Efficacy and safety of S-1 maintenance therapy in advanced nonsmall-cell lung cancer patients
Xiao-Wei Cheng, Wen-Hua Leng, Chun-Ling Mu
Xiao-Wei Cheng, Wen-Hua Leng, Department of Respiratory and Critical Care Medicine,Panzhihua Central Hospital, Panzhihua 617067, Sichuan Province, China
Chun-Ling Mu, Department of Ultrasound, Panzhihua Central Hospital, Panzhihua 617067,Sichuan Province, China
Abstract BACKGROUND Previous reports have demonstrated that S-1 has remarkable effects in the maintenance treatment of advanced non-small-cell lung cancer (NSCLC), and has less toxic and side effects than conventional drugs.AIM To investigate the efficacy and safety of S-1 maintenance therapy in patients with advanced NSCLC.METHODS Ninety-four patients with NSCLC admitted to our hospital from September 2015 to April 2018 were included in the study and divided into the S-1 group (47 cases)and the gemcitabine group (47 cases) by random digital table method. The S-1 group was treated with S-1, while the gemcitabine group received gemcitabine treatment. The clinical efficacy and quality of life of the patients after treatment in the two groups were evaluated.RESULTS There was no significant difference in the total effectiveness rate between the two groups (P = 0.519). The quality-of-life scores indicated that there was no significant difference between the two groups in terms of four dimensions of the GQOLI-74 questionnaire (P = 0.518, 0.094, 0.338, 0.418). The incidence of nausea and vomiting, granulocytopenia and diarrhea in the S-1 group was significantly lower than that in the gemcitabine group (P = 0.001, 0.001 and 0.001, respectively).There was no significant difference in the incidence of thrombocytopenia (P =0.366), the progression-free survival (P = 0.064), and the survival between the two groups (P = 0.050).CONCLUSION S-1 maintenance therapy shows a significant therapeutic effect in patients with advanced NSCLC. It has the same clinical efficacy as gemcitabine, but with less toxic and side effects than conventional drugs.
Key Words: Advanced non-small cell lung cancer; Maintenance therapy; S-1; Gemcitabine;Efficacy; Toxic and side effects
INTRODUCTION
Lung cancer is one of the major fatal diseases in China, and the incidence and mortality of lung cancer have been on the rise over the last 50 years. Epidemiological statistics show that the mortality and morbidity of lung cancer in men are higher than in women, and the incidence of lung cancer in cities is lower than in rural areas[1].Lung cancer is known to be related to long-term smoking, air pollution, and other factors, but its specific pathogenesis has still not been determined.
Non-small-cell lung cancer (NSCLC), the main type of lung cancer, accounts for up to 75%-80% of the total number of lung cancer, and NSCLC patients without symptoms at an early stage or without typical symptoms can hardly be diagnosed[2].Although radical resection has an excellent therapeutic effect in the early-stage and middle-stage NSCLCs, a large number of NSCLC patients still need to receive other treatments because they have missed the opportunity of radical resection.
Chemotherapy is the predominant treatment for advanced NSCLC patients.Cisplatin, paclitaxel, and other commonly applied drugs can control the progress of the disease to a certain extent. After chemotherapy, drugs like gemcitabine and docetaxel are usually used to treat advanced NSCLC patients to improve their quality of life and extend their survival time[3]. In recent years, some reports have shown that S-1 has remarkable effects in the maintenance treatment of advanced NSCLC with less toxic and side effects than conventional drugs[4,5]. To verify this finding, a comparative study was conducted to observe the efficacy and safety of S-1 maintenance therapy in advanced NSCLC patients.
