Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion
Marijan Kolovrat, Slaven Gojkovic, Ivan Krezic, Dominik Malekinusic, Borna Vrdoljak, Katarina Kasnik Kovac,Tamara Kralj, Domagoj Drmic, Ivan Barisic, Katarina Horvat Pavlov, Andreja Petrovic, Antonija Duzel,Mario Knezevic, Ivan Mirkovic, Antonio Kokot, Alenka Boban Blagaic, Sven Seiwerth, Predrag Sikiric
Marijan Kolovrat, Slaven Gojkovic, lvan Krezic, Dominik Malekinusic, Borna Vrdoljak, Katarina Kasnik Kovac, Tamara Kralj, Domagoj Drmic, lvan Barisic, Katarina Horvat Pavlov, Andreja Petrovic, Antonija Duzel, Mario Knezevic, lvan Mirkovic, Antonio Kokot, Alenka Boban Blagaic,Sven Seiwerth, Predrag Sikiric, Departments of Pharmacology and Pathology, School of Medicine, University of Zagreb, Zagreb 10000, Croatia
Abstract BACKGROUND The Pringle maneuver [portal triad obstruction(PTO)] provides huge disturbances during ischemia and even more thereafter in reperfusion.Contrarily, a possible solution may be stable gastric pentadecapeptide BPC 157,with already documented beneficial effects in ischemia/reperfusion conditions.Recently, BPC 157, as a cytoprotective agent, successfully resolved vessel occlusions in rats (ischemic colitis; deep vein thrombosis, superior anterior pancreaticoduodenal vein; bile duct cirrhosis) through rapid collateral vessel recruitment to circumvent vessel occlusion.Thereby, medication BPC 157 regimens were administered as a single challenge before and during ischemia or,alternatively, at various time points during reperfusion.AIM To introduce BPC 157 therapy against pringle maneuver-damage.METHODS In deeply anesthetised rats, the portal triad was clamped up for 30 min.Rats then underwent reperfusion for either 15 min or 24 h.Medication [(10 μg, 10 ng/kg)regimens, administered as a single challenge] picked (a) ischemia, PTO period [at 5 min before (ip) or at 5 or 30 min of ligation time (as a bath to PTO)] or (b)reperfusion, post-PTO period [at 1 or 15 min (bath during surgery) or 24 h (ip)reperfusion-time].We provided gross, microscopy, malondialdehyde, serum enzymes, electrocardiogram, portal, caval, and aortal pressure, thrombosis and venography assessments.RESULTS BPC 157 counteracts electrocardiogram disturbances (increased P wave amplitude, S1Q3T3 QRS pattern and tachycardia).Rapidly presented vascular pathway (portal vein-superior mesenteric vein-inferior mesenteric vein-rectal veins-left ileal vein-inferior caval vein) as the adequate shunting immediately affected disturbed haemodynamics.Portal hypertension and severe aortal hypotension during PTO, as well as portal and caval hypertension and mild aortal hypotension in reperfusion and refractory ascites formation were markedly attenuated (during PTO) or completely abrogated (reperfusion); thrombosis in portal vein tributaries and inferior caval vein or hepatic artery was counteracted during portal triad obstruction PTO.Also, counteraction included the whole vicious injurious circle [i.e., lung pathology (severe capillary congestion), liver(dilated central veins and terminal portal venules), intestine (substantial capillary congestion, submucosal oedema, loss of villous architecture), splenomegaly, right heart (picked P wave values)] regularly perpetuated in ischemia and progressed by reperfusion in Pringle rats.CONCLUSION BPC 157 resolves pringle maneuver-damage in rats, both for ischemia and reperfusion.
Key words: BPC 157; Pringle maneuver; Rats; Portal hypertension; Caval hypertension;Ischemia
INTRODUCTION
We focused on the therapy of the Pringle maneuver in rats[1], so far not described severe preportal hypertension[1], the temporary portal triad obstruction (PTO),ischemia, the short and prolonged reperfusion, the lack of adequate portocaval shunting as the most detrimental feature that should be counteracted.With stable gastric pentadecapeptide BPC 157[2-6], we suggest the resolution of the damages, either those following occlusion or those following re-opening of the hepatic artery, portal vein and bile duct.
Therapy is the recovering effect it has on occluded vessels, bypassing the occlusion as the specific effect of BPC 157 in ischemia/reperfusion[7-11].There is benefit arising from BPC 157 therapy of the deep vein thrombosis, inferior caval vein occlusion,colitis ischemia/reperfusion, duodenal venous congestion and cecum perforation[7-10].Recently, after induction of liver cirrhosis due to both bile duct ligation and portal hypertension, prevention and reversal of the already pre-existing portal hypertension to normal values[11]have become possible.
Therefore, in the PTO-syndrome in rats, the rapidly activated way, portal veinsuperior mesenteric, vein-inferior mesenteric vein-rectal vein-left iliac vein-inferior caval vein, would appear as a specific activation of the collateral circulation, as the bypassing loop that can rapidly circumvent occlusions and decompress PTO-rats upon BPC 157 administration.That solution in Pringle-rats with ischemia and reperfusion goes with the resolution of oxidative stress, hemodynamic disturbances,severe portal and caval hypertension, aortic hypotension, rapid cloth formation in the portal vein, superior mesenteric vein, lienal vein, inferior caval vein, hepatic artery,ascites, peaked P waves, tachycardia; increased serum values; gross intestine, liver,lung, spleen and heart lesions.Especially, it goes with the agent application during reperfusion.
Contrarily, in preportal hypertension studies in chronically made portal veinstenotic rats, the high-grade portal-systemic shunting[12]is unable to decompress even the slow development of steady mild portal hypertension[13-16].The PTO-rat studies are all without portal hypertension assessment[17-21].They are only reperfusion-induced injury studies[16-20].Pre-existing ischemia was not investigated[17-21].Finally, without the agent’s application during reperfusion, all require preconditioning during the ischemia (i.e., purportedly attenuated ischemia to attenuate reperfusion)[17-21].
On the other hand, the resolution of all these points in Pringle rats mandates BPC 157 pleiotropic beneficial effects[2-6].This includes those it has on the liver (including portal hypertension) and intestinal (i.e., simultaneously induced lesions by NSAIDs[22-25], insulin[26], or alcohol[27]), lung[28-30], venous and arterial thrombosis[9,31]as well as heart disturbances[32-36].BPC 157 counteracts the free radical formation and lesions in distinctive targets (i.e., liver[11,37]and gastrointestinal tract[7,8,10,38], vessels[9],brain[39], sphincters[40], bladder[41]).Namely, BPC 157 is an original cytoprotective antiulcer peptide rapidly acting in particular to protect the endothelium, used in ulcerative colitis and now in a multiple sclerosis trial, with lethal dose (LD1) not achieved[2-6].
Ultimately, using the regimens effective in previous studies[7-11], rats before, during and after the Pringle maneuver used several distinct BPC 157 regimens to resolve ischemia (PTO-ligation-period) and reperfusion-related injury (post-PTO-period) and demonstrated a direct beneficial effect with regard to either injury.
MATERIALS AND METHODS
Animals
Study protocols were conducted in male Albino Wistar rats, body weight 200 g, 12 wk old, randomly assigned, used in all of the experiments, with six rats/group/interval,approved by the local Ethics Committee (case number 380-59-10106-17-100/290) and by the Directorate of Veterinary (UP/I-322-01/15-01/22).They were in-house bred -Pharmacology animal facility, School of Medicine, Zagreb, Croatia.The animal facility registered by the Directorate of Veterinary; Reg.No: HR-POK-007.Laboratory rats were acclimated for 5 d and randomly assigned to their respective treatment groups.They were housed in PC cages in conventional laboratory conditions at a temperature of 20 °C-24 °C, a relative humidity of 40%-70% and a noise level 60 DCB.Each cage was labelled according to study number, group, dose, number and sex of each animal.Fluorescent lighting provided illumination 12 h/d.Standard Good Laboratory Practice diet and fresh water were providedad libitum.Animal care was in compliance with the SOPs of the Pharmacology Animal facility; the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes(ETS 123).
Ethical principles of the study ensured compliance with European Directive 010/63/E, the Law on Amendments to Animal Protection Act (Official Gazette 37/13), the Animal Protection Act (Official Gazette 135/06), Ordinance on the Protection of Animals used for Scientific Purposes (Official Gazette 55/13), FELASA recommendations and recommendations of the Ethics Committee School of Medicine,University of Zagreb.Experiments were assessed by observers unaware of the given treatment.
Drugs
As previously[15-19]medication, without carrier or peptidase inhibitor, included stable gastric pentadecapeptide BPC 157 (a partial sequence of the human gastric juice protein BPC, freely soluble in water at pH 7.0 and in saline).
It was prepared as a peptide with 99% (HPLC) purity (1-des-Gly peptide was the main impurity; manufactured by Diagen, Ljubljana, Slovenia, GEPPPGKPADDAGLV,M.W.1419) (dose and application regimens as described previously[2-11].
Surgery
In deeply anesthetised rats [thiopental (Rotexmedica, Germany) 40 mg/kg ip, apaurin(Krka, Slovenia) 10 mg/kg ip], the portal triad was exposedviaa midline laparotomy and then clamped up for 30 min.Rats then underwent reperfusion for either 15 min or 24 h.
