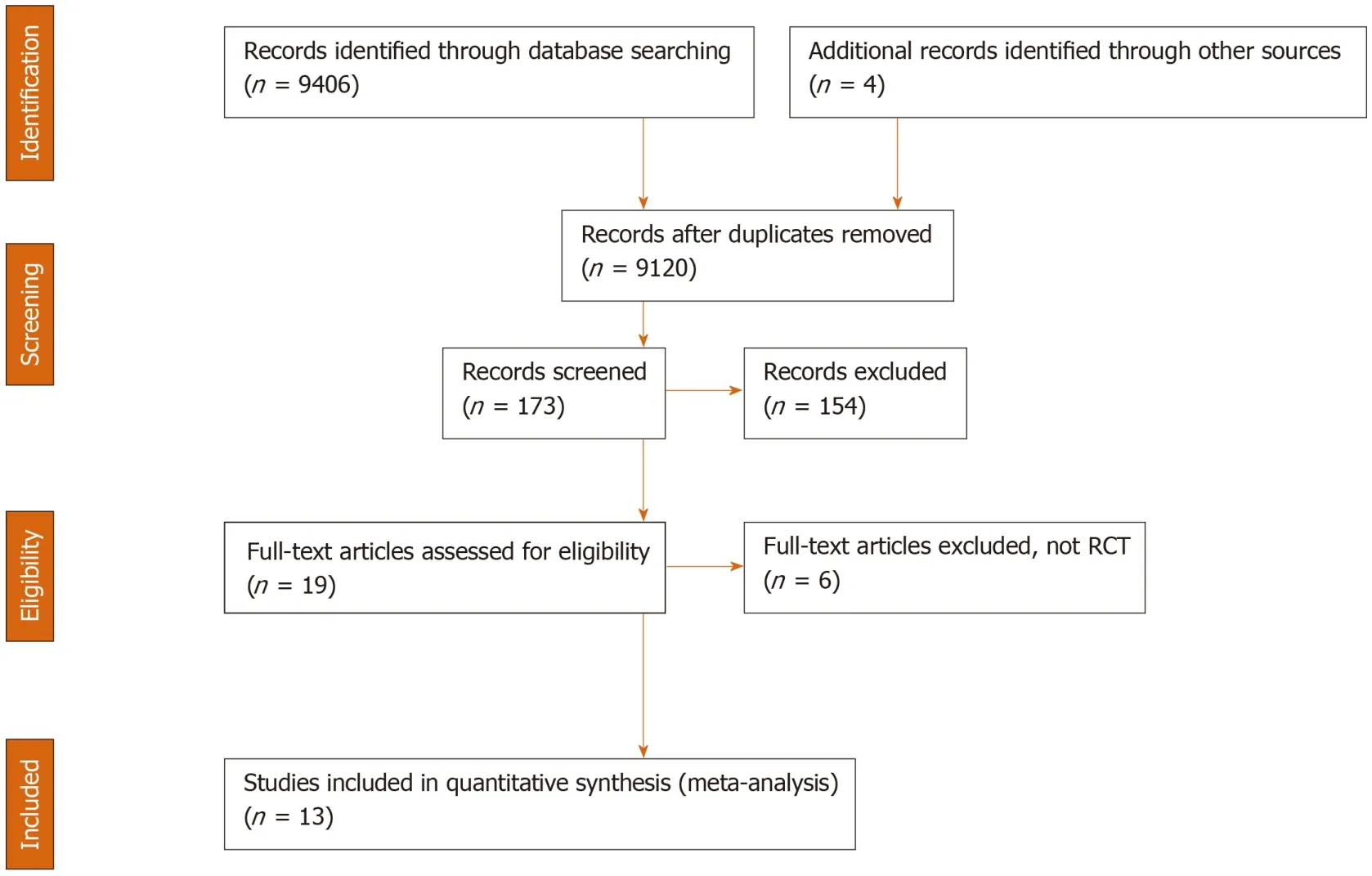
Figure 1 Flow chart of assessment of studies identified in the network meta-analysis.
