Successful treatment of a high-risk nonseminomatous germ cell tumor using etoposide, methotrexate, actinomycin D,cyclophosphamide, and vincristine: A case report
Jina Yun, Sang W Lee, Sung H Lim, Se H Kim, Chan K Kim, Seong K Park
Jina Yun, Sung H Lim, Se H Kim, Chan K Kim, Seong K Park, Department of Internal Medicine,Division of Hematology-Oncology, Soonchunhyang University Bucheon Hospital,Soonchunhyang University School of Medicine, Bucheon 14584, South Korea
Sang W Lee, Department of Urology and Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University School of Medicine, Bucheon 14584, South Korea
Abstract BACKGROUND Choriocarcinoma is an infrequent entity and the most aggressive subtype of germcell tumors. Because of early metastatic spread and rapid disease progression,choriocarcinoma patients display poor prognosis. Although etoposide,methotrexate, actinomycin D, cyclophosphamide, and vincristine (EMA-CO)regimen is widely used to treat gestational trophoblastic tumors in females, its role in treating male choriocarcinoma is seldom reported.CASE SUMMARY A 32-year-old man was diagnosed with burned-out primary germ cell tumors(GCT) with retroperitoneum, liver and lung metastases. Biopsy of the liver revealed pure choriocarcinoma. The patient received bleomycin, etoposide, and cisplatin chemotherapy. After two cycles of treatment, response evaluation revealed the mixed response. EMA-CO regimen was used in the second-line therapy. After eight cycles, the patient showed a potentially resectable state and thus, all residual masses were surgically removed. The patient was completely cured, and 10 years later, he is leading a healthy life without complications.CONCLUSION This paper is the first case of high-risk nonseminomatous GCT in a male patient to be successfully treated with the EMA-CO regimen. The EMA-CO regimen can be used actively in patients with high-risk nonseminomatous GCT.
Key Words: Antineoplastic combined chemotherapy protocols; Choriocarcinoma;Testicular neoplasms; Cyclophosphamide; Methotrexate; Case report
INTRODUCTION
Choriocarcinoma is an extremely rare germ cell tumor (GCT) of trophoblastic cells.Unlike other GCTs, choriocarcinoma is commonly observed in extensive metastatic diseases, marked β human chorionic gonadotropin (β-HCG) elevations, and rapid disease progressions. Most GCTs are highly sensitive to cisplatin-based chemotherapy,with cure rates of approximately 80% for advanced diseases. However, pure choriocarcinoma of the testis with serum β-HCG > 50000 mIU/mL and with nonpulmonary visceral metastases accounts for the relatively poor prognosis[1,2]. These patients require early aggressive treatment to improve their chance of survival[3]. We report a case of cisplatin-refractory high-risk non seminomatous GCT, which was treated successfully with a seldom used second-line etoposide, methotrexate,actinomycin D, cyclophosphamide, and vincristine (EMA-CO) regimen followed by residual surgical resection.
CASE PRESENTATION
Chief complaints
A 32-year-old male visited our hospital (soonchunhyang university bucheon hospital)due to painful and palpable abdominal mass in the left upper quadrant.
History of present illness
The patient suffered from indigestion and left upper quadrant abdominal pain onemonth prior to the hospital visit, and the symptoms continued to worsen.
History of past illness
The patient had an unremarkable past medical history.
Physical examination
He was afebrile, and physical examinations revealed a large, tense mass extending from the left upper quadrant to the left lower quadrant.
Laboratory examinations
The baseline levels of serum β-HCG and α-fetoprotein were 239335.0 mIU/mL and 2.6 ng/mL, respectively.
Imaging examinations
The initial contrast-enhanced computed tomography (CT) and magnetic resonance imaging scans revealed a huge left retroperitoneal mass measuring 16 cm and multiple metastatic liver and lung masses (Figure 1). A scrotal ultrasound revealed a heterogeneous echoic intratesticular mass in the left testis, without any abnormality associated with the right testis.