Medication
To evaluate lesions and blood vessel presentation by gross, microscopic and venographic assessment, electrocardiogram (ECG), contrast ink application,thrombosis, serum enzymes level assessment and free radicals in liver tissue assessment, medication [BPC 157 (10 μg/kg, 10 ng/kg) or saline (5 mL/kg) (controls)]was applied as a bath at the clamped area immediately after portal triad clamping in rats with PTO.Likewise, the same medication [BPC 157 (10 μg/kg, 10 ng/kg) or saline(5 mL/kg) (controls)] was applied as a bath at the area that used to be clamped immediately after clamping removal and reperfusion initiation.For portal vein, caval vein and abdominal aorta pressure recording, medication [BPC 157 (10 μg/kg, 10 ng/kg) or saline (5 mL/kg) (controls)] was applied intraperitoneally in rats at 5 min before PTO, as a bath in rats with PTO at 5 min or 30 min of ligation time, as a bath in rats that had PTO, in the post-PTO-period, at 1 minute or at 24 h of reperfusion time.For portal vein, caval vein venography or yellow ink contrast application, the medication [BPC 157 (10 μg/kg, 10 ng/kg) or saline (5 mL/kg) (controls)] was immediately applied before as a bath in rats with PTO at 15 min of ligation time, as a bath in rats used that had PTO, in the post-PTO-period, at 15 min reperfusion time.
Portal vein and abdominal aorta pressure recording
In deeply anesthetised and laparatomised rats, the recording lasted 5 min, with a cannula (BD Neoflon™ Cannula) (assessed in 1-min intervals) connected to a pressure transducer (78534C MONITOR/TERMINAL Hewlett Packard), inserted into the portal vein, inferior caval vein and abdominal aorta at the level of bifurcation at 5 or 30 min of ligation time in rats with PTO or in rats that had PTO at 5 min or 24 h of reperfusion time.
Of note, normal rats exhibit a portal pressure between 3 and 5 mmHg[34]or like the pressure in the inferior caval vein (providing at least 1 mmHg higher values in the portal vein) and abdominal aorta blood pressure values between 100 and 120 mmHg at the level of bifurcation[17].
ECG recording
In deeply anesthetised rats, the ECG was recorded continuously in all three main leads by positioning stainless steel electrodes on all four limbs, using an ECG monitorviaa 2090 Medtronic programmer (Minneapolis, MN, United States) connected to a digital oscilloscope (LeCroy waverunner LT342, Chestnut Ridge, NY, United States),which enabled precise recordings, measurements and analysis of the ECG parameters[32-36].
Vessels, intestine, liver presentation
Using a camera attached to a USB microscope (Veho discovery VMS-004 deluxe), in deeply anaesthetised rats, we directly recorded the presentation of the vessels.We assessed vessels [filled/appearance or cleared out/disappearance (hollow)] at the stomach and between the arcade vessels on the ventral and dorsal sides at a 1-cm long segment of the duodenum, jejunum, ascending colon and between 10 vessels from the proximal to the distal cecum throughout the experiment.Assessments were made at selected time points before and after therapy - with regard to the point immediately before therapy (as 100%) - in rats with PTO at 5, 15 and 30 min of ligation time and in rats that had PTO at 5, 10 and 15 min of reperfusion time.
We grossly assessed yellow or pale areas in the liver [(1): Normal liver presentation with no yellow or pale areas; (2) Only small yellow or pale areas; (3) Mild yellow or pale areas; and (4) Extensive yellow or pale areas].We assessed hemorrhagic congestive areas in stomach, duodenum, jejunum, cecum and colon ascendens, scored upon opening 1-4, (1) normal mucosa presentation; (2) only small hemorrhagic areas;(3) advanced hemorrhagic areas; and (4) extensive and severe hemorrhagic areas.Assessments occurred before sacrifice at 30 min of ligation time in rats with PTO or at 15 min or 24 h of reperfusion time in rats that had PTO.
Using the described camera attached to a USB microscope, we monitored the application of yellow or orange ink (KOH-I-NOR HARDTMUTH, Ceske Budejovice,Czech Republic) in rats with a PTO-ligation into the portal vein below the point of ligation or up to this point before its entry into the liver or the inferior caval vein.This was done to verify, upon application, the rapid presentation (or absence) of contrast in the liver, increased vessel-branching or tortuous veins of portosystemic shunts (of portal vein-superior, mesenteric vein-inferior, mesenteric vein-rectal, veins-left iliac,vein-inferior caval vein, azygos vein).Thereby, we used a simple scoring system (1)presentation not different from healthy; (2) presentation shows moderate increase; (3)presentation shows mild increase; and (4) presentation shows abundant increase.Assessments were performed at 15 min of ligation time in rats with PTO or at 15 min of reperfusion time in rats that had PTO.We used a total injection volume of 1 mL (0.1 mL/s) in the portal vein or of 2 mL (0.3 mL/s) in the inferior caval vein.
Venography
Venography, in a fluoroscopy unit (Shimadzu type C-VISION PLUS, Tokyo, Japan)[17],was performed (1) in rats with a PTO-ligation or (2) in rats that had PTO, during reperfusion.Warmed non-ionic contrast medium (Iohexol; OMNIPAQUE 350, GE Healthcare, Chicago, United States) was injected (A) in rats with a PTO-ligation into the (1) portal vein below obstruction [total injection volume of 1 mL (0.1 mL/s)]; (2)portal vein up to obstruction [total injection volume of 1 mL (0.1 mL/s)]; and (3)inferior volume of 2 mL (0.3 mL/s).The contrast medium was visualised under real time to assure adequate filling.The subtraction mode was used to record the images(14 frames per second).At 15 min of ligation time, or at 15 min of reperfusion-time,venograms were taken, captured and digitised onto a personal computer file,followed by analysing using the ISSA (VAMSTEC- Software Company, Zagreb,Croatia) image program.We assessed the number of rats presenting (1) full presentation of the portal vein-superior, mesenteric vein-inferior, mesenteric veinrectal, vein-left iliac, vein-inferior caval, vein pathway (portal vein venography below obstruction); (2) complete filling of the hepatic venous vascular bed, hepatic vein,inferior caval vein and the right atrium of the heart (portal vein venography up to obstruction); and (3) blood flow through the hepatic veins into the liver, and the liver fully presented (inferior caval vein venography at the level of bifurcation) or (B) the time to liver presentation in reperfusion with inferior caval vein venography at the level of bifurcation.
Microscopy
In rats with PTO, at 30 min, and in rats after PTO, in the post-PTO period, in reperfusion at 15 min and 24 h of reperfusion time, tissue specimens from liver,spleen, stomach, duodenum, ileum, cecum, ascending colon, cecum, liver and heart were obtained.The tissue specimens were fixed in buffered formalin (pH 7.4) for 24 h,dehydrated and embedded in paraffin wax, followed by staining with hematoxylineosin.Tissue injury was evaluated microscopically by a blinded examiner.
Liver and spleen weight, ascites
Liver and spleen weight were expressed as percentages of the total body weight(presenting in normal rats, for liver 3.2%-4.0% and 0.20%-0.26% for spleen).Likewise,ascites (mL) were assessed.
Thrombus assessment
At death, the portal vein, the superior mesenteric vein (up to the inferior anterior pancreaticoduodenal vein), the lienal vein inferior and the caval vein, as well as a hepatic artery were removed, and the clot was weighed[17].
Bilirubin and enzyme activity
To determine the serum levels of aspartate transaminase (AST), alanine transaminase(ALT, IU/L) and total bilirubin (μmol/L), blood samples were collected immediately after euthanasia and centrifuged for 15 min at 3000 rpm.All tests were performed using an Olympus AU2700 analyser with original test reagents (Olympus Diagnostica, Lismeehan, Ireland)[28-34,42].Since bilirubin levels were not increased, the data are not shown.
Oxidative stress in liver
At the end of the experiment and at 30 min of PTO ligation time or in the post-PTO ligation period, at 15 min and 24 h of reperfusion time, oxidative stress in the collected tissue samples was assessed by quantifying thiobarbituric acid-reactive species as malondialdehyde (MDA) equivalents, as described previously[15-19,43,44].
For this, the tissue samples were homogenised in PBS (pH 7.4) containing 0.1 mmol/L butylated hydroxytoluene (Tissue Ruptor, Qiagen, United States) and sonicated for 30 s in an ice bath (Ultrasonic bath, Branson, United States).
Trichloroacetic acid (10%) was added to the homogenate, the mixture was centrifuged at 3000 rpm for 5 min, and the supernatant was collected.Then, 1% TBA was added,and the samples were boiled (95 °C, 60 min).The tubes were then kept on ice for 10 min.Following centrifugation (14000 rpm, 10 min), the absorbance of the mixture was determined at a wavelength of 532 nm.The concentration of MDA was estimated based on a standard calibration curve plotted using 1,1,3,3’-tetraethoxypropane.The extent of lipid peroxidation was expressed as MDA, using a molar extinction coefficient of 1.56 × 105mol/L/cm.The protein concentration was determined using a commercial kit; the results are expressed in nmol per mg of protein.