The network of all intervention comparisons analyzed for efficacy of risk reduction of death is shown in Figure 3A.The network meta-analysis for the relative effects of each treatment is shown in Figure 3B.SUCRA interpretations of the rank probability for efficacy is shown in Figure 3C and, in ascending order, the treatments ranked as(1) rifaximin; (2) ciprofloxacin; (3) norfloxacin; (4) TMP-SMX; and (5) placebo.The median inconsistency factors for norfloxacin/placebo/TMP-SMX and ciprofloxacin/norfloxacin/placebo were -0.22 [95%CI: (-1.64, 0.56)] and -0.20 [95%CI:(-1.39, 0.50)], which met the criteria for consistency.Comparison data from the node split model did not show significant differences between the direct and indirect effects(ciprofloxacinvsnorfloxacin,P= 0.25; ciprofloxacinvsplacebo,P= 0.21; norfloxacinvsplacebo,P= 0.09; norfloxacinvsTMP-SMX,P= 0.35; placebovsTMP-SMX,P=0.20) supporting the consistency of the network meta-analysis.
DISCUSSION
In this systematic review and meta-analysis, we compared and assessed the efficacy of different antibiotic treatments for SBP prophylaxis in individuals with advanced cirrhosis.This was done in order to validate current treatment recommendations and to perform indirect comparisons of active treatments where no or few direct randomized comparison trials existed.Among the four antibiotics and placebo included in the meta-analysis, rifaximin was the most effective in preventing SBP,followed by ciprofloxacin, TMP-SMX, norfloxacin, and placebo.Similarly, rifaximin ranked highest in reducing the risk of death.
Current guidelines from the AASLD and EASL recommend prophylactic treatment with intravenous ceftriaxone or oral norfloxacin for the prevention of SBP in the setting of GI bleeding and severe liver disease[10].Norfloxacin is recommended for primary prophylaxis in cirrhotic patients with low ascitic fluid protein concentration and/or high serum bilirubin levels as they are at high risk of developing a first episode of SBP.Furthermore, norfloxacin is also recommended for secondary prophylaxis because recurrent SBP is common[7,10].Our study validates results from two meta-analyses by Goelet al[17]and Sidhuet al[18], which found a reduction in the development of SBP with the use of rifaximin compared to the recommended norfloxacin regimens.A recent network meta-analysis by Facciorussoet al[2]reported moderate evidence for norfloxacin and ciprofloxacin in primary prophylaxis of SBP,and low quality evidence for the use of rifaximin.This difference may be accounted for by the inclusion of studies that included both patients with primary prophylaxis and with a history of SBP in our study.Such studies were included in our primary outcome of combined primary and secondary prevention, but not in our subgroupanalyses due to lack of subgroup randomization and incomplete information.Analyses of treatment effects in these subgroups are therefore subject to additional biases when compared to complete cohorts[36].Our network meta-analysis provides evidence for superiority of rifaximin over the other studied antibiotics, which could otherwise not be compared by direct meta-analysis.Furthermore, ciprofloxacin and TMP-SMX ranked higher than norfloxacin in reducing the risk of SBP.Ciprofloxacin also ranked higher than norfloxacin in reducing the risk of death.Selective decontamination likely reduces the incidence of bacterial translocation of causative microflora through the gut.Evidence suggests that this effect may be compounded by or contributed to decreased expression of bacterial virulence factors and adhesion molecules[37,38].Increased antibiotic efficacy with rifaximin has been seen in other GI diseases such as small intestinal bacterial overgrowth and traveler’s diarrhea, which may also be working preferably in the setting of SBP prophylaxis[39,40].
