Free and liposome form of gallic acid improves calvarial bone wound healing in Wistar rats
Ahmet Altan, Hatice Balci Yuce, Őzkan Karataş, Mehmet Murat Taşkan, Fikret Gevrek, Sefa Çolak, Nihat Akbulut
1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Gaziosmanpaşa University, Tokat, Turkey
2Department of Periodontology, Faculty of Dentistry, Gaziosmanpaşa University, Tokat, Turkey
3Department of Histology and Embryology, Faculty of Medicine, Gaziosmanpaşa University, Tokat, Turkey
ABSTRACT Objective: To evaluate the effect of free and liposome form of gallic acid on bone regeneration in critical defects in Wistar rats.Methods: Thirty-two female Wistar rats were divided into four study groups: group 1, negative control; group 2, positive control; group 3, gallic acid powder; group 4, gallic acid liposome. A critical-sized defect was created in all rats. Groups 2 to 4 had xenograft, autograft and membrane placement while negative control rats did not receive any treatment. The defect area was sutured and rats were kept alive for 30 d. At the end of the study, a bone specimen including the defect area was removed from calvaria. All specimens were evaluated under the stereomicroscope, then underwent histological analysis. Inflammatory cell counts, osteoblast, osteoclast counts, receptor activator of nuclear factor κ-B (RANKL), osteoprotegerin (OPG), runt-related transcription factor 2 (Runx2), bone morphogenetic protein-2 (BMP-2), and alkaline phosphatase were determined.Results: The biggest unhealed defect area was observed in the negative control group and the smallest was observed in the gallic acid liposome group. There were no differences between the positive control group vs. the gallic acid powder group and the gallic acid powder group vs. the gallic acid liposome group. The severity of inflammation was the highest in the negative control group and the lowest in the gallic acid liposome group with significant differences between the groups. All groups had similar osteoblast counts while osteoclast counts were the highest in the positive control group. Gallic acid groups had a lower number of osteoclasts compared with the positive control group. Runx2 and alkaline phosphatase levels were similar in the groups while OPG and BMP-2 levels exhibited a significant increase compared with the negative control group and the positive control group. RANKL was similar in the negative control group, the positive control group, and the gallic acid powder groups but decreased in the gallic acid liposome group.Conclusions: Gallic acid powder and liposome significantly improve bone regeneration in Wistar rats with calvarial defects. The improvement in healing is evident with decreased inflammation and RANKL expressions and increased OPG and BMP-2 expressions.
KEYWORDS: Anti-inflammatory; Antioxidants; Bone regeneration; Gallic acid
1. Introduction
Bone is a dynamic tissue and undergoes continuous formation and resorption which is also called the remodeling process[1]. Wound healing in bone generally occurs as the regeneration of the original tissue and bone tissue can compensate for the deficiencies to a certain level. However, the bone formation may not occur at the desired level under certain conditions such as fractures, surgical interventions to eliminate pathologies or extract third molar teeth or chronic inflammatory diseases like periodontitis[2]. In such cases, regenerative procedures should be performed in order to stimulate bone healing[3].
Although there are several major differences in the metabolism of bone compared to the connective tissue, the basic principles of wound healing also apply to bone tissue[1]. The first step of the healing is inflammation which is the biological response of the host against the bacterial challenge, irritants or tissue damage[4]. The inflammation is followed by granulation tissue formation and then the repair mechanisms. However, persistent inflammation in bone prevents the repair phase. The resolution of inflammation improves the healing period and assists the host defense systems[5,6]. In this regard, anti-inflammatory agents might provide significant efficacy. Antioxidants especially flavonoids are potent modulators of inflammatory pathways through blocking nuclear factor κ-B (NFκB) pathway and arachidonic acid metabolites[7,8]. Accordingly, gallic acid as a strong antioxidant and anti-inflammatory agent, was shown to down-regulate pro-inflammatory mediators such as lipid mediators, nitric oxide, cyclooxygenase-2, lipoxygenase expressions[7]. In addition to the inflammatory mediators, gallic acid also decreased inflammation by preventing chemotaxis and inflammatory cell recruitment[7-9]. Studies also reported reduced levels of cytokines such as inducible NO synthase, tumor necrosis factor-α, and interleukins[7,8,10]. Decreased inflammation is not enough alone to promote bone formation, stimulation of osteoblastic activity is also required[11]. Gallic acid can also induce osteoblastic activity and bone formation which were reported by previous studies[10,12-14]. Up-regulated calcium-phosphor metabolism, alkaline phosphatase (ALP) activity, and increased osteoblast proliferation were reported by Chauhan et al.[12]. Another study by Jin et al. reported increased osteoblastic activity with cell viability, proliferation, and mineralization[13]. Along with increased bone formation, prevention of bone destruction was also reported in vitro and in vivo[14].