Statistical analysis
Statistical analysis was performed by parametric one-way ANOVA with post hoc Newman-Keuls test and non-parametric Kruskal-Wallis followed by the Mann-WhitneyU-test to compare groups.Values are presented as the mean ± SD and as the minimum/median/maximum values.To compare the frequency difference between the groups, theχ2test or Fischer's exact test was used;aP< 0.05 was considered statistically significant.
RESULTS
We focused on the stable gastric pentadecapeptide BPC 157 and the recruitment of the portal vein-superior, mesenteric vein-inferior, mesenteric vein-rectal, vein-left iliac,vein-inferior caval vein pathway to recover Pringle rats in ischemia.Likewise, the focus was on BPC 157 and the counteraction of the reperfusion-induced worsening when applied in reperfusion.
All BPC 157 administration regimens (μg- and ng-regimens) were effective in ischemia and reperfusion (Figures 1-14).The portal hypertension assay (Figure 1) and the disturbances course documented a marked attenuation when it was given before(5 min) PTO, much like in the rats with PTO and pre-existing severe portal hypertension and systemic hypotension (seen in the abdominal aorta) (at 5 or 30 min of ligation time).In reperfusion, the worsening that simultaneously appeared and persisted, the huge portal hypertension and, even more, the caval hypertension and aortic pressure not compensated (increasing to values of 80 mmHg) completely disappeared with BPC 157 medication (given at 1 min and 24 h of reperfusion time)(Figure 1).Likewise, unlike the controls with peaked P waves and tachycardia, QRS complexes such as right bundle branch block (RBBB) pattern in all rats, in ischemia and reperfusion, the peaked P waves and tachycardia either did not appear or, if preexisting, they rapidly disappeared with all BPC 157 regimens (Figure 1).The RBBB pattern was absent (Fisher´s exact probability testaP< 0.05 at leastvscontrol) and sinus rhythm appeared in the normal range of heart frequency (Figure 2).As visualised grossly, in ischemia and in reperfusion, with BPC 157, increased blood vessel branching rapidly appeared in the serosa of all organs affected (Figures 3-5),while the splenic veins were particularly less congested and tortuous (Figure 2), much like the azygos vein, indicating the counteraction of the right heart malfunction(Figure 5).In a period of 30 min of PTO, progressive thrombosis occurred in controls(i.e.in the portal vein, the lienal vein, the superior mesenteric vein and the inferior caval vein as well as in the hepatic artery) (Figure 6).Contrarily, strong attenuation occurred in the veins and artery of the BPC 157 rats presenting only considerably smaller clots (Figure 6).Likewise, in ischemia and reperfusion, BPC 157 rats had much less ascites formation (Figure 6).Serum ALT and AST values in ischemia and in reperfusion increased in controls and lessened in rats along with BPC 157 administration in either ischemia or reperfusion (Figure 7).Administration of BPC 157 in either ischemia or reperfusion markedly declined gross lesions in the liver(yellowish areas in ischemia; grey areas in reperfusion) and in the gastrointestinal tract (hemorrhagic lesions mostly exaggerated in the stomach and the duodenum in ischemia and in reperfusion) (Figures 7-9); splenomegaly was abolished in ischemia,but presented in reperfusion (Figure 7).
The MFDA-levels in the liver may be indicative.Regularly, PTO increased MDA levels in the liver, and reperfusion additionally increased them, unless BPC 157 administration resulted in MDA levels in the liver within a normal, healthy range in both ischemia and reperfusion (Figure 6).
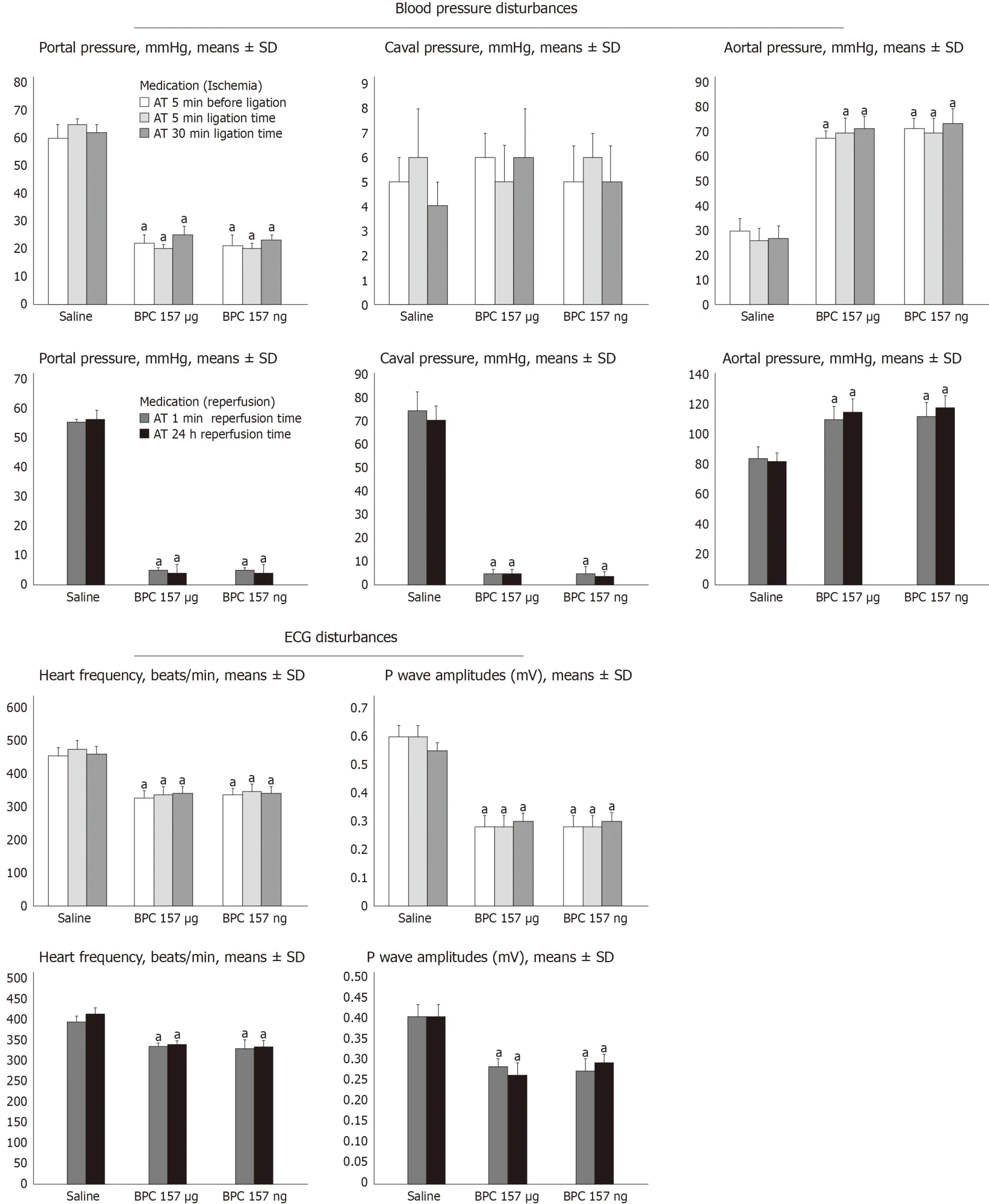
Figure 1 Counteracting effect of BPC 157 on portal, caval hypertension and aortal hypotension, sinus tachycardia, peaked P waves, in (1) ischemia and (2)reperfusion.
Figure 2 BPC 157 effect on electrocardiogram-disturbances (left) and lienal veins (right) presentation.
Further, with respect to the portal vein-superior, mesenteric vein-inferior, superior mesenteric vein-rectal, veins-left iliac, vein-inferior, caval vein shunt along with portal hypertension, persisting (controls) or quickly counteracted (BPC 157), portal vein venography (Figure 10) or orange ink contrast (Figure 10) application below ligation likely revealed the portosystemic shunt non-functioning (controls) or functioning(BPC 157) presentation.Controls presented such portosystemic shunt only weakly(Fisher´s exact probability testaP< 0.05 at leastvscontrol) (Figure 10A), inferior mesenteric vein with the tortuous presentation (Figure 10A).The BPC 157 rats fully presented portosystemic shunt as portal vein-superior, mesenteric vein-inferior,superior mesenteric vein-rectal, veins-left iliac, vein-inferior caval vein way (Figure 10B), rectal inferior mesenteric vein with increased branching (Figure 11a2).Likewise,after BPC 157 application, as a function of time, portal vein venography up to ligation(Figure 10) or yellow ink contrast (Figure 11) application, before its entry into the liver, revealed complete filling of the hepatic venous vascular bed, hepatic vein,inferior caval vein and the right atrium of the heart (Figure 10D).There was an immediate presentation of the yellow contrast in the liver (Figure 11, b2 and b4),which further progressed.Contrarily, in the controls, there was no filling of the hepatic vascular bed or any other blood vessels except for the portal vein (note, as administration of contrast continued, the portal vein ruptured) (Fisher´s exact probability testaP< 0.05 at leastvscontrol).Also, no yellow contrast appeared in the liver; as the administration of contrast continued, the portal vein ruptured (Figure 11,b1 and b3).Thus, a particular functioning of intrahepatic vasculature capacity occurs in rats that received BPC 157 therapy.Also, after BPC 157 application, inferior caval vein venography or yellow ink contrast application at the bifurcation site (Figures 7,10E) demonstrated blood flow through the hepatic veins into the liver, and the liver fully presented.There was an immediate and highly abundant presentation of yellow contrast in the liver and in the hepatic veins.Contrarily, venography could regularly not find blood flow through the hepatic veins into the liver, and the liver was not presented in controls (Figure 7) (Fisher´s exact probability testaP< 0.05 at leastvscontrol), while the yellow contrast demonstrated an immediate, but very scant,presentation in the liver and hepatic veins, which would later disappear (Figure 10E).Consequently, it is likely that BPC 157 affects hepatic vein contribution, thereby counteracting the absence of blood flow in the liver during PTO.