Further diagnostic work-up
Left orchiectomy was performed along with a pathological study compatible with the scar tissues in the surrounding atrophic testis. An ultrasound-guided percutaneous liver biopsy was conducted for histological confirmation. Histopathologic features were consistent with pure choriocarcinoma. Immunohistochemistry was positive for an antibody to β-HCG.
FINAL DIAGNOSIS
We diagnosed the burned-out choriocarcinoma as retroperitoneum, liver and lung metastases.
TREATMENT
The patient underwent two cycles of bleomycin, etoposide, and cisplatin chemotherapy [bleomycin, etoposide, and cisplatin (BEP); B, 30 U; E, 20 mg/m2; and P,100 mg/m2]. Imaging studies demonstrated an 18% reduction in the retroperitoneal tumor mass (133 mm); however, the size of metastatic liver masses increased, and a new lesion was observed in S6 (Figure 2). The patient underwent second-line chemotherapy (EMA-CO; E, 100 mg/m2; M, 300 mg/m2; A, 0.5 mg; C, 600 mg/m2; and O, 1.0 mg/m2). After eight cycles of chemotherapy, the total tumor mass regressed by 25%, and the hematogenous multiple metastatic lung nodules disappeared. The patient showed a potentially resectable status (Figure 3). The levels of serum β-HCG were 77.2 mIU/mL (Figure 4). We surgically removed all remnant massviaS 5,6 sectionectomy, left nephrectomy, and excision of the huge retroperitoneal mass. The pathologic report revealed nearly total necrotic tissue without residual tumor.
OUTCOME AND FOLLOW-UP
The patient underwent regular follow-up CT and serum β-HCG and alpha-fetoprotein level tests. The patient is now alive with no evidence of disease for 10 years since the operation, and is leading a healthy life without complications.
DISCUSSION
Choriocarcinoma typically presents in female genital tract following gestational events such as hydatidiform mole, normal pregnancy, abortion, and ectopic pregnancy[4].Therefore, it belongs to the malignant end of the spectrum in gestational trophoblastic disease[5,6]. However, it is also classified as a germ cell tumor and may arise in the testis or ovary.
In male choriocarcinoma, the primary lesion is usually localized to the testes;however, lesions have also been reported in extragonadal primary sites such as the mediastinum, pineal body, retroperitoneum, and in other visceral organs[5,7]. The histogenetic origin of primary extragonadal germ cell tumors is still a matter of debate,and it remains uncertain whether such tumors develop primarily in the extragonadal sites or represent metastases of a primary testicular tumor[8]. A homogeneous scar with remote hemorrhage is the only evidence of a primary tumor in that location in a few patients with metastatic disease and no gross evidence of testicular tumor[6]. This phenomenon is commonly seen in choriocarcinomas and is called “burned-out tumor”[9]. The term ‘burned-out’ tumor of the testis refers to spontaneous and fully regressed testicular tumor that appears during the metastatic stage. These metastases may involve retroperitoneal, mediastinal, supraclavicular, cervical and axillary lymph nodes, lungs, and liver. These sites also show primary lesions of extragonadal choriocarcinoma, so it is difficult to distinguish between primary and metastatic lesion based on cancer location alone. According to Abellet al[10], the criteria for a diagnosis of primary extragonadal GCT include that the lesion is located high in the retroperitoneum with adjacent lymph node involvement but without involvement of the lower aortic, iliac, or pelvic lymph nodes. In this case, the patient has his retroperitoneal mass below the renal hilum on the left side corresponding to the landing zone of his left primary burnt-out GCT. Therefore, by definition, we diagnosed the burned-out testicular choriocarcinoma with retroperitoneum, liver and lung metastases, and not as an extragonadal primary retroperitoneal choriocarcinoma.
The prognosis of male choriocarcinoma is abysmal, with a cumulative survival rate of 30%, and the course of the disease is rapid; 23.8% and 45.4% of the patients show 1-mo and 6-mo mortality, respectively[11]. Notably, the extragonadal site and pure choriocarcinoma histology of male choriocarcinoma lead to a worse prognosis[12].However, these data are also available in case reports. Patients mentioned in case reports were treated regardless of prognosis, and the drugs used in each patient also varied, and therefore, it is still difficult to establish a treatment consensus.