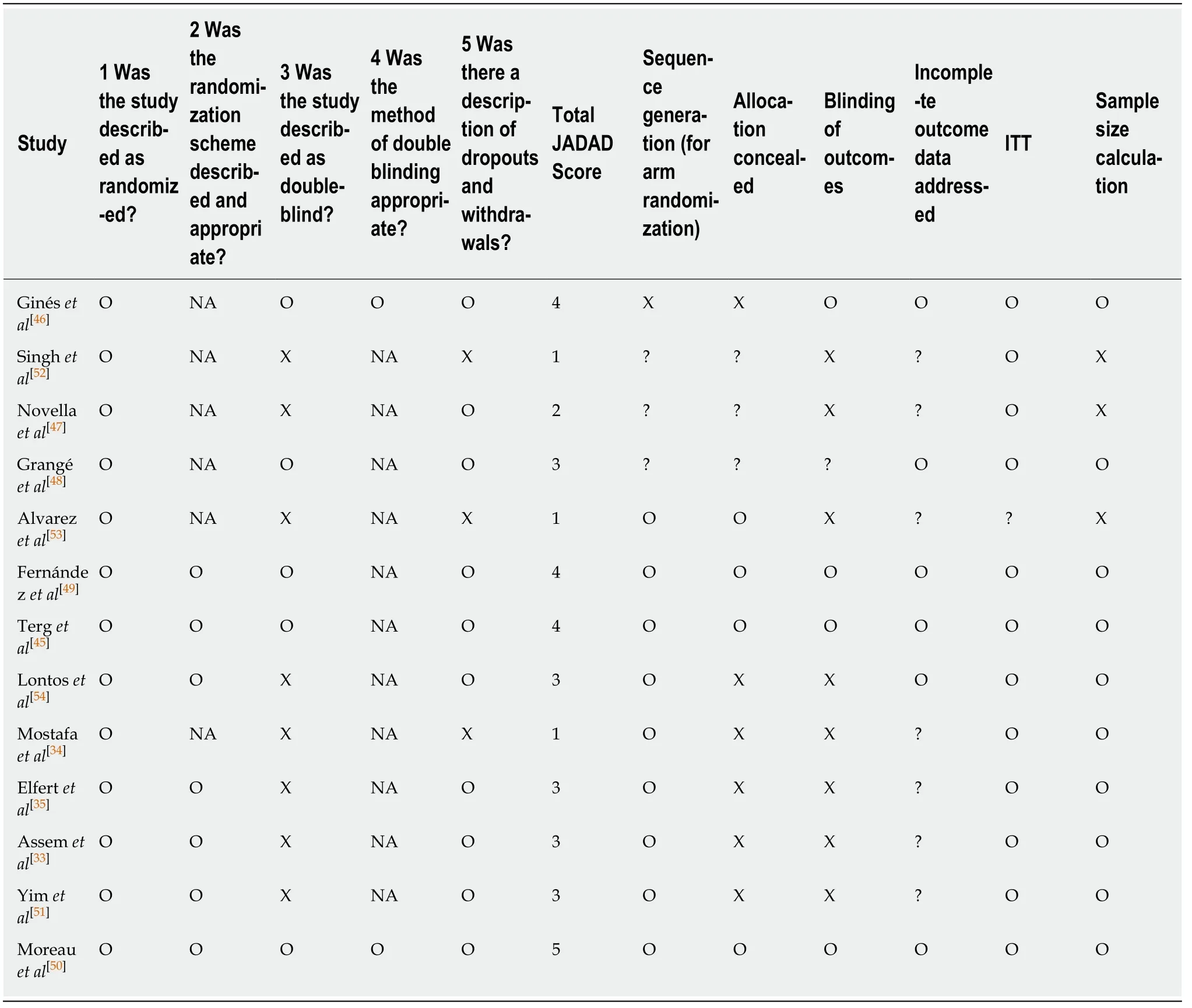
Table 2 JADAD and Cochrane meta-analysis bias scores
Rifaximin also has a favorable side effect profile compared to other antibiotics,particularly with respect to the development of antibiotic resistant flora.The use of fluoroquinolones such as norfloxacin, which have traditionally been used for SBP prophylaxis, is associated with the development of resistant bacterial strains.Concurrently, there has been a recent shift in cases of documented SBP from being caused by gram-negative organisms to being caused by gram-positive organisms[41,42].This is particularly seen in cases of SBP in patients on norfloxacin prophylaxis and may contribute to the increased efficacy of rifaximin seen in trialsvsnorfloxacin, as the infective organisms are more likely to be may be gram-positive that fall under the spectrum covered by rifaximin.