Based on the beneficial effects of gallic acid[15], the present study hypothesized that gallic acid would decrease inflammation and increase bone regeneration in critical-sized defects in Wistar rats. Therefore, the present study aimed to evaluate the effect of free and liposome form of gallic acid on bone regeneration in critical defects in Wistar rats. Local expressions of receptor activator of NFκB (RANKL), osteoprotegerin (OPG), runt-related transcription factor 2 (Runx2), bone morphogenetic protein-2 (BMP-2), and ALP were also determined along with the inflammatory cell infiltration, osteoblast and osteoclast counts in the wound area.
2. Materials and methods
2.1. Chemicals and reagents
Gallic acid powder was procured from Sigma Aldrich, Missouri, USA. Xenograft and collagen membrane were purchased from Tutogen Medical GmbH, Brand, Germany. Ketamine and xylazine were obtained from Eczacibasi IlacSanayi, Istanbul, Turkey. The antibodies used were anti-RANKL antibody, anti-OPG antibody, anti-Runx2 antibody, anti-BMP-2 antibody, and anti-ALP. Anti-RANKL antibody, anti-OPG antibody, and anti-Runx2 antibody were procured from Thermo fisher scientific, Massachusetts, USA. Anti-BMP-2 antibody and anti-ALP were purchased from Abcam, Cambridge, UK.
2.2. Animals and grouping
Thirty-two female Wistar rats (230-250 g) were used and divided into four study groups: Group 1, negative control group (NC, n=8); Group 2, positive control group (PC, n=8); Group 3, gallic acid powder group (GA-P, n=8); Group 4, gallic acid liposome group (GA-L, n=8).
Bone defects were created at calvaria of the rats. Defects were prepared as critical-size defects of 5 mm. All defects were prepared via a physiodispensor under general anesthesia which was provided via intraperitoneal injection of ketamine (Eczacibasi IlacSanayi, Istanbul, Turkey) (100 mg/kg) and xylazine (Eczacibasi IlacSanayi, Istanbul, Turkey) (0.5 mg/kg). All procedures were carried out by an experienced researcher. Firstly, rats were stabilized and the surgical area was disinfected by the povidone-iodine solution. A 10-mm incision was performed via surgical blade #15c and a fullthickness flap was elevated. The defect area was marked and a bone defect was created using a trephine burr of 5 mm diameter. The defects in the NC group were left untreated and the flaps were closed via 4-0 silk ligatures. The defects in the PC group received xenograft (Tutogen Medical GmbH, Brand, Germany) and autograft combination (w/w) and covered via a collagen membrane (Tutogen Medical GmbH, Brand, Germany). The rats in the GA-P group received gallic acid powder (5 mg) in addition to the same graft and membrane treatment. The rats in the GA-L group received gallic acid liposome (containing 5 mg gallic acid) in addition to the same graft and membrane treatment. All wounds were closed with interrupted sutures with 4-0 silk ligature. A topical antibiotic pomade was applied to the wounds and intramuscular antibiotic injections were applied for 3 d after the surgery.
Rats were kept in individual cages in a room with 12 hours of light/dark cycles and received food and water ad libitum. All rats were observed every day for 3 d and once a week afterward in case of any complication.
2.3. Gallic acid liposome preparation
Gallic acid liposomes were prepared according to a liposome protocol described by Vitonyte et al. with slight differences[16]. Briefly, gallic acid powder (Sigma Aldrich, Missouri, USA), soy lecithin, as the phospholipid source, and cholesterol were dissolved in trichloromethane. Gallic acid was used at 5 mg/mL as reported by Vitonyte et al.[16]. After a homogenous solution was achieved, the solvent was evaporated with a rotary evaporator. The thin film obtained after evaporation was treated with the phosphate buffer solution and stirred with an ultrasound shaker for 10 min. The mixture was then centrifuged at 6 000 rpm for 15 min and filtered with a standard filter paper.
2.4. Characterization of the liposomes
2.4.1. Particle size, polydispersity index (PDI) and zeta potential
The particle size and PDI were determined by a dynamic light scattering zeta sizer (Perkin Elmer, Massachusetts, USA)[17].