In reperfusion, we assume that also intrahepatic vasculature capacity during reperfusion could be rescued.Namely, in reperfusion, portal vein venography (Figure 10) or yellow ink contrast (Figure 11) application into the inferior caval vein at the bifurcation site demonstrated a distinctive reperfusion in BPC 157 rats, which exhibited more extensive and faster reperfusion (Figures 10H and 11d2).This may be an interesting finding with respect to BPC 157 administration in reperfusion, along with the pre-existing ECG-disturbances, severe portal hypertension, and even more caval hypertension and not compensated aortal hypotension and high MDA-level liver, which were all counteracted, and organ lesions markedly attenuated.A comparative venography assessment upon contrast application showed faster liver presentation in BPC 157 rats [5.06 ± 0.1 (μg), 5.16 ± 0.2 (ng)vs11.55 ± 0.1 (control) s];aP< 0.05, at leastvscontrol.Consistently, a comparable application of yellow ink revealed that BPC 157 rats showed abundant presentation of yellow contrast in the liver before the controls.
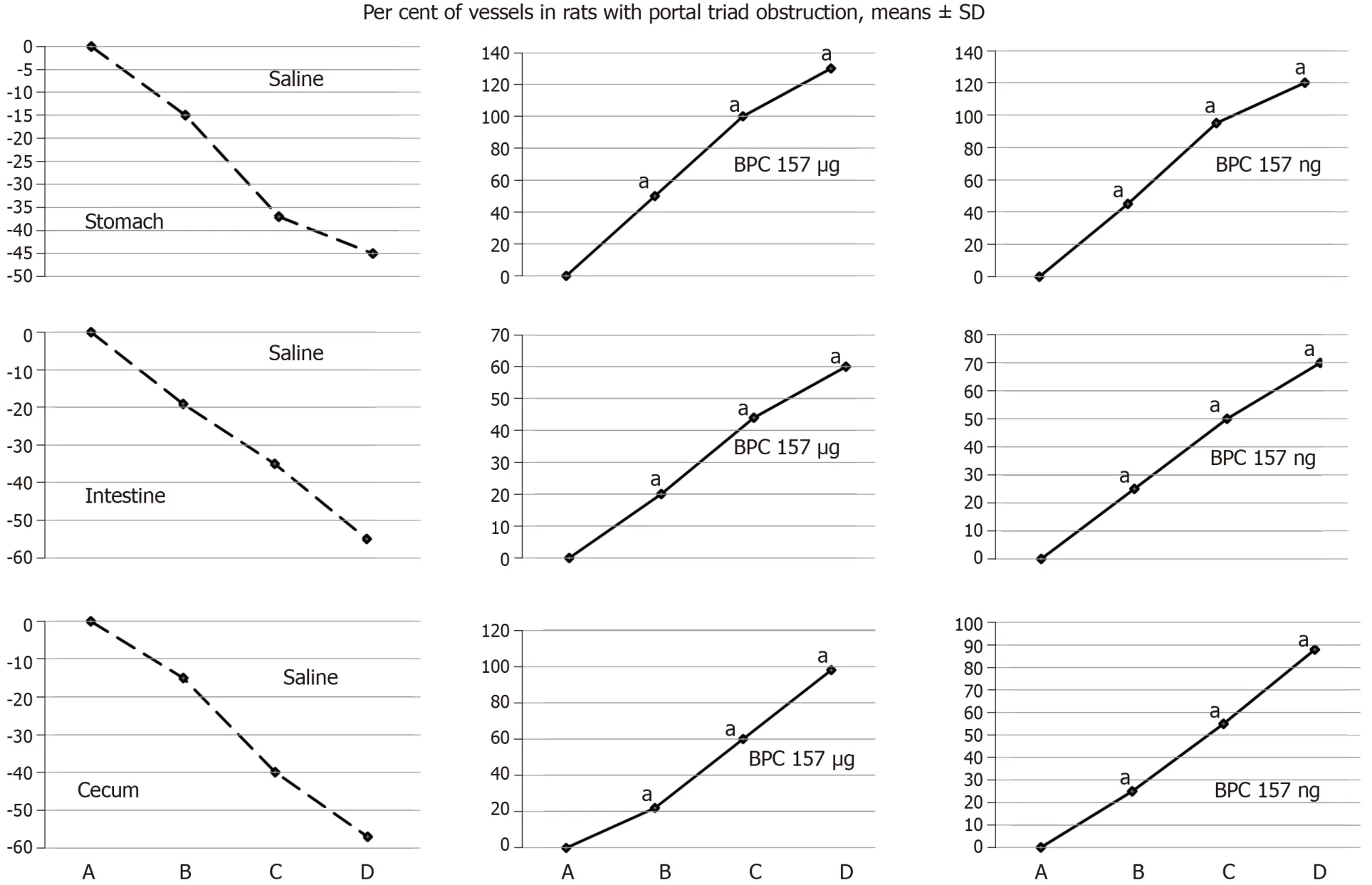
Figure 3 BPC 157 improves the vessels presentation during ischemia.
Regularly, PTO rats, much like post-PTO rats, appeared with a considerable amount of similar lesions.Illustratively, in livers, the substantial congestion of the central vein, as well as the branches of the terminal portal venules, are the most prominent findings (Figure 8), along with submucosal edema, substantial capillary congestion, extravasation of erythrocytes and erythrocytes with ischemic changes consistently present in the stomach, duodenum, jejunum, cecum and ascending colon(Figures 12 and 13).Specifically, lifting of the surface of the mucosal epithelial layer appears in the stomach, along with the loss of villous architecture, loss of surface epithelium in the duodenum, jejunum and cecum and focal loss of epithelium in the ascending colon.The BPC 157 therapy largely attenuated all of the noted changes in PTO-rats (Figures 12 and 13).In the liver, BPC 157 rats showed no congestion of the central vein or branches of the terminal portal venules (Figure 8), as well as less submucosal edema, capillary congestion and preserved mucosal architecture throughout the whole intestine (Figures 12 and 13).Lungs presented with preserved architecture, but mild to severe capillary congestion in alveolar septa, progressing during the reperfusion, particularly with a prolonged period, a course markedly counteracted in BPC 157 rats (Figure 14).
In the spleen, all rats exhibited sinusoidal congestion and enlargement of the red pulp, leading to a reduction of the white pulp at the end of the ischemia (data not shown).
Likewise, as expected, no morphological changes were found in the myocardium,mainly because changes found on an ECG were the result of acute right ventricular overload (data not shown).
Generally, these results indicate the success of BPC 157 therapy (Figures 1-14).With BPC 157 given either before or during PTO, there is the resolution of ischemia-induced disturbances.Likewise, with BPC 157 given during reperfusion,after PTO, at post-PTO time, there is a counteraction of the reperfusion-induced disturbances in rats.