MATERIALS AND METHODS
Clinical data
Ninety-four patients with NSCLC admitted to our hospital from September 2015 to April 2018 were included, who all met the diagnostic criteria for NSCLC inClinical Guidelines for the Diagnosis and Treatment of Lung Cancer by the Chinese Medical Association (2018 Edition). Inclusion criteria: (1) Patients with tumor-node-metastasis(TNM) staging at phase IIIB-IV stage; (2) Patients with stable conditions after 4 cycles of combined chemotherapy with platinum-containing drugs; (3) Patients with no heart failure, respiratory failure, and other serious diseases; (4) Patients who were informed of the medication program and volunteered to participate; and (5) Patients with consciousness, who were willing to complete quality of life questionnaire. Exclusion criteria: (1) Patients who withdrew from the treatment plan halfway and were transferred to another hospital; (2) Patients would die 3 mo later according to pretreatment estimation; and (3) Patients with incomplete general data. As shown in Table 1, there was no significant difference in the clinical data between the two groups of patients. The study was approved by the Ethics Committee of our hospital.
Gemcitabine group
The drug was purchased from Broad Medicine Huangshi Feiyun Pharmaceutical Co.,Ltd., Chinese Medicine Approval No. H20133194. It was delivered by intravenous drip at a dose of 1000 mg/m2on d 1 and d 8. The regimen was repeated every 21 d until the patient's disease progressed.
S-1 group
The drug was purchased from Fuzhou Haiwangfu Pharmaceutical Co., Ltd., Chinese Medicine Approval No. H20140020. The drug was delivered orally at a dose of 80 mg/m2per day on d 1 to d 14. The regimen was repeated every 21 d for 1 cycle until the patient's disease progressed.
Observation indicators
Curative effect evaluation:Complete remission: The patient's target lesion and clinical symptoms basically disappeared. Remission: The target lesion reduced by ≥ 30%, and the clinical symptoms improved significantly after treatment. Controlled: The target lesion reduced by < 30% or by < 20% after treatment. Progressed: New lesions appeared, the total diameter of the lesion increased by ≥ 20%, and the total effectiveness rate of treatment = (total remission cases + remission cases)/total number of cases × 100%.
The life quality of patients was assessed by the GQOLI-74 questionnaire, which covered four dimensions, namely, material, society psychology and body. A higher score indicated the higher quality of life.
The incidence of toxic and side effects such as thrombocytopenia, nausea and vomiting, granulocytopenia, and diarrhea in the two groups during the treatment period was analyzed.
The patients were followed up, whose progression-free survival and survival were analyzed. The progression-free survival was defined as the period from the startof maintenance treatment to the cease of disease progression, and the survival was defined as the period from the start of maintenance treatment to death.
Statistical analysis
The observation data such as quality-of-life score and incidence of toxic and side effects were analyzed and processed using the SPSS statistical software. The measurement data (± s) were subjected to independent samplettest if the normal distribution was satisfied, and the non-normal data indicated count data with Median(IQR) and percentage (%) and were subjected toχ2test.P< 0.05 indicated significant differences between groups.
RESULTS
Comparison of clinical effects between the two groups
There was no significant difference in the total effectiveness rate of treatment between the S-1 group and the gemcitabine group (P= 0.519) (Table 2).
Comparison of quality-of-life scores between the two groups
There was no significant difference in quality-of-life scores in multiple dimensions between the two groups (P= 0.518, 0.094, 0.338, 0.418) (Table 3 and Figure 1).
Comparison of survival and progression-free survival between the two groups
There was no significant difference in progression-free survival and survival between the two groups (P= 0.064, 0.050) (Table 4).
Comparison of toxic and side effects between the two groups
The incidence of granulocytopenia, nausea, and vomiting, and diarrhea in the S-1 group was lower than that in the gemcitabine group (P= 0.001, 0.001, 0.001), and there was no significant difference in the incidence of thrombocytopenia between the twogroups (P= 0.366) (Table 5).