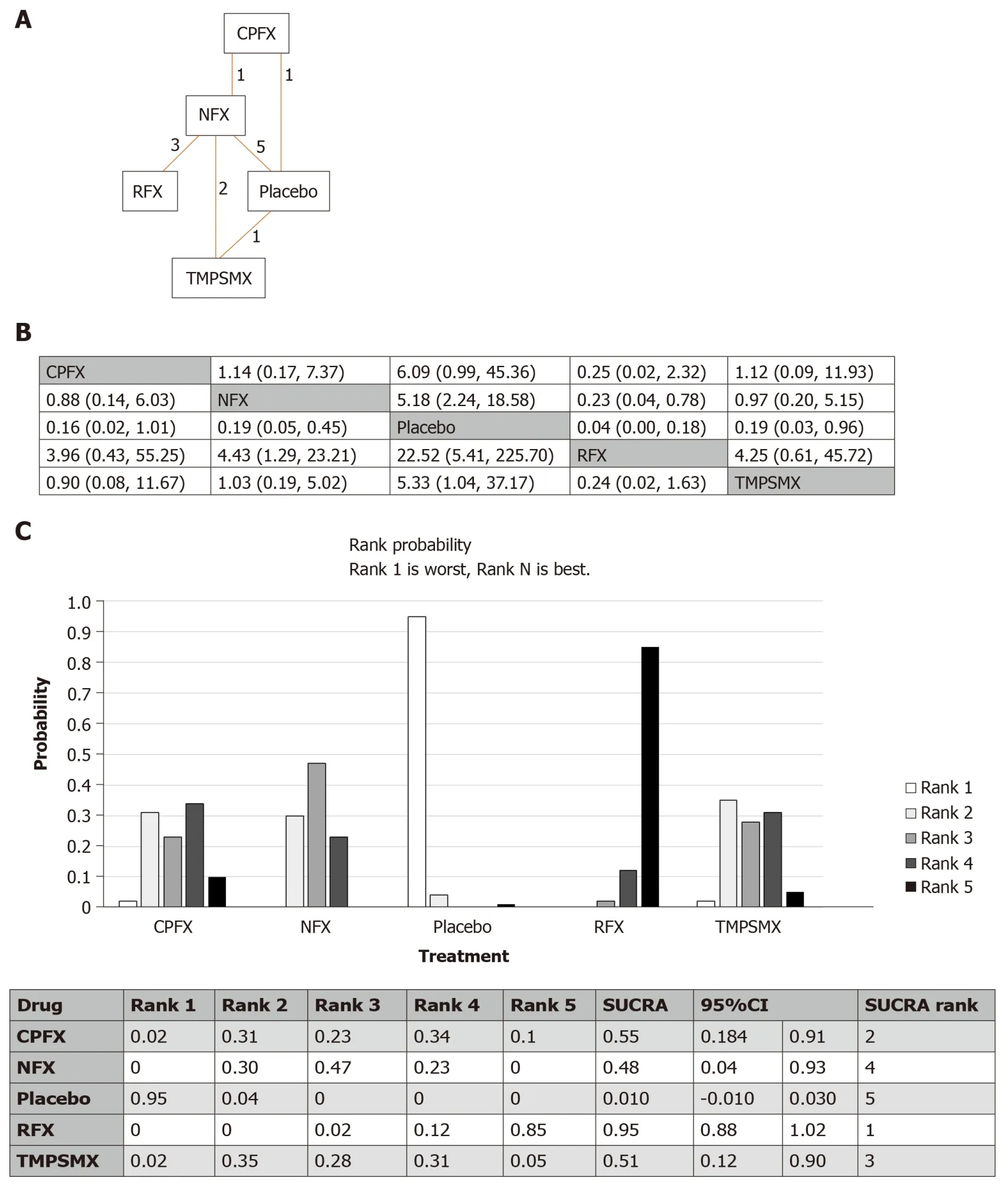
Figure 2 Network meta-analysis of studies assessing the risk of spontaneous bacterial peritonitis.