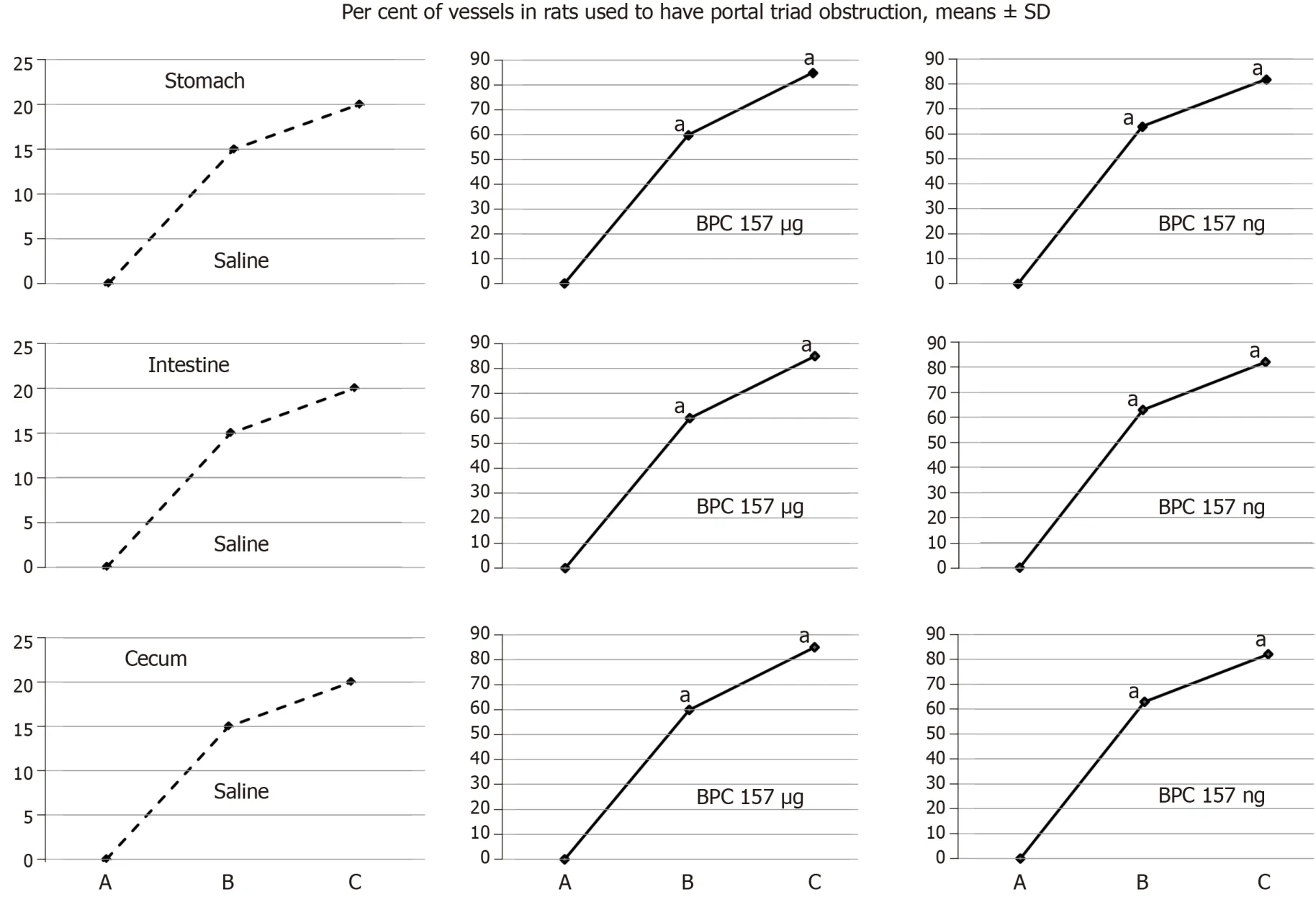
Figure 4 BPC 157 improves the vessels presentation during reperfusion.
DISCUSSION
We emphasize the resolving of the Pringle maneuver, the stable gastric pentadecapeptide BPC 157[2-6]therapy in ischemia and in reperfusion, its portal veinsuperior mesenteric vein-inferior mesenteric vein-rectal vein-left iliac vein-inferior caval vein pathway recruitment (Figures 10 and 11).This therapeutic effect rapidly decompressed portal hypertension and related disturbances in Pringle rats (Figure 1),much like its particular effect on the occluded vessels, the bypassing of the occlusion and the reestablishment of the blood flow[7-11].Supportive effectiveness analogy goes with the successful ischemic/reperfusion therapy demonstrated in the rats with the infrarenal occlusion of the inferior caval vein, ischemic/reperfusion colitis, duodenal congestion and cecum perforation injuries[7-10], as well as bile-duct-induced liver cirrhosis with portal hypertension[11].Here, BPC 157 administration in ischemia promptly attenuates portal hypertension (effective prophylactically (portal hypertension development prevented); a therapeutic effect against pre-existing shortlasting and long-lasting portal hypertension, both decreased instantaneously).Moreover, in the worst reperfusion condition, as therapy efficacy progressed, BPC 157 completely eliminated both portal and caval hypertension and aortal hypotension(Figure 1).
Illustratively, inferior mesenteric vein presentation is tortuous in controls, unlike in BPC 157 rats (Figure 11).In BPC 157-rats, in ischemia and in reperfusion, the end result of the oxidative stress (MDA level) in the liver is continuously within the normal values.Contrarily, in the control livers, high MDA values during PTO further progressed in reperfusion (Figure 6)[2-6].Control rats exhibited rapid clot formation (in the portal vein, superior mesenteric vein, lienal vein, inferior caval vein, hepatic artery) (Figure 6) and ascites (consistently present in the ischemia and reperfusion period) (Figure 6).These disturbances contrast with apparently less venous and less arterial clots and less ascites in BPC 157 rats, as emphasized previously[9,11,31].Moreover, controls presented with immediately peakedPvalues, tachycardia and an RBBB pattern of QRS complexes as the identifiers of the right heart failure (Figures 1 and 2) [and thereby, congested azygos vein (Figure 5) and lung congestion (Figure 14)].This failure contrasts with the ECG disturbances completely abrogated (and thereby, non-congested azygos vein) and less lung congestion in BPC 157-rats[2-6].Therefore, the immediate presentation of adverse effects and the immediate therapeutic effect may suggest the essential immediate cause-consequence chain of events and, in particular, the relation to the liver as the prime organ affected[2-6], liver and liver circulation failure, presentation (controls) and counteraction (BPC 157) in Pringle rats.All controls exhibited gross lesion progression, in ischemia and in reperfusion (Figure 7), increased enzyme serum values (Figure 7) and a dilated central vein and terminal portal venules (Figure 8).This contrasts with the markedly spared gross liver presentation, less gross lesions in the ischemia and in the reperfusion,lower serum enzyme values and no congestion of the central vein or branches of the terminal portal venules in BPC 157-rats.
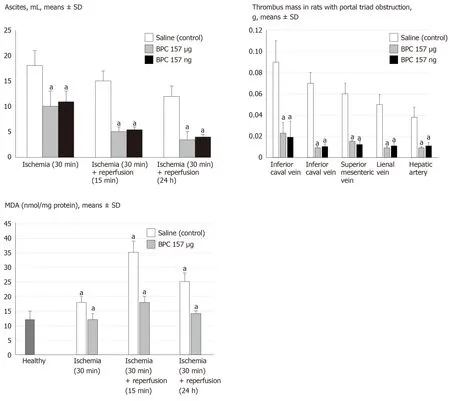
Figure 6 BPC 157 counteracts ascites (mL) (upper), increased MDA-level (nmol/mg protein) in liver (low), in ischemia and reperfusion, and thrombus presentation (thrombus mass, g) in veins [inferior caval vein, portal vein, superior mesenteric vein, lienal vein] and artery (hepatic artery) (middle) during portal triad obstruction.
First, while the quick activation of the portal vein-superior mesenteric vein-inferior mesenteric vein-rectal vein-left iliac vein-inferior caval vein pathway may rapidly decompress portal hypertension, this may reflect the regular absence of blood in the sinusoid[45]and reversal with BPC 157 therapy.Unlike controls, in BPC 157 rats, there is retrograde blood entry through the hepatic veins and liver visibility or yellow contrast presentation in hepatic veins, as well as rapid abundant presentation in the liver (inferior caval vein venography or yellow contrast application in inferior caval vein, during PTO) (Figures 10 and 11).Consequently, the anoxic effect of ischemia on sinusoidal endothelial cells, Kupffer cells and hepatocytes, was accentuated (controls),or counteracted (BPC 157), and thereby, enhanced liver dysfunction was accentuated(controls) or counteracted (BPC 157), as reflected in the more (controls) or markedly less (BPC 157) increase in liver enzymes (Figure 7) (including also the consequence of reduced bile flow rate)[45].
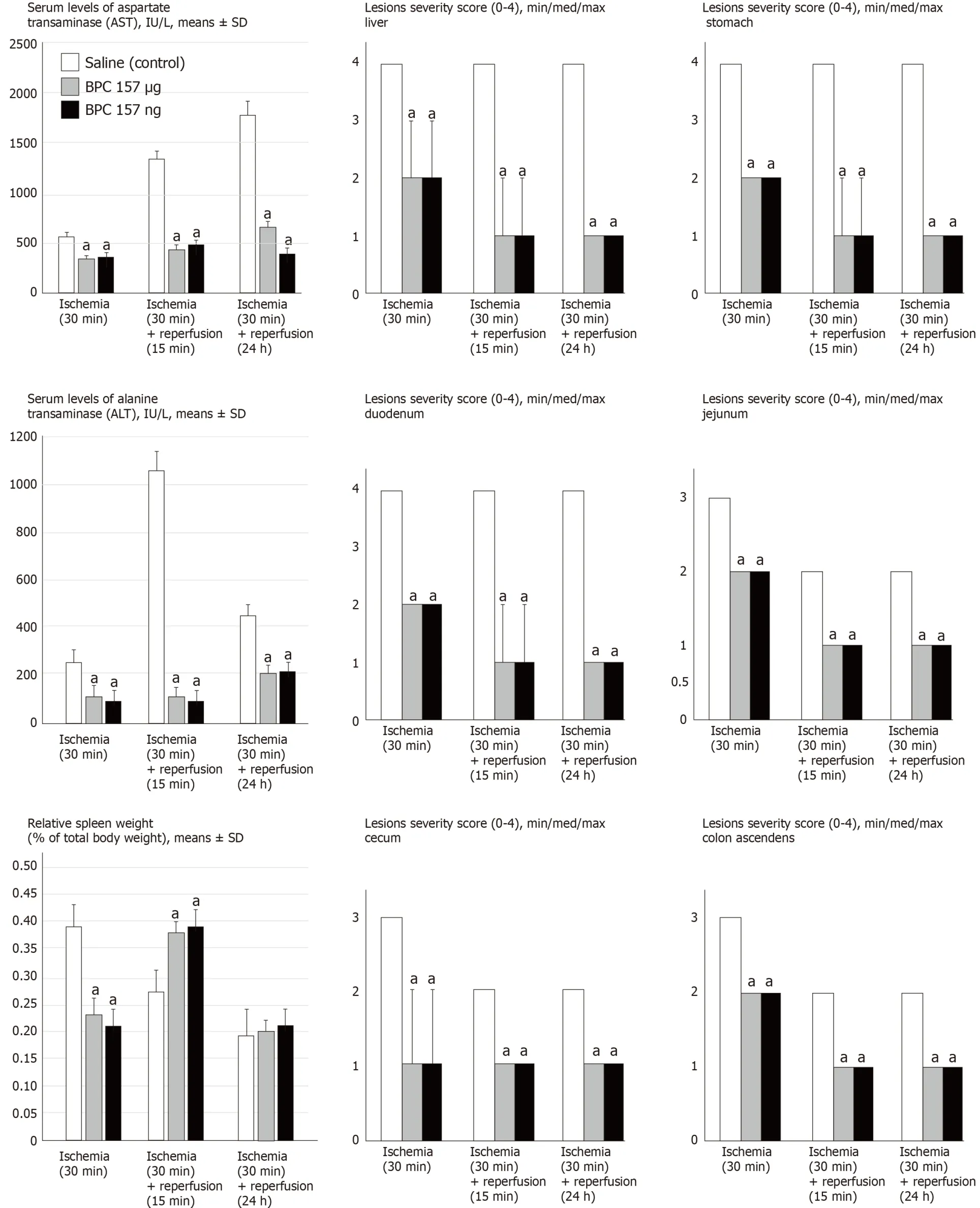
Figure 7 BPC 157 counteracts increased serum enzymes values (lU/L, mean ± SD), gross lesions severity (liver, stomach, duodenum, jejunum, cecum,ascending colon) scored 0-4, Min/Med/max, splenomegaly (relative spleen weight as % of total body weight, mean ± SD) in ischemia, (splenomegaly appears in early reperfusion).