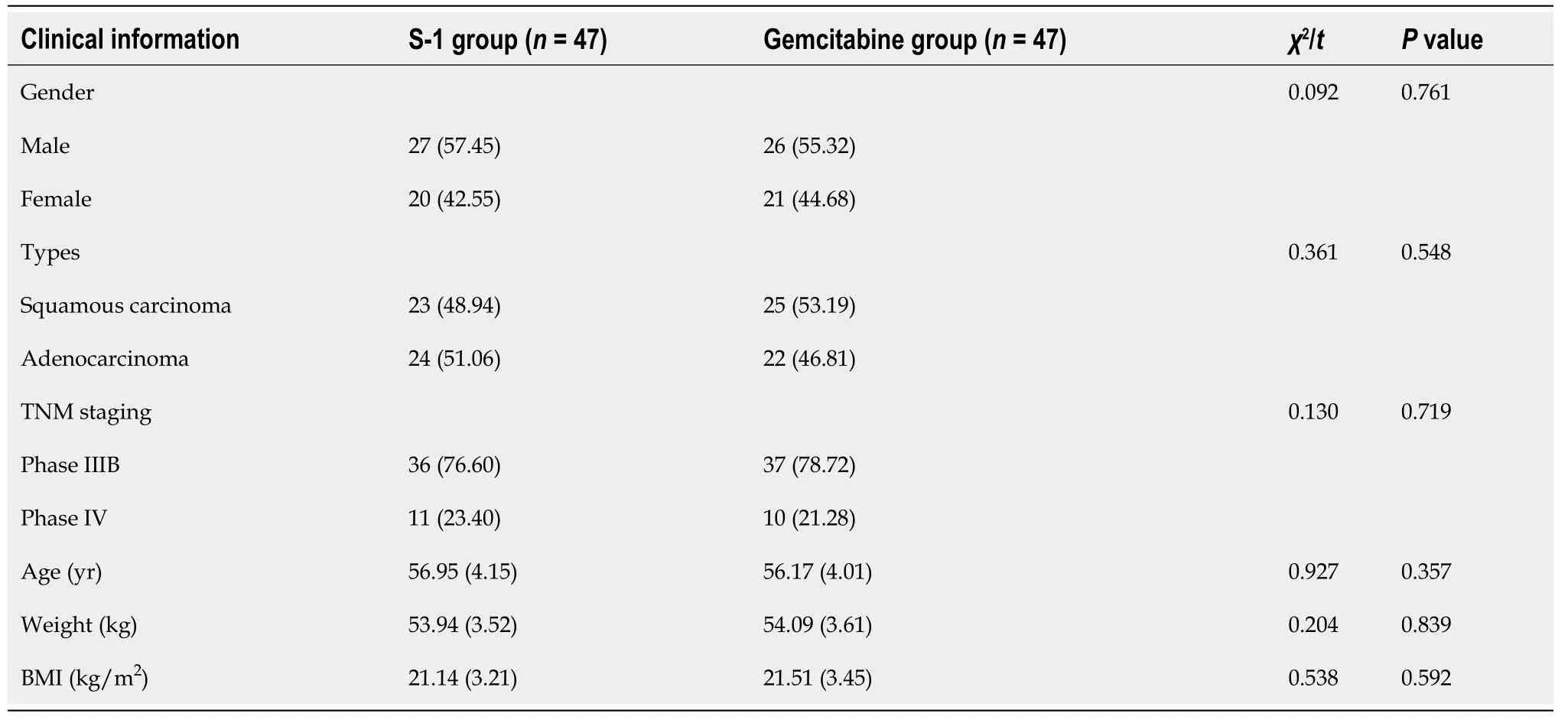
Table 1 Comparison of clinical data between the two groups, n %
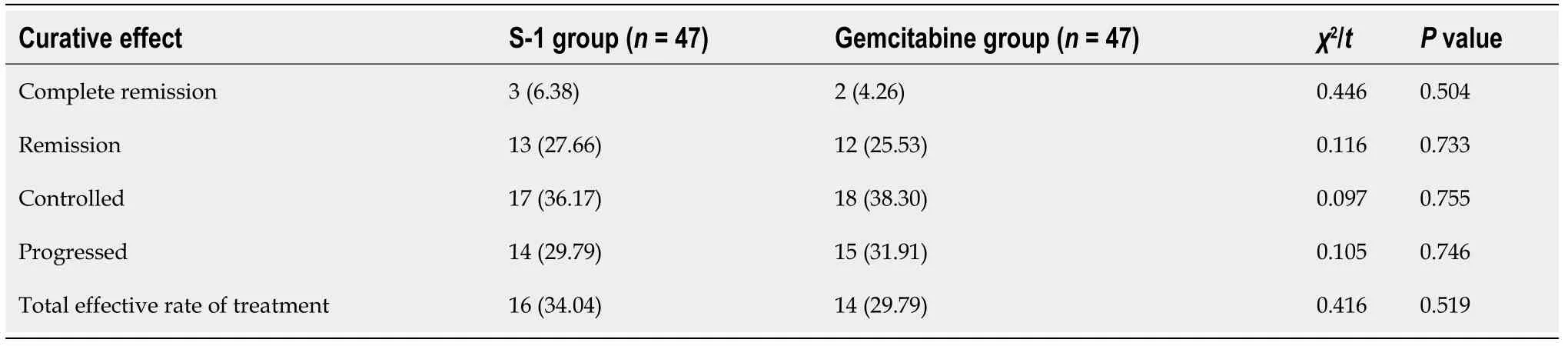
Table 2 Comparison of efficacy between the two groups, n %
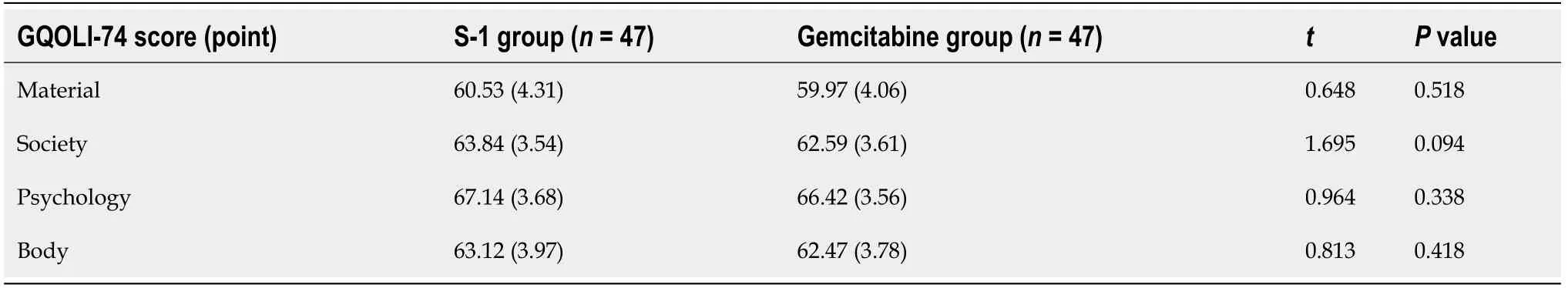
Table 3 Comparison of quality-of-life scores between the two groups
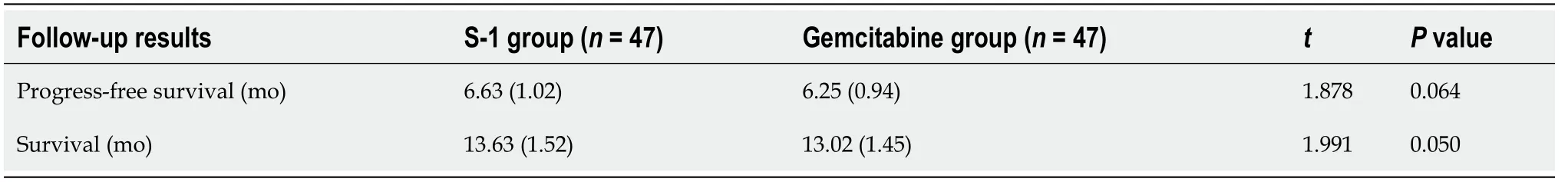
Table 4 Comparison of survival and progression-free survival between the two groups
DISCUSSION
Lung cancer is one of the most fatal and malignant tumors worldwide. With the development of modern industrial society, the air quality continues to deteriorate, andthe incidence of lung cancer is increasing year by year. Lung cancers can be divided into the peripheral type and the central type according to the anatomical site,squamous cell carcinoma and adenocarcinoma according to histological classification,and small cell lung cancer (SCLC) and NSCLC according to clinical characteristics[6]. The last classifying method is common in clinical diagnosis and treatment. NSCLC is the main type of lung cancer, with a high mortality rate, and its diagnosis and treatment have been the research focus[7].