2.4.2. Encapsulation efficiency
The encapsulation efficiency of gallic acid liposomes was evaluated via ultracentrifugation technique[18]. The liposomes were centrifuged with 130 000×g, for 3 h at 4 ℃. Centrifugation unloaded and separated gallic acid liposomes. Then the liposome pellet was subjected to phosphate buffer solution and Triton-X (0.5%, v/v). Afterwards, the amount of gallic acid was determined at 280 nm. The encapsulation efficiency (EE) was then calculated according to the following equation: EE%=W2/W1×100%; where W1is the total gallic acid weight added in liposome preparation, mg; W2is the weight of encapsulated gallic acid, mg.
2.4.3. Scanning electron microscopy (SEM) of the liposomes
Samples were crushed with agate for homogenized size, taken up on a stap with carbon tape and coated with gold. After coating, a high vacuum analysis was carried out in SE Mode with an ETD detector (Fei Quanta FEG 450, Thermo Fisher Scientific, Massachusetts, USA). Analyses were performed at the appropriate voltage and Z heights in terms of image clarity and sample size. In general, powder samples were used at the height of approximately 10-20 mm (to the electron gun) and spot 3 in the range of 1-7 in terms of electron application field spot image resolution. The device operated with a maximum voltage of 30 kV, and analyses were carried out at appropriate voltage values (10-15 kV for sample).
2.5. Measurement of the bone defect area
All rats were kept alive for 30 d and euthanized with anesthetic overdose. A bone specimen of 10 mm including the bone defect was excised with physiodispensor. Bone specimens were evaluated via a stereomicroscope (Stemi 2000 and Axiovison 4.8, Carl Zeiss, Jena, Germany) under 10× magnification and standardized photographs were taken. After the evaluations, all specimens underwent histological tissue processing. The healed and unhealed bone defect was measured on standardized photographs via an image analysis program (Stemi 2000 and Axiovison 4.8, Carl Zeiss, Jena, Germany).
2.6. Histopathological analysis
All tissues were rehydrated with ethanol series and then cleared with xylene series. Then tissues were embedded in paraffin and serial sections were obtained. Total inflammatory cells and osteoblast and osteoclast counts in the wound area were determined on H&Estained sections. Three measurements were performed for each section and a mean value was calculated and recorded. Inflammatory cells counted were macrophage, neutrophil, eosinophil, T lymphocytes and plasma cells[19,20] The cells which were not easily differentiated were not counted.
2.7. Determination of RANKL, OPG, Runx2, BMP-2, and ALP by immunohistochemical assay
Firstly, three sections were selected from each rat and rehydrated through ethanol series. Then all sections were cleared with xylene and endogenous peroxidase activity was suppressed with hydrogen peroxide (3%). After washing three times for 5 min (3×5) with phosphate-buffered solution (PBS), sections were incubated with rabbit serum for 30 min. After serum incubation, sections were washed 3×5 with PBS and incubated with primary antibodies overnight at 4 ℃ in a humidified dark room. The antibodies used were anti-RANKL antibody (Thermo fisher scientific, Massachusetts, USA), anti-OPG antibody (Thermo fisher scientific, Massachusetts, USA), anti-Runx2 antibody (Thermo fisher scientific, Massachusetts, USA), anti-BMP-2 antibody (Abcam, Cambridge, UK), and anti-ALP antibody (Abcam, Cambridge, UK) and dilution ratio was 1:250. After primary antibody incubation, sections were washed again 3×5 with PBS and incubated with biotinylated secondary antibody immunoglobulin G for 30 min. Then, all sections were washed again and treated with a streptavidin-horseradish peroxidase-conjugated reagent for 30 min. After washing again with 3×5 PBS, 3-amino-9-ethylcarbazole (AEC) chromogen was applied to visualize immunoreactivity for 5 min. After AEC treatment, sections were washed again with 3×5 PBS, counterstained with
Gill’s hematoxylin, washed with distilled water and then mounted.