Second, portal vein venography (or yellow contrast application) up to the ligation before its entry into the liver indicates the particular functioning (BPC 157) [or nonfunctioning (controls)] of intrahepatic vasculature capacity during PTO in rats.Unlike in the controls, with BPC 157 therapy, as a function of time, there was the complete filling of the hepatic venous vascular bed, the hepatic vein, the inferior caval vein and the right atrium of the heart and an immediate presentation of yellow contrast in the liver, which further progressed (Figures 10 and 11).
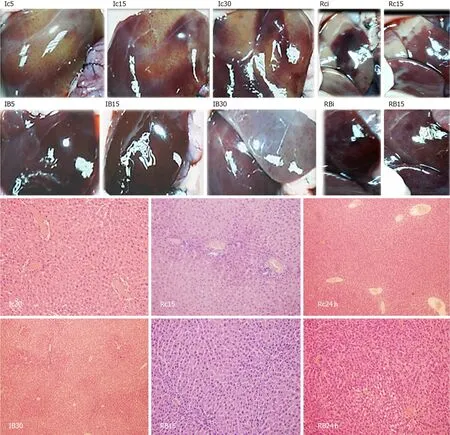
Figure 8 BPC 157 effect on liver gross presentation (left, lc5, lc15, lc30, Rci, Rc15, lB5, lB15, lB30, RBi, RB15) and liver microscopy [right, lc30, Rc15, Rc24 h, lB30, RB15, RB24 h (white letters)] presentation.
As a final point, the acknowledged increased vascular incapacitation of the liver during reperfusion as a follow up of PTO (and thereby, persistent severe portal hypertension, complicated with even more caval hypertension), the therapy application in reperfusion could either further accentuate (saline, controls) or rapidly resolve (BPC 157) debilitated intrahepatic vascular capacity.Therefore, in BPC 157 rats, the rescue is documented with the inferior caval vein venography, much like with the yellow contrast application in the inferior caval vein during reperfusion.The BPC 157 rats exhibited more rapid and extensive full liver presentation and yellow contrast in the liver (Figures 10 and 11); they also microscopically counteracted substantial congestion of the central vein as well as branches of the terminal portal venules (Figure 5).Simultaneously, as already mentioned above, just opposite to the downhill course in controls, portal and caval hypertension and aortal hypotension disappeared (Figure 1), peaked P waves and tachycardia disappeared (Figure 1), lung congestion decreased (Figure 14) and MDA liver values became normal (Figure 6).In addition, it may be the prompt antithrombotic effect of BPC 157 application[9,31,43,46]that provided the abrogated thrombosis[1]as an important factor to rescue the disturbed hepatic microcirculation and increased hepatic vascular resistance.Likely, BPC 157 acts to counteract Virchow, as it did in the rats with inferior caval vein occlusion[9].Thus, BPC 157 may counteract the regular venous return decrease to the heart and failure of spontaneous decompression of the portal system (note, caval hypertension was consistently elevated in comparison to the portal hypertension, with a gradient of at least 10 mmHg).Such a special effect may be that BPC 157 specifically interacts with the NO-system[44], counteracts NOS-blocker L-NAME-induced hypertension as well as NOS-substrate L-arginine-induced hypotension[43], potassium over-dose and severe hyperkalemia arrhythmias and hypertension[33]or doxorubicin-induced chronic heart failure and hypotension[34].
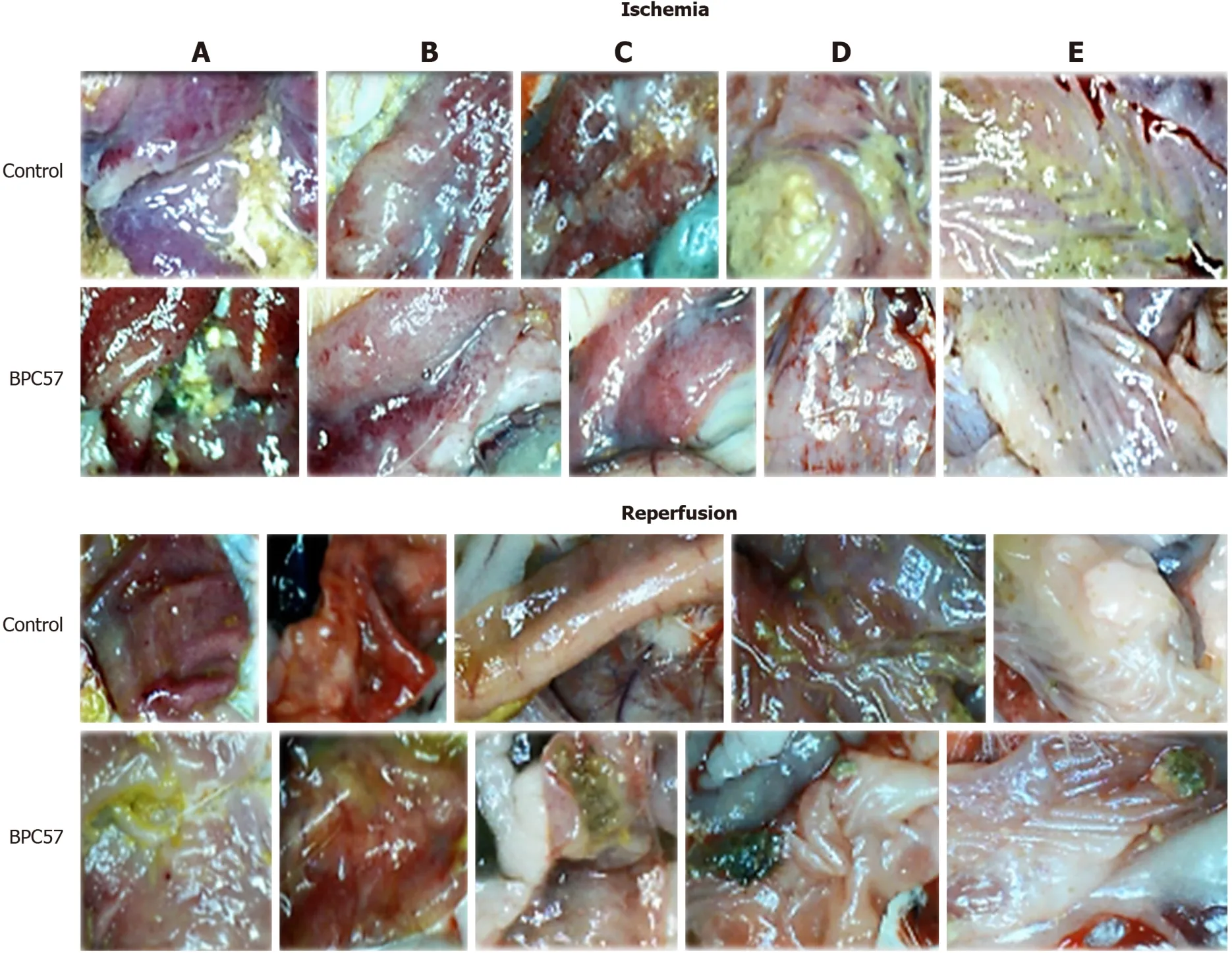
Figure 9 BPC 157 effect on gastrointestinal lesions gross presentation in ischemia (upper) and in reperfusion (lower).