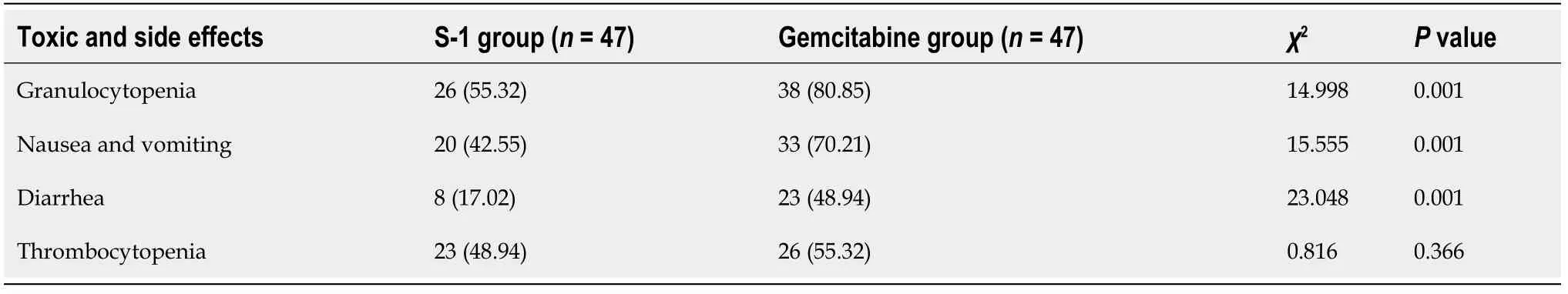
Table 5 Comparison of toxic and side effects between the two groups, n (%)
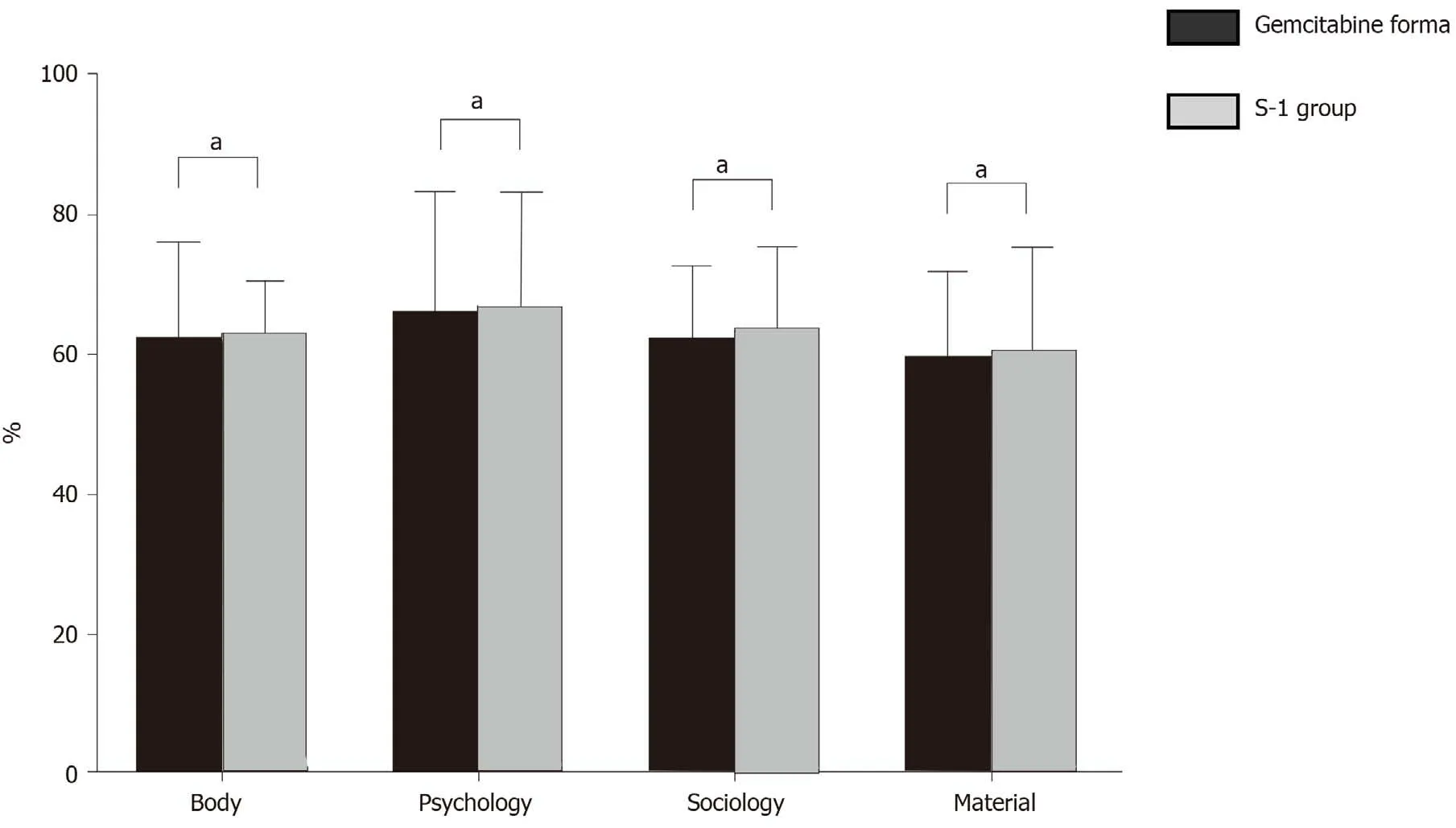
Figure 1 Comparison of quality-of-life scores between the two groups. aP < 0.05.
Clinical treatment of NSCLC mainly includes surgery, chemotherapy, and radiotherapy. The surgical treatment is to control the progress of the disease which is suitable for patients with stage I to II lung cancer. However, as NSCLC cannot be diagnosed due to absence of symptoms or specific symptoms observed in the early stage, the disease generally progresses to the middle and late stages, and radical surgery can hardly achieve satisfactory outcome[8].
Chemotherapy is the most common treatment for advanced NSCLC. Since the 1990s, the combination of two chemotherapeutic drugs containing platinum has been adopted as the first-line standard chemotherapy regimen to control the rapid growth of tumors and prolong the survival time of patients[9-13]. According to a clinical survey,the current mainstream chemotherapy regimen resulted in a clinical remission rate of about 15 to 36%, the survival time of patients who have received chemotherapy is only 8 to 10 mo, and less than 40% of patients have a survival period of more than one year.Maintenance therapy aims to strengthen the effect of chemotherapy, prolong life, and improve the quality of life of patients after chemotherapy[14].
Currently, drugs for maintenance treatment in patients with NSCLC include gemcitabine, docetaxel and gefitinib[15,16]. Gemcitabine belongs to a cytosine nucleoside derivative, which has the same action mechanism with arabinoside. Once injected into the body, gemcitabine is converted into arabinoside triphosphate and diphosphate under the activating effect of deoxidized cytosine kinase. Arabinoside triphosphate can significantly inhibit the synthesis of DNA polymerase and thereby prevent the synthesis of cancer cell DNA. In contrast, arabinoside diphosphate can hinder the formation of deoxycytidine diphosphate, thus avoiding the polymerization and synthesis of tumor cell DNA and retarding tumor growth[17].Clinical studies confirmed that patients having received gemcitabine maintenance had progressionfree survival of about 5 to 7 mo and survival of 12 to 15 mo, which further prolonged the survival of patients after chemotherapy[18]. However, gemcitabine also has many toxic and side effects, such as granulocytopenia, and thrombocytopenia.