To date, the treatment of male choriocarcinoma is based on the guidelines for testicular GCTs. The most commonly used chemotherapy regimens are BEP, EP(etoposide and cisplatin), and VIP [VP-16 (etoposide) or vinblastine plus ifosfamide and cisplatin][13]. According to the IGCCCG risk classification, our patient was classified as poor risk because of nonpulmonary visceral metastases[1]. The standard chemotherapy regimen for poor-risk patients with advanced nonseminoma is 4 cycles of BEP[14]. Therefore, our patient also received BEP chemotherapy as a first-line treatment. After two cycles of BEP, the anticancer drug of this patient was changed to EMA-CO regimen, and of course, there is an issue on whether it was adequate to change the anticancer drug because β-HCG decreased from 317449 mIU/mL to 3354 mIU/mL. However, the size of the nonpulmonary visceral metastatic sites, liver metastatic masses increased and new lesions were found, so anticancer drugs were forced to be changed. It still remains to be seen whether the response would have been different with the continued treatment of BEP regimen.
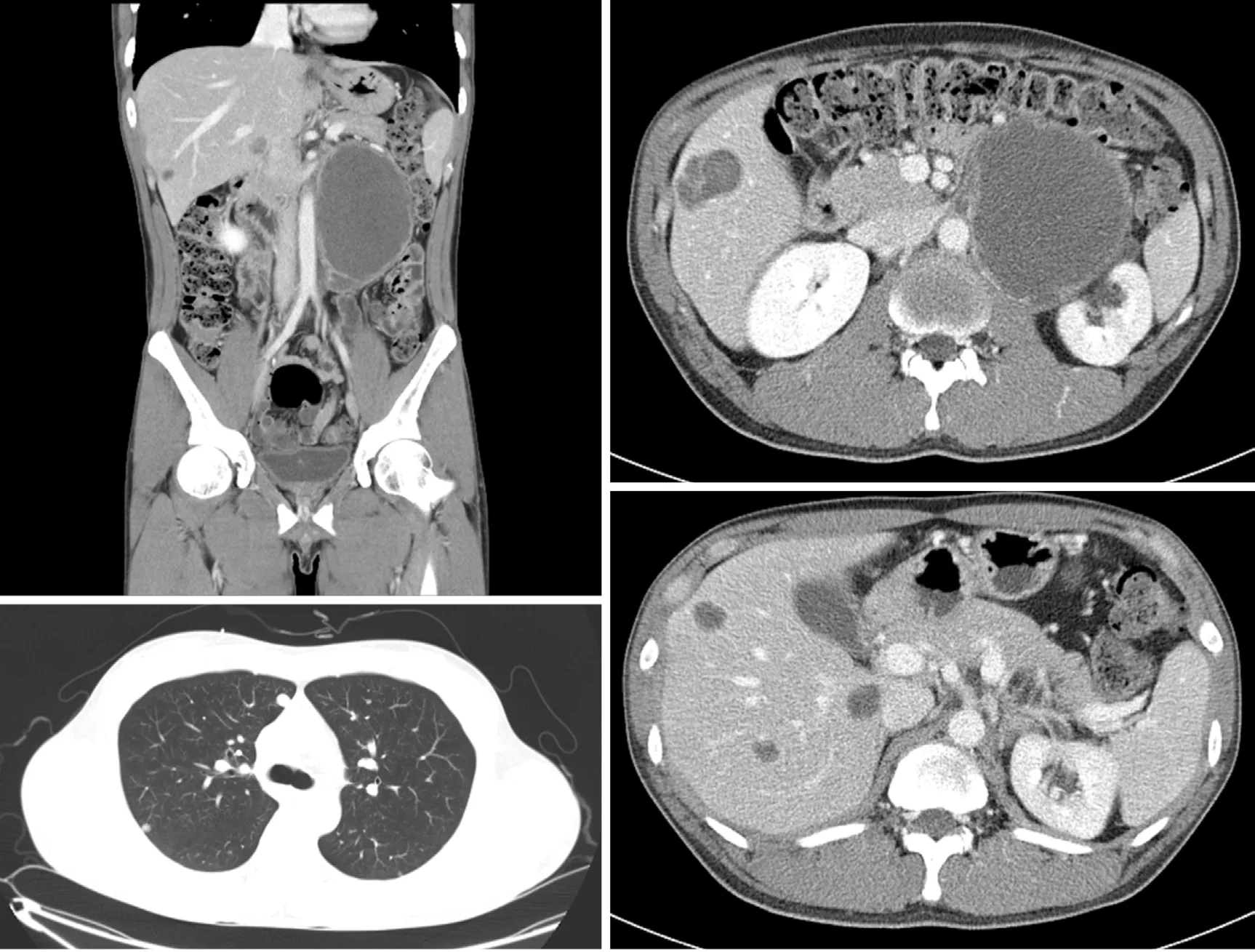
Figure 2 A follow-up computed tomography scan after 2 cycles of bleomycin, etoposide, and cisplatin chemotherapy, showing that the left retroperitoneal mass had decreased in size, but that liver metastatic lesions had increased in size and a new lesion in S6 had developed.