AEC provided a red color in the slides with different shades from pale red to dark red. Immunohistochemistry was evaluated by 1 000× magnification (Nikon, Tokyo, Japan). A cell counting frame of 10 000 µm2was created and all cells within were marked according to their staining density. The staining density was recorded from 0 to 3 as no staining-0, slight staining-1, mild staining-2, and dense staining-3. To compare the results, all stained and non-stained cells were converted to a numeric value, H score which provided statistical comparison. The conversion was performed based on a formula, [∑Pi(i+l)]. In the formula i: represents the intensity score of the staining and Pi: represents the percentage of the stained cells. An experienced blinded researcher performed all immunohistochemical evaluations from three different points on each slide and a mean value for each animal was recorded[5,20,21].
2.8. Statistical analysis
A power analysis was performed, and the power of the study was calculated as 90%. All data were analyzed with the IBM SPSS program (IBM, New York, USA) and presented as mean and standard deviation. All data were firstly analyzed with the Kolmogorov-Smirnov test for normality. The immunohistochemical results were analyzed with non-parametric tests, Mann Whitney U and Kruskal Wallis tests. The stereomicroscope measurements, inflammatory cells, osteoblast, and osteoclast counts were analyzed with parametric tests, One Way ANOVA and Tukey. P<0.05 was considered statistically significant.
2.9. Ethical statement
The s tudy protocol and all experimental designs were reviewed and approved by Animal Ethics Committee of Tokat Gaziosmanpasa University’s School of Medicine, Turkey (Project no: 2019-HADYEK-02). And the study was conducted at Tokat Gaziosmanpasa University Faculty of Dentistry. All experimental procedure was performed following the guidelines of the European Communities Council Directive of November 24, 1986 (86/609/EEC) and the manuscript was created according to the ‘NC3Rs ARRIVE Guidelines, Animal Research: Reporting of In Vivo Experiments’.
3. Results
The calvarial defect model was a traumatizing procedure for rats. Two rats were lost during the procedure and immediately replaced. No rats were lost apart from those two and the healing period was uneventful. All rats functioned normally and no changes were observed in the behavior of the rats after the procedure.
3.1. Characters of liposomes
The zeta potential was measured in folded capillary cells at 25 ℃and found to be -50.2 mV. The spherical structure of the liposomes and the size were observed on the scanning electron microscopy images. The encapsulation efficiency was found 95%.
3.2. Morphometric evaluation
The NC group had the highest unhealed defect area compared to the other groups (P<0.05). The PC group showed significantly lower defect area compared to the NC group (P<0.05) but similar to the GA-P group (P>0.05). The GA-L group had the lowest defect area (P<0.05) and almost all of the bone defect was found to be filled with newly formed bone (Table 1, Figure 1).
3.3. Histological analysis
Inflammatory cell infiltration was higher in the NC group than those of the other groups (P<0.05). The PC had lower inflammatory cell infiltration compared to the NC but higher inflammatory cell infiltration compared to the GA-P group (P<0.05). Gallic acid powder significantly decreased inflammatory cell infiltration (P<0.05) however the decrease was greater in the GA-L group compared to the GA-P group (P<0.05) (Table 1, Figure 2).
Osteoblast counts were similar in all groups (P>0.05) while osteoclast numbers were significantly different (P<0.05). The NC group had the lowest osteoclast counts (P<0.05). The counts increased in the PC group (P<0.05) and both gallic acid groups showed significantly lower osteoclast counts compared to the PC group (P<0.05). The counts in the GA-P and GA-L groups were similar (P>0.05) (Table 1, Figure 2).
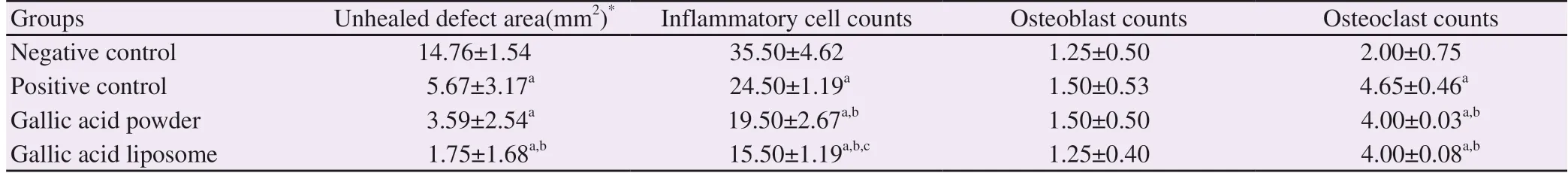
Table 1. Unhealed defect area, inflammatory cell counts, osteoblast and osteoclast cell counts in the study groups.