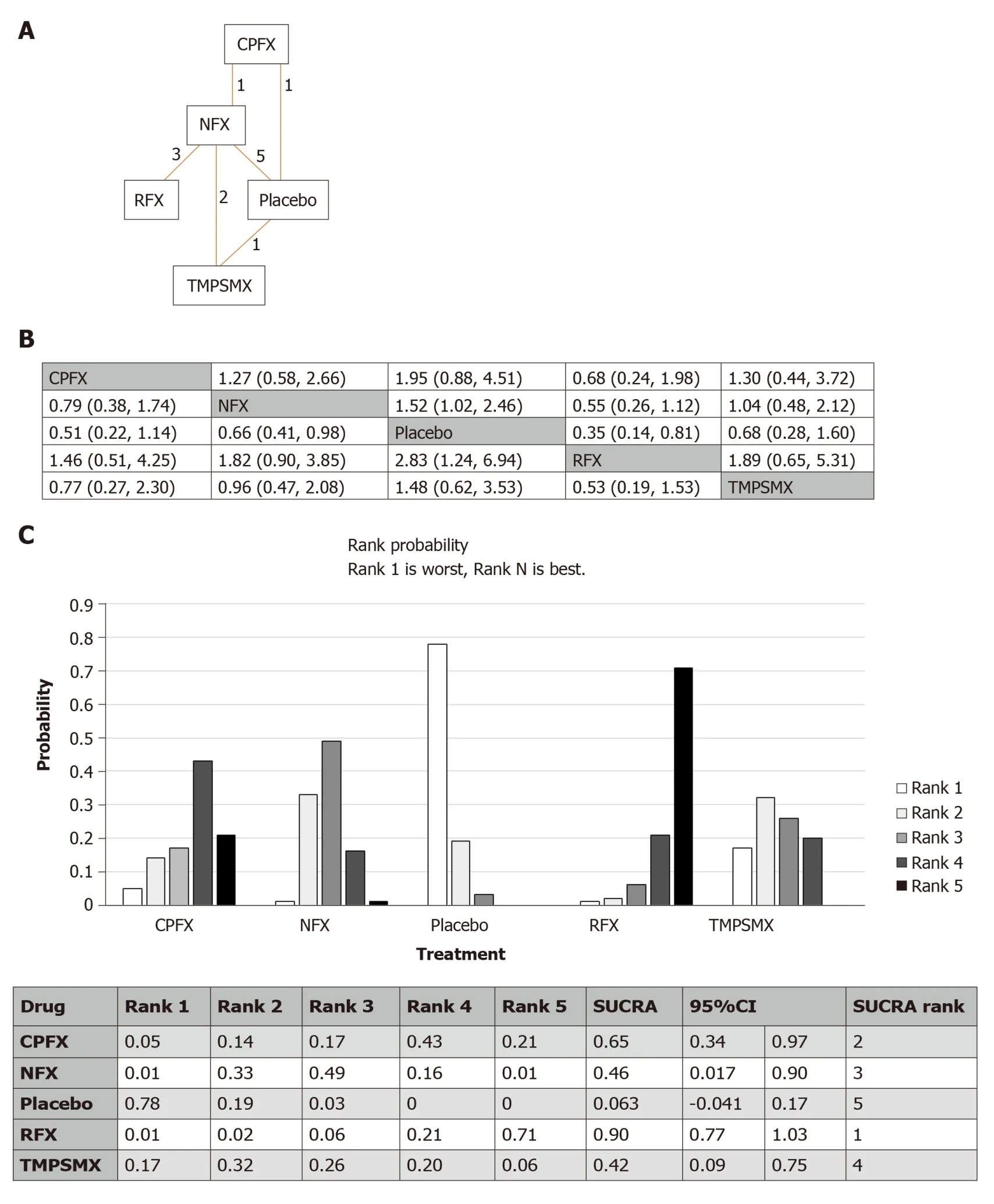
Figure 3 Network meta-analysis of studies assessing the risk of death/transplant.
A major limitation of our study is the rather sparse geometry of the network due to the small number of RCTs.This effect is compounded by the range in quality of the studies used, with a JADAD score of less than three in 4 of the 11 studies used.Scores were most often lowered by the fact that practitioners and participants were not blinded to treatments and outcomes in some of the studies.However, we confirmed that a similar result was found when excluding studies with low quality scores.Several studies also contained elements indicative of bias and heterogeneity as determined by Cochrane meta-analysis criteria, and many of the studies analyzed were relatively smaller in size.The studies that included rifaximin all compared its efficacy to norfloxacin and there were no studies comparing rifaximin to placebo or other antibiotics, therefore limiting direct comparison with other agents.This further affirms the need for network meta-analyses in order to simultaneously compare the efficacy of multiple agents.Disagreement between direct and indirect comparisons may raise concerns for the validity of a network meta-analysis, however, the robustness of our network meta-analysis was supported by the inconsistency model that demonstrated no such inconsistency.Rank probabilities identified in this network meta-analysis can be plotted against the possible ranks for all competing treatments[43,44].We used SUCRA as a numerical summary to supplement the cumulative ranking[44], however, the results should be interpreted with caution as there is no means to statistically assess the difference of the SUCRA values[44].Most studies did not differentiate between primary and secondary prophylaxis, but we found similar results when network meta-analysis was limited to studies using antibiotics for either primary or secondary prophylaxis.The time span of included studies ranged from the 1990s to 2018 which may have seen a change in bacteriology of organisms causing SBP, however subgroup analysis including studies that were reported after 2010 demonstrated similar outcome.The results of the secondary outcome in our network meta-analysis, the reduction in the risk of death/transplant,needs to be approached with caution as it was not a primary outcome in any of the included studies.The included studies did not take other decision points into account,such as cost or quality of life.Furthermore, other factors such as demographics,concomitant proton inhibitor use, or past antibiotic use, which could confound outcomes, could not be assessed in the present study.