Another question that kept arising while treating this patient was the consideration of the appropriate duration of EMA-CO chemotherapy treatment. After 6 cycles of EMA-CO, pulmonary metastasis was not seen in CT and was subsequently determined to be resectable. However, β-HCG was still high at 164 mIU/mL. When two more cycles of EMA-CO chemotherapy were added and the residual masses were completely removed, the β-HCG fell to 8.1 mIU/mL. Then, a month later, it reached normal level. Mohleret al[15]suggested that phagocytosis of necrotic tumor apparently released entrapped β-HCG resulting in a false positive tumor marker. The pathological examination of mass removed in this case also confirmed all necrotic lesions. When we additionally stained the necrotic tissue, the β-HCG was strongly stained. The lingering HCG is thought to have been sequestration or entrapment in the necrotic tumor.
EMA-CO is the most widely used regimen in patients with high-risk gestational choriocarcinoma. The response rates with EMA-CO treatment range from 70% to 90%,and the overall survival rate is 86.2%[16]. However, the EMA-CO regimen is not routinely recommended in non-gestational choriocarcinoma, such as male choriocarcinoma, despite similar histology. Yoonet al[7]reported using the EMA-CO regimen for two male patients diagnosed with primary gastric choriocarcinoma. However, the treatment failed. Boweret al[17]showed that cyclical POMB/ACE (cisplatin, vincristine,methotrexate, bleomycin, actinomycin D, cyclophosphamide and etoposide) regimen appears to be better than conventional BEP chemotherapy in poor prognosis nonseminomatous GCT patients. The 3-year overall survival was 75% for the poorprognosis group using POMB/ACE regimen. Because EMA-CO and POMB/ACE regimens are similar, we expected EMA-CO to have comparable efficacy to POMB/ACE.
CONCLUSION
Our case report is the first of its kind demonstrating successful treatment using the EMA-CO regimen in a male diagnosed with retroperitoneal choriocarcinoma with widespread metastases. We suggest that the EMA-CO regimen can be used more actively in patients with retroperitoneal choriocarcinoma.

Figure 3 A follow-up computed tomography scan, after 8 cycles of etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine regimen, showing that the left retroperitoneal mass and metastatic liver lesions had markedly decreased in size and that metastatic lung lesions had nearly disappeared.