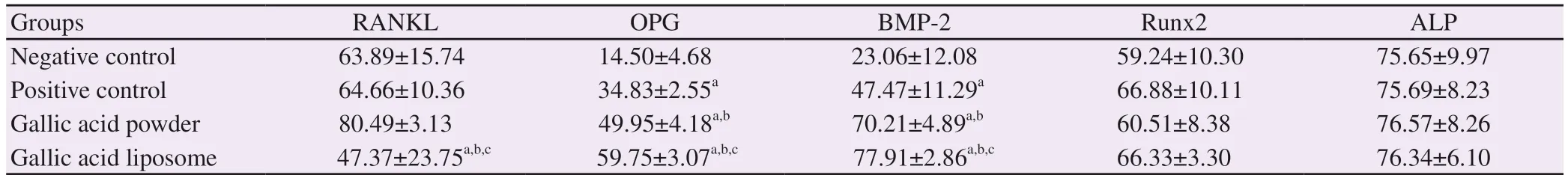
Table 2. Immunohistochemistry staining results in the study groups.
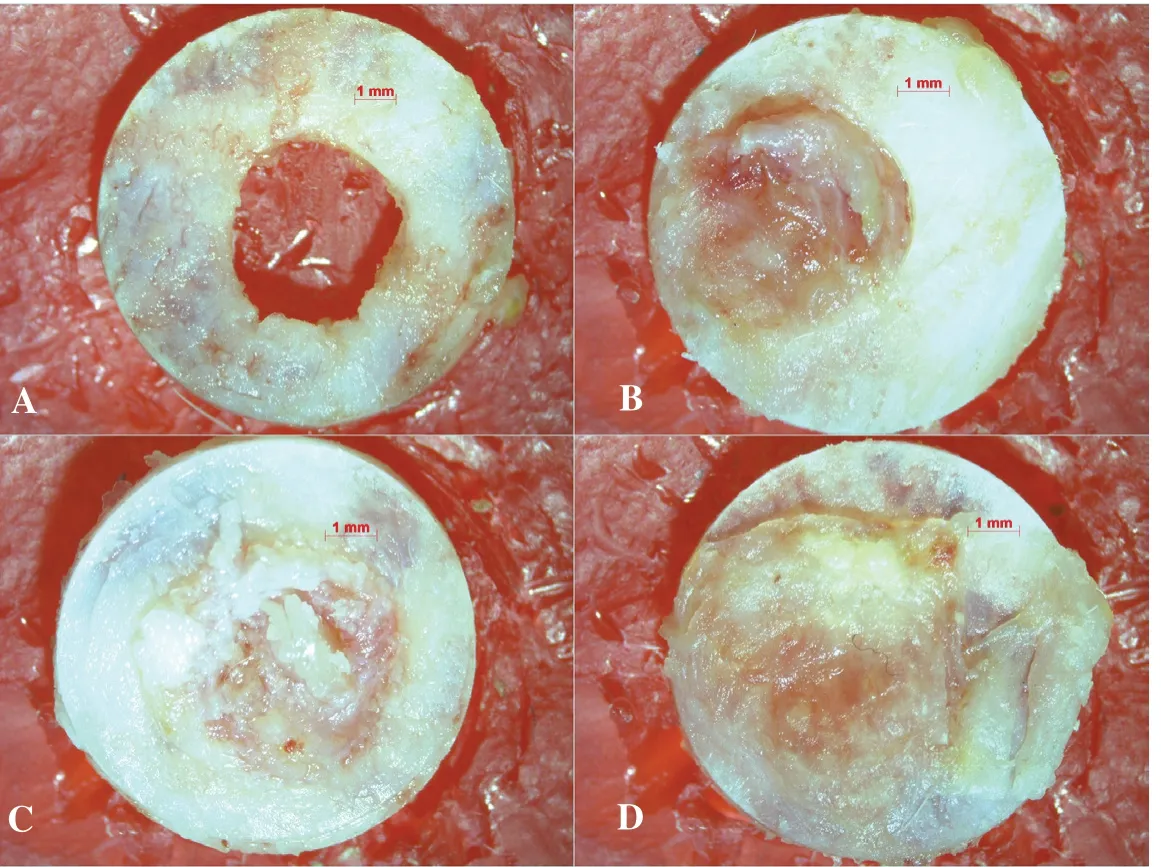
Figure 1. Representative stereomicroscope images of the study groups under 8× magnification. A: Negative control group, B: Positive control group, C: Gallic acid powder group, D: Gallic acid liposome group.