In conclusion, this systematic review and network meta-analysis of RCTs comparing multiple antibiotics for prophylaxis of SBP suggests that rifaximin is the most effective for the outcomes of preventing SBP and reducing all-cause mortality in high risk cirrhotic patients.Further comparative studies, particularly with appropriate randomization and larger power, are warranted to confirm these findings.
ARTICLE HIGHLIGHTS
Research background
Spontaneous bacterial peritonitis (SBP) confers significant mortality with high rates of recurrence.Prevention is therefore indicated and of great importance in cirrhotic individuals with ascites and either significant hepatic disease, gastrointestinal (GI) bleeding, or history of SBP.
Research motivation
Yet data is sparse regarding the choice of antibiotic when comparing the previous gold standard,norfloxacin, to other agents including ciprofloxacin, trimethoprim-sulfamethoxazole (TMPSMX),and the GI selective agent rifaximin.The network meta-analysis technique allows us to make indirect comparisons across studies using common comparators.
Researc h objectives
Our present study uses this technique to rank and evaluate recommended therapies for primary and secondary prophylaxis of SBP.
Research methods
Thirteen randomized control trials including a total of 1757 patient were analyzed.Individual meta-analyses showed superiority of rifaximin over norfloxacin as well as norfloxacin and TMPSMX over placebo.Network meta-analysis demonstrated the rank of efficacy in reducing the combined primary and secondary risk of SBP as: Rifaximin, ciprofloxacin, TMP-SMX,norfloxacin, and placebo/no comparator.Rifaximin ranked highest in sensitivity analyses limited to studies of either primary or secondary prophylaxis alone, and in studies reported after 2010.Similarly, rifaximin ranked highest in reducing the risk of death/transplant.
Research results
This study provides new evidence for superiority of rifaximin compared to norfloxacin in both primary and secondary SBP prophylaxis.In summary, this conclusion is supported by decreased mortality when rifaximin is used for primary or secondary prophylaxis compared to norfloxacin,ciprofloxacin, and TMP-SMX as shown in individual and network meta-analyses.Other new insights from this study were that rifaximin still performed best in a subgroup analysis of studies done after the year 2010, after the recommendation was made for rifaximin use in hepatic encephalopathy.
Research conclusions
Therefore, this study proposes the new hypothesis that the common use of rifaximin for hepatic encephalopathy in decompensated cirrhosis does not decrease its effectiveness in SBP prophylaxis.Additional molecular and biochemical data is needed to explain the beneficial effect of rifaximin.However, our data supports the hypothesis that rifaximin’s selective decontamination of the GI tract, favorable resistance profile, and ability to decrease bacterial translocation across the gut may all contribute to its superiority for prophylaxis.Implications of these results for clinical practice include reconsideration of current AASLD guidelines to recommend rifaximin over norfloxacin as the first line agent for SBP prophylaxis.
Research perspectives
The next steps in this area of study should include additional data from large studies with direct comparisons between each antibiotic.Randomized control trial methods should be used in future research studies in order to confirm our meta-analysis findings.
 World Journal of Hepatology2020年5期
World Journal of Hepatology2020年5期
- World Journal of Hepatology的其它文章
- Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion
- Drug and herbal/dietary supplements-induced liver injury: A tertiary care center experience
- Usefulness of Mac-2 binding protein glycosylation isomer in noninvasive probing liver disease in the Vietnamese population
- Epidemiological profile of alcoholic liver disease hospital admissions in a Latin American country over a 10-year period
- Transmission of cryptococcosis by liver transplantation: A case report and review of literature