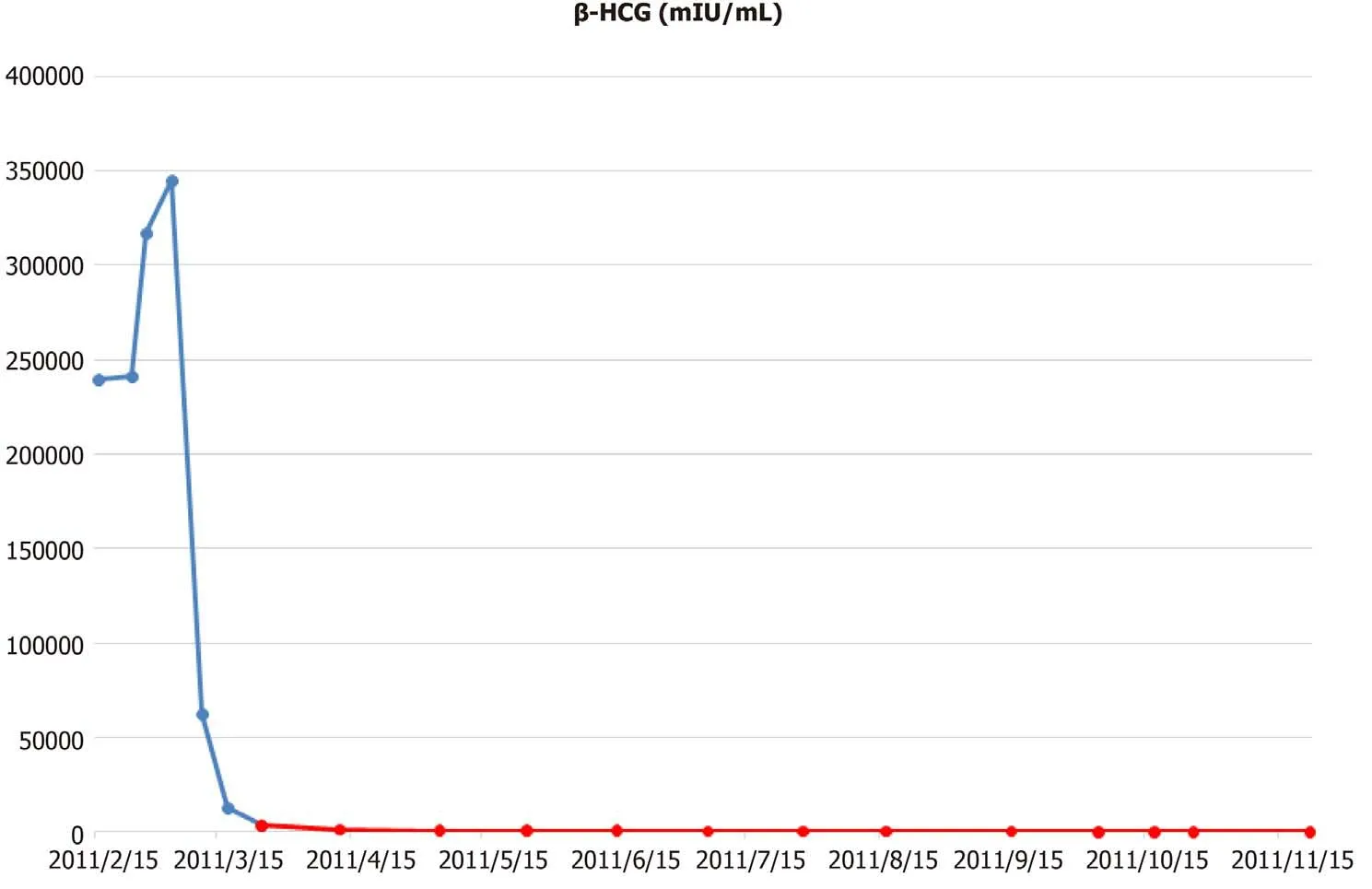
Figure 4 Macroscopic features of the resected specimen, showing that tumors removed from liver and retroperitoneum, were relatively well-marginated and round-shaped lesions. β-HCG: β human chorionic gonadotropin.
 World Journal of Clinical Cases2020年21期
World Journal of Clinical Cases2020年21期
- World Journal of Clinical Cases的其它文章
- Strategies and challenges in the treatment of chronic venous leg ulcers
- Peripheral nerve tumors of the hand: Clinical features, diagnosis,and treatment
- Treatment strategies for gastric cancer during the COVID-19 pandemic
- Oncological impact of different distal ureter managements during radical nephroureterectomy for primary upper urinary tract urothelial carcinoma
- Clinical characteristics and survival of patients with normal-sized ovarian carcinoma syndrome: Retrospective analysis of a single institution 10-year experiment
- Assessment of load-sharing thoracolumbar injury: A modified scoring system