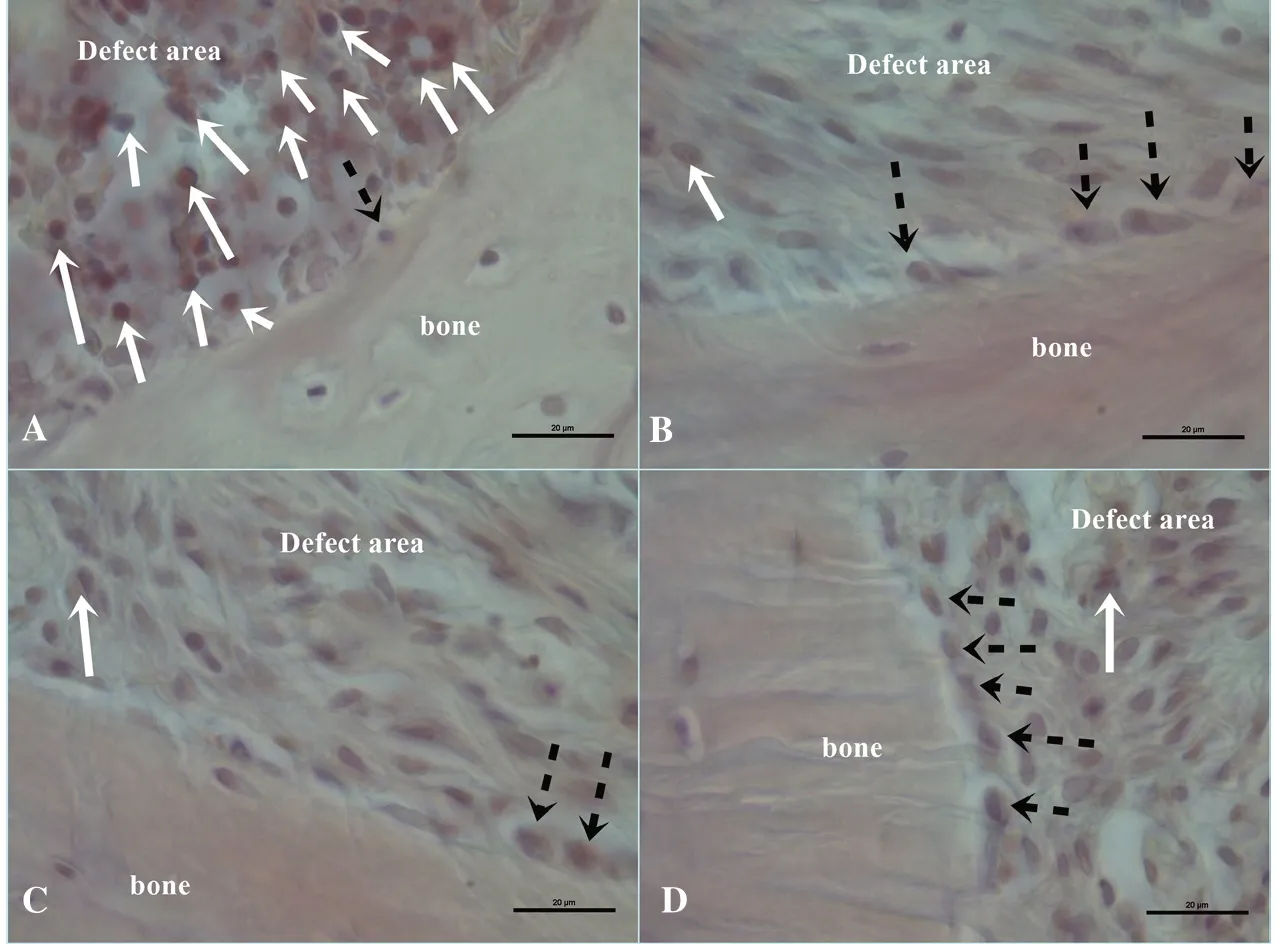
Figure 2. Representative hematoxylinhematoxylin-eosin staining images of the study groups. A: Negative control group, B: Positive control group, C: Gallic acid powder group, D: Gallic acid liposome group. Straight white arrows indicate inflammatory cells and interrupted black arrows indicate osteoblast cells. Scale bar: 20 μm.