S-1 was developed by Japanese scholars in 1991 and belongs to oral fluorouracil,with the main components including tegafur, interacial, and gimeracil. Its clinical medicinal mechanism includes three aspects: (1) Tegafur, a prodrug of 5-fluorouracil(5-FU), is converted into 5-FU after intake by the body; and (2) Gimeracil, a dihydropyrimidine dehydrogenation inhibitor, can directly inhibit the catabolism of 5-FU, and prolong the effective concentration of 5-FU in the body after the intake of tegafur, thus having an effect similar to that of continuous intravenous drip[19,20].
In the present study, the clinical efficacy, quality of life, survival and progressionfree survival of the gemcitabine group were similar to those of the S-1 group,indicating that S-1 had the same effect as gemcitabine in treating advanced NSCLC.However, the S-1 group had significantly lower incidence of granulocytopenia, nausea and vomiting and diarrhea than the gemcitabine group, suggesting that S-1 was superior to gemcitabine in terms of toxic and side effects and safety. Li Ting compared the clinical efficacy of S-1 with that of gemcitabine in the treatment of advanced pancreatic cancer, and found that the clinical efficacy of the two drugs tended to be consistent, but the incidence of adverse reactions in patients receiving S-1 was lower than that in patients receiving gemcitabine. There was no significant difference in the clinical efficacy, quality of life and progression-free survival between the S-1 group and gemcitabine group, and the S-1 group had lower incidence of adverse reactions such as vomiting and diarrhea than the experimental group.
CONCLUSION
S-1 has the same curative effect with gemcitabine in the maintenance treatment of patients with advanced NSCLC, but S-1 is less toxic and its application should be preferentially promoted.
ARTICLE HIGHLIGHTS
Research background
Chemotherapy is the predominant treatment for advanced non-small-cell lung cancer(NSCLC) patients. Cisplatin, paclitaxel, and other commonly applied drugs can control the progress of the disease to a certain extent. After the chemotherapy, drugs like gemcitabine and docetaxel are usually used to treat advanced NSCLC patients, so as to improve their quality of life and extend their survival time.
Research motivation
S-1 has remarkable effects in the maintenance treatment of advanced NSCLC with less toxic and side effects than conventional drugs.
Research objectives
To investigate the efficacy and safety of S-1 maintenance therapy in patients with advanced NSCLC.
Research methods
Ninety-four patients with NSCLC admitted to our hospital from September 2015 to April 2018 were included in the study and divided into the S-1 group (47 cases) and the gemcitabine group (47 cases). S-1 group was treated with S-1, while the gemcitabine group was treated with gemcitabine. The clinical efficacy and quality of life of the patients after treatment in the two groups were evaluated.
Research results
There was no significant difference in the total effective rate between the two groups (P= 0.519). The quality-of-life scores indicated that there was no significant difference between the two groups. The incidence of nausea and vomiting, granulocytopenia and diarrhea in the S-1 group was significantly lower than that in the gemcitabine group.There was no significant difference in the incidence of thrombocytopenia(P=0.366),the progression-free survival (P= 0.064), and the survival between the two groups (P=0.050).
Research conclusions
S-1 maintenance therapy shows a significant therapeutic effect in patients with advanced NSCLC. It has the same clinical efficacy as gemcitabine, but with less toxic and side effects, and is more effective and safer than conventional drugs.
Research perspectives
Randomized control trails are required to further validate the effect of S-1 maintenance therapy in patients with advanced NSCLC.
 World Journal of Clinical Cases2020年21期
World Journal of Clinical Cases2020年21期
- World Journal of Clinical Cases的其它文章
- Strategies and challenges in the treatment of chronic venous leg ulcers
- Peripheral nerve tumors of the hand: Clinical features, diagnosis,and treatment
- Treatment strategies for gastric cancer during the COVID-19 pandemic
- Oncological impact of different distal ureter managements during radical nephroureterectomy for primary upper urinary tract urothelial carcinoma
- Clinical characteristics and survival of patients with normal-sized ovarian carcinoma syndrome: Retrospective analysis of a single institution 10-year experiment
- Assessment of load-sharing thoracolumbar injury: A modified scoring system