Resolution with BPC 157 therapy abrogated all congestive hemorrhagic intestinal lesions, from the stomach to the ascending colon (Figures 7, 9, 12 and 13).Providing resolution of hemodynamic disturbances (Figure 1), in addition to the known beneficial BPC 157 effect on liver and gastrointestinal lesions[2-6], there is a supportive chain of events in the counteraction of lesions in ischemia and reperfusion.This occurs along with the rapid activation of a bypassing loop and, consequently, portal hypertension counteraction (Figure 1).Along with this is a “honeycomb” smaller vessel network, which appears at the intestinal serosa (Figures 3-5), a finding noticed in rats with ischemic/reperfusion colitis, duodenal venous congestion lesions or inferior caval vein occlusion[7-10].Otherwise, intestinal ischemia[42]appears as the final consequence of blood pooling in the splanchnic bed, inducing portal hypertension and multivisceral edema; mesenterial venous occlusion could induce intestinal injury as early as within 5 min, both of which are inflow and outflow alterations[47].
Probably, these findings, when summarized, would need relations that are more precise.On the other hand, the recovery of the Pringle rats, in the ischemia and in the reperfusion, could explain the previously described pleiotropic effect on all of the affected systems[2-6], venous and arterial thrombosis[9,31]and free radical formation[2-6].
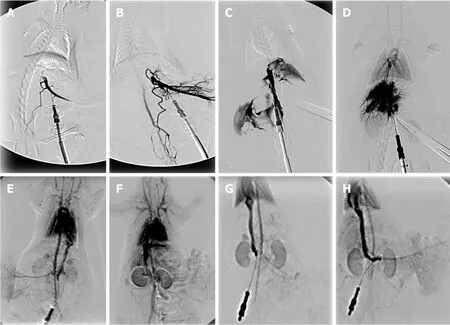
Figure 10 BPC 157 and venography assessment.
Namely, BPC 157 affects different molecular pathways noted in counteracting tumor cachexia[48], vascular occlusion[9], and various lesions (i.e., skin[49,50], tendon[51,52],muscle[53]).Of note, the progressive activation of the mTOR signalling pathway is likely a pathogenic mechanism of portal hypertension, and thereby a blockade of mTOR as a possible therapy[54].Also, a release of TNF from the liver by the injured hepatic tissue, delivered to the pulmonary circulation as the first vascular bed, could be responsible for lung lesions[55].Therefore, it is indicative that in counteracting tumor-induced muscle cachexia, BPC 157 counteracts the expression of p-mTOR,much like it counteracts the increase of pro-inflammatory and pro-cachectic cytokines such as IL-6, TNF-α and the expression of FoxO3a, p-AKT and P-GSK-3β[48].
Thus, the administration of BPC 157 resolves the adverse effects of the Pringle maneuver.The immediate recovery of collaterals results in the bypassing of the obstruction, even in the worst conditions of PTO disturbances.Accordingly, the reversal of the signs of the right heart failure occurs.Portal hypertension markedly decreased and completely disappeared.Likely, this combined effect resolves the further circle of injuries (i.e., the specific pathology in the liver, intestine, spleen, lung and heart).Specifically, the disturbed hemodynamics (i.e., the severe portal hypertension and severe aortal hypotension during PTO as well as the severe portal and caval hypertension and mild aortal hypotension in reperfusion, along with refractory ascites formation), thrombosis and ischemia, perpetuated and progressed by reperfusion in the Pringle rats, would be attenuated or completely abrogated.The rapid reestablishment of blood flow in both the ischemic and reperfusion conditions accompanied the reduction of the increased MDA values in liver tissues to the normal values.Thus, these new insights into PTO and related disturbances, and into portal hypertension, suggest the effective BPC 157 use in future therapy.Otherwise, the abrupt PTO and severe portal hypertension (> 60 mmHg) [and in reperfusion, caval hypertension (> 70 mmHg)] makes spontaneous decompression of the portal system by a portocaval shunt hardly possible as well as severe aortal hypotension in the ischemia not compensated in the reperfusion.Further, these notations likely correlate with important pulmonary pathological alterations (i.e., the adult respiratory distress syndrome) associated with human liver transplantation[56], portopulmonary hypertension common in cirrhosis with refractory ascites[57], as well as with a high incidence of caval hypertension in patients with portal hypertension[58].
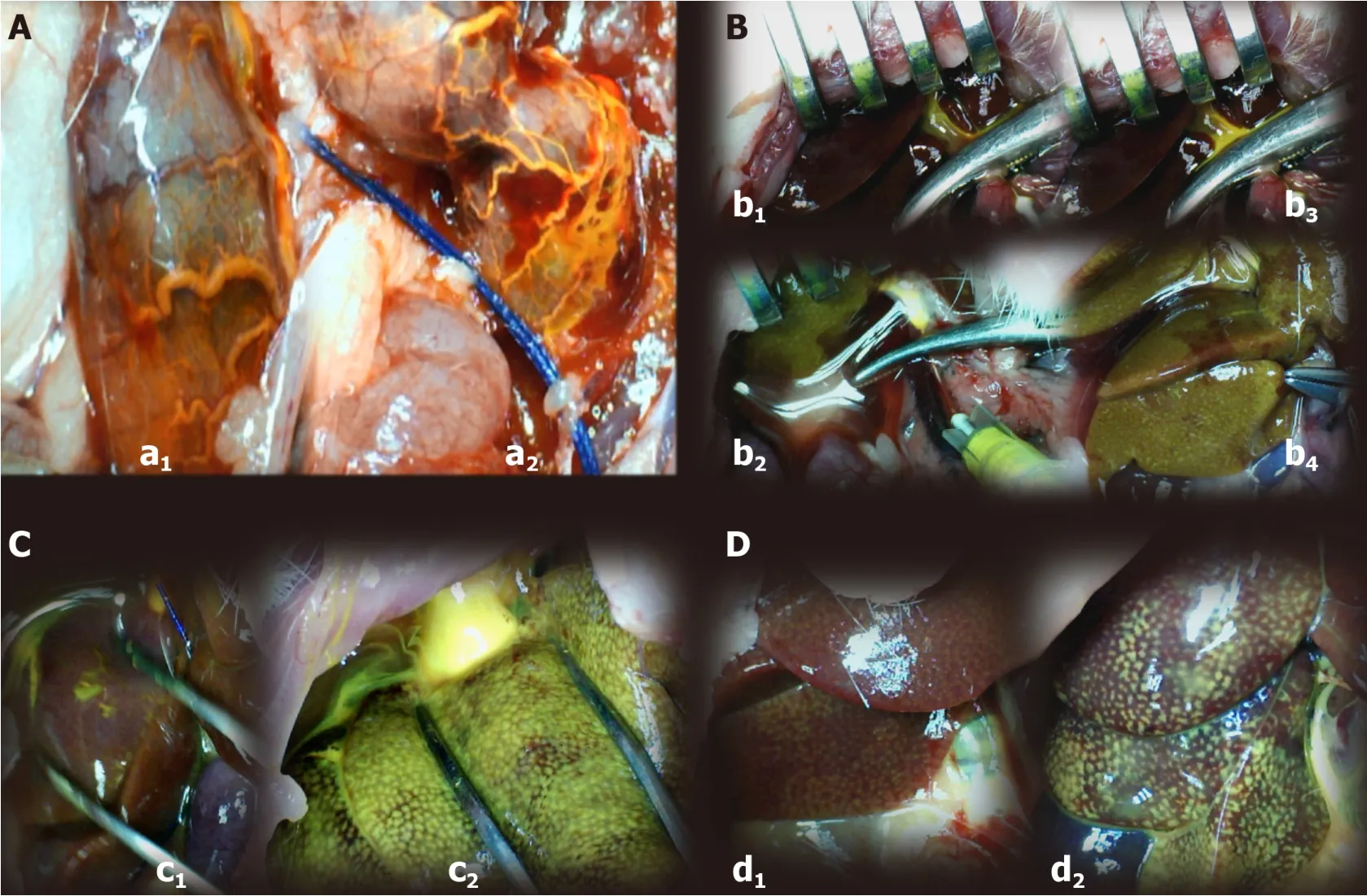
Figure 11 BPC 157 and yellow (orange) contrasts assessment.