3.4. Immunohistochemical result of RANKL, OPG, Runx2, BMP-2, and ALP
RANKL expressions were similar in the NC and PC groups (P>0.05). The GA-P group showed a slight increase but the difference was not significant (P>0.05). The GA-L had significantly lower expressions of RANKL compared to the other groups (P<0.05) (Table 2, Figure 3).
OPG expressions were the lowest in the NC group (P<0.05). The values significantly increased in the PC group (P<0.05). The GA-P and the GA-L groups had higher OPG levels compared to the NC and PC groups (P<0.05). The difference between the GA-P and the GA-L groups was also significant (P<0.05) (Table 2, Figure 3).
BMP-2 levels presented a similar pattern to the OPG levels. The lowest values were observed in the NC group (P<0.05). The PC had significantly higher values (P<0.05). The levels increased in the GA-P and the GA-L groups and the difference between the GA-P and the GA-L groups was also significant (P<0.05) (Table 2).
Runx2 and ALP expressions did not exhibit any significant differences among the groups (P>0.05). Although there were slight differences in Runx2 expressions, ALP levels were almost the same in all the groups (Table 2, Figure 3).

Figure 3. Representative immunohistochemistry staining of RANKL, OPG, and Runx2 in the study groups. RANKL: receptor activator of nuclear factor κ-B, OPG: osteoprotegerin, Runx2: runt-related transcription factor 2. Scale bar: 50 μm.
4. Discussion
This study evaluated the effects of gallic acid in powder and liposome forms on critical-sized calvarial bone defects in Wistar rats. The primary outcome was the bone healing which was determined by the unhealed defect area. The results revealed that both the positive control group and gallic acid groups provided significant bone fill and the smallest defect area was observed in the GA-L group. The secondary outcomes were the osteoblast, osteoclast, and inflammatory cell counts and the bone markers which were determined histologically. Gallic acid liposomes provided significantly lower RANKL and inflammatory cell counts and higher osteoclast counts and BMP-2 and OPG expressions while osteoblast counts, Runx2 and ALP expressions were similar among the groups. Bone remodeling is a life-long process and requires a balance between bone formation and bone resorption[1,3]. Bone healing after surgeries, trauma, and metabolic bone diseases might alter the remodeling process and persistent inflammation and infection in the healing period might compromise the bone formation[2]. As a powerful antioxidant and anti-inflammatory agent, gallic acid accelerated bone formation which was demonstrated by Lee et al[22]. Their results revealed that gallic acid stimulated bone healing by increasing osteoblastic cell adhesion to orthopedic implants and preventing fibroblast proliferation which resulted in a better osteointegration and biocompatibility[22]. In addition, Huang et al. showed that gallic acid elevated osteoblastic activity and osteogenic markers Runx2, ALP, OCN and increased collagen Ⅰ and extracellular matrix synthesis in vitro[23]. Increased osteoblast proliferation was also demonstrated[23]. Gallic acid also reduced fracture risk and bone fragility and up-regulated osteogenic activity reported by Chauhan et al.[12]. The present results also revealed promoted bone formation in the calvarial defects. The stereomicroscope measurements revealed the smallest defects in the GA-L group followed by the GA-P, PC, and NC groups. However, the results of GA-P were similar to the PC even though there was a slight decrease in the defect size. The effect of gallic acid on bone formation was also evident in increased osteoblastic activity through BMP-2 expressions. GA-L group had the highest BMP-2 expressions compared to the other groups. GA-P also increased BMP-2 levels but not to the level of GA-L group. However, the results did not show any improvement in the osteoblast counts, Runx2 and ALP expressions in the groups. All groups had similar values in these parameters. In contrast, Hou et al. recently reported that a gallic acid derivative increased Runx2 expression in osteoblasts[24]. A possible explanation might be the study duration which was 4 weeks in the present study while 8 weeks in Hou et al.’s. Despite Runx2 expressions, the present results are compatible with the findings of Hou et al. who also used a calvarial model to assess the effects of gallic acid on bone metabolism[24]. Increased defect fill, osteoblast proliferation, and activity were demonstrated in the results of the present study and Hou et al.[24].
The effect of gallic acid on bone metabolism is not limited to bone formation. Gallic acid also prevented osteoclast formation which was reported by Baek et al. through Akt and Btk-PLCγ 2-Ca2+signaling pathway[14]. Shim et al. demonstrated that plant extracts containing gallic acid suppressed the NF-κB pathway and RANKL and prevented osteoclastogenesis through the NFATc1 pathway resulting in attenuation in bone loss[25]. The decrease in osteoclast counts and bone resorptive activity were also reported in experimental osteoporosis[26]. Oka et al. found that gallic acid derivatives also decreased osteoclast differentiation and osteoclastic activity via down-regulated tartrate-resistant acid phosphatase and matrix metalloproteinase levels[27]. Osteoclast counts in the present study also increased with gallic acid in both GA-P and GA-L groups. Nonetheless, the form of gallic acid, either free or liposome, did not affect the osteoblast counts. However, the RANKL expressions were significantly lower in the GA-L group compared to the other groups. NC, PC and GA-P groups had higher expressions of RANKL. As for OPG, gallic acid provided significantly higher expressions than those of the other groups. The improvement was better in the GA-L group compared to the GA-P group. Increased osteoclast counts along with the decreased RANKL and increased OPG expressions indicate the preventive effect of gallic acid on osteoclastic activity which was reported in previous studies[14,25,27,28].
One of the most pronounced biological effects of gallic acid is the anti-inflammatory effect which occurs through blocking the NF-κB pathway. Gallic acid was reported to suppress NF-κB and inhibit inflammation[8,9,14]. Gallic acid also down-regulated significant inflammatory mediators such as inducible NO synthase, cyclooxygenase-2, and neutrophil adhesion molecules[9]. Proinflammatory cytokines such as IL-6 also decreased after gallic acid treatment which was reported by Rong et al.[29]. Reduced skin wound inflammation, improved wound healing, and decreased inflammatory cell infiltration[30] were also reported after gallic acid application. The present study evaluated the inflammation as the inflammatory cell infiltration in the defect area. The results showed significantly decreased inflammation with gallic acid application. The effect of gallic acid was more evident in the GA-L group. Furthermore, RANKL is the receptor activator of NF-κB, and decreased expressions might indicate decreased NF-κB activity.
The present study used gallic acid in two different forms as the free gallic acid, gallic acid powder, and the gallic acid liposome. A liposome is a spherical vesicle with two lipid layers encapsulating the agent inside[16,31]. The encapsulation of compounds such as gallic acid protects the structure and prolongs the effects which were demonstrated by Vitonyte et al.[16]. The most significant benefit is the protection of the active agent encapsulated within the liposome. However, in the topical use of encapsulation, the release of the agent might be slower than the free form, and therefore, the efficacy might decrease[31]. In the present study, liposome provided significantly better efficacy in terms of anti-inflammatory effect, RANKL, OPG, and BMP-2 expressions.
Nevertheless, the present results should be interpreted considering
the limitations of animal studies. Animal biology differs from human biology and pharmacokinetic features of agents used might be different. Also, the present study evaluated the local expressions of the markers, performing different analyses such as RT-PCR, immunofluorescence, or Western blotting would provide more insights.
Gallic acid is a potent anti-inflammatory flavonoid and was shown to promote bone healing in the present experimental model. Unhealed bone defect, inflammatory cell counts and RANKL expressions were significantly lower in the gallic acid administered groups and BMP-2 and OPG expressions were higher. Gallic acid liposome provided better results except for unhealed defect area and osteoclast counts which were similar between the GA-P and GA-L groups. This study evaluated the bone healing morphometrically and other parameters histologically. Future studies involving a cell culture study to observe the effect of gallic acid on osteoblasts and osteoclasts with different parameters would be beneficial.
Conflict of interest statement
We declare that there is no conflict of interest.
Authors’ contributions
A.A. and H.B.Y. made substantial contributions to conception and design and interpretation of data and writing the manuscript. O.K. and M.M.T. contributed to collection of data. F.G. performed histopathological analysis. S.Ç. and N.A. carried out experiments. H.B.Y. performed statistical analysis. A.A. performed critical revisions of the manuscript and prepared the final approved version for publication. All authors read and approved the final manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年4期
Asian Pacific Journal of Tropical Biomedicine2020年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Identification and investigation of Calodium hepaticum in rodents and insectivores from Wuhan section of the Yangtze River in China
- Antibacterial activity of bacillomycin D-like compounds isolated from Bacillus amyloliquefaciens HAB-2 against Burkholderia pseudomallei
- Anti-Acinetobacter baumannii activity of Rumex crispus L. and Rumex sanguineus L. extracts
- A novel polyherbal formulation containing thymoquinone attenuates carbon tetrachloride-induced hepatorenal injury in a rat model