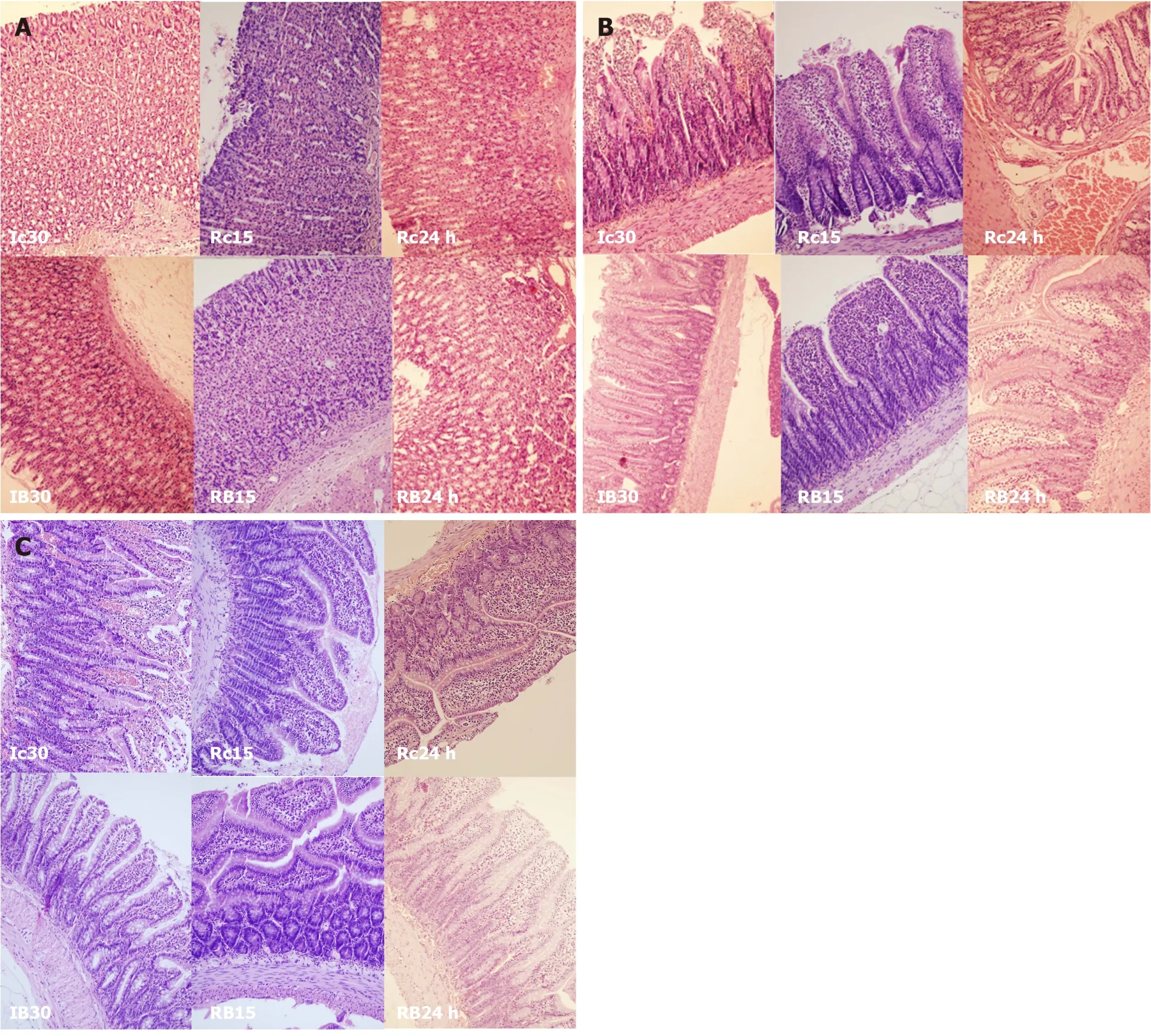
Figure 12 BPC 157 effect on gastrointestinal lesions microscopy presentation [stomach, duodenum, ileum].
Figure 13 BPC 157 effect on gastrointestinal lesions microscopy presentation (cecum, colon).
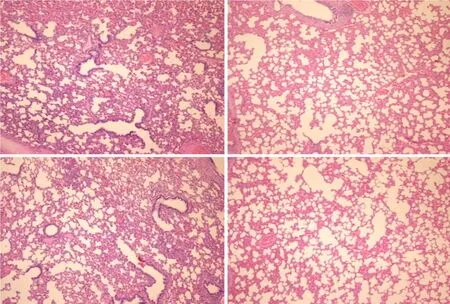
Figure 14 Lung (HE staining, × 4).
ARTICLE HIGHLIGHTS
Research background
The Pringle maneuver (portal triad obstruction) provides huge disturbances during ischemia and even more thereafter in reperfusion.Contrarily, a possible solution may be stable gastric pentadecapeptide BPC 157, with already documented beneficial effects in ischemia/reperfusion conditions.Recently, BPC 157, as a cytoprotective agent, successfully resolved vessel occlusions in rats (ischemic/reperfusion colitis; deep vein thrombosis, superior anterior pancreaticoduodenal vein; bile duct cirrhosis) through rapid collateral vessel recruitment to circumvent vessel occlusion.Likely, as a new effect, BPC 157 application may be useful when applied in the ischemia condition much like when given in the reperfusion condition.Thereby, medication BPC 157 regimens were administered as a single challenge before and during ischemia or,alternatively, at various time points during reperfusion.
Research motivation
We focused on the therapy of the Pringle maneuver in rats, so far not described severe preportal hypertension, the temporary portal triad obstruction (PTO), ischemia, the short and prolonged reperfusion, the lack of adequate portocaval shunting as the most detrimental feature that should be counteracted.With stable gastric pentadecapeptide BPC 157, we suggest the resolution of the damages, either those following occlusion or those following re-opening of the hepatic artery, portal vein and bile duct.
Research objectives
The first objective in the PTO-syndrome in rats is the rapidly activated way, portal vein-superior mesenteric, vein-inferior mesenteric vein-rectal vein-left iliac vein-inferior caval vein, supposed to appear as a specific activation of the collateral circulation, as the bypassing loop that can rapidly circumvent occlusions and decompress PTO-rats upon BPC 157 administration.The additional objective is verification that that solution in Pringle-rats with ischemia and reperfusion goes with resolution of the whole syndrome.In this, there are the resolution of oxidative stress, hemodynamic disturbances, severe portal and caval hypertension, aortic hypotension, rapid cloth formation in the portal vein, superior mesenteric vein, lienal vein,inferior caval vein, hepatic artery, ascites, peaked P waves, tachycardia; increased serum values;gross intestine, liver, lung, spleen and heart lesions.The final objective is demonstration that it goes also with the agent application during reperfusion.
Research methods
In the Pringle-rats with the PTO occluded or reopen, assessment includes gross (USB camera)and microscopic observations, venography, blood pressure and electrocardiogram assessment,bilirubin and enzyme activity, levels of nitric oxide, malondialdehyde in the liver.With the mentioned methods, assessed was the activated pathway, portal vein-superior mesenteric, veininferior mesenteric vein-rectal vein-left iliac vein-inferior caval vein.Then, we assessed the resolution of the oxidative stress, hemodynamic disturbances, severe portal and caval hypertension, aortic hypotension, rapid cloth formation in the portal vein, superior mesenteric vein, lienal vein, inferior caval vein, hepatic artery, ascites, peaked P waves, tachycardia;increased enzymes serum values; gross intestine, liver, lung, spleen and heart lesions.
Research results
BPC 157 counteracts electrocardiogram disturbances (increased P wave amplitude, S1Q3T3 QRS pattern and tachycardia).BPC 157 administration rapidly presented portal vein-superior mesenteric vein-inferior mesenteric vein-rectal veins-left ileal vein-inferior caval vein vascular pathway as the adequate shunting.As evidenced, this vascular pathway recovery means the immediately recovered disturbed hemodynamic.Portal hypertension and severe aortal hypotension during PTO, as well as portal and caval hypertension and mild aortal hypotension in reperfusion and refractory ascites formation were markedly attenuated (during PTO) or completely abrogated (reperfusion); thrombosis in portal vein tributaries and inferior caval vein or hepatic artery was counteracted during PTO.Likewise, the whole vicious injurious circle was counteracted [i.e., lung pathology (severe capillary congestion), liver (dilated central veins and terminal portal venules), intestine (substantial capillary congestion, submucosal edema, loss of villous architecture), splenomegaly, right heart (picked P wave values)], otherwise regularly perpetuated in ischemia and progressed by reperfusion in Pringle rats.
Research conclusions
BPC 157 resolves Pringle maneuver in rats, both for ischemia and reperfusion.
Research perspectives
The reported evidence that the administration of BPC 157 resolves the adverse effects of the Pringle maneuver means the immediate recovery of collaterals, which results in the bypassing of the obstruction, even in the worst conditions of PTO disturbances.The key importance of this therapy effect verifies the reversal of the signs of the right heart failure much like the severe portal hypertension and severe aortal hypotension during PTO as well as the severe portal and caval hypertension and mild aortal hypotension in reperfusion, along with refractory ascites formation, thrombosis and ischemia markedly decreased and completely disappeared.The rapid reestablishment of blood flow in both the ischemic and reperfusion conditions accompanied the reduction of the increased malondialdehyde values in liver tissues to the normal values.Likely,this combined effect resolves the further circle of injuries (i.e., the specific pathology in the liver,intestine, spleen, lung and heart).Thus, these new insights into Pringle maneuver and related disturbances, and into portal hypertension, suggest the effective BPC 157 use in future therapy.Otherwise, the abrupt PTO and severe portal hypertension (> 60 mmHg) [and in reperfusion,caval hypertension (> 70 mmHg)] makes spontaneous decompression of the portal system by a portocaval shunt hardly possible as well as severe aortal hypotension in the ischemia not compensated in the reperfusion.
 World Journal of Hepatology2020年5期
World Journal of Hepatology2020年5期
- World Journal of Hepatology的其它文章
- Drug and herbal/dietary supplements-induced liver injury: A tertiary care center experience
- Usefulness of Mac-2 binding protein glycosylation isomer in noninvasive probing liver disease in the Vietnamese population
- Epidemiological profile of alcoholic liver disease hospital admissions in a Latin American country over a 10-year period
- Systemic review and network meta-analysis: Prophylactic antibiotic therapy for spontaneous bacterial peritonitis
- Transmission of cryptococcosis by liver transplantation: A case report and review of literature
