Anti-Acinetobacter baumannii activity of Rumex crispus L. and Rumex sanguineus L. extracts
Verica Aleksic Sabo, Emilija Svircev, Neda Mimica-Dukic, Dejan Orcic, Jelena Narancic, Petar Knezevic✉
1Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad, Trg Dositeja Obradovica 3, 21 000 Novi Sad, Vojvodina, Serbia
2Department of Chemistry, Biochemistry and environmental protection, Faculty of Sciences, University of Novi Sad, Trg Dositeja Obradovica 3, 21 000 Novi Sad, Vojvodina, Serbia
ABSTRACT Objective: To examine the effect of Rumex crispus (R. crispus) and Rumex sanguineus (R. sanguineus) plant extracts against isolates of Acinetobacter baumannii (A. baumannii) from wounds, including multidrug-resistant strains. Methods: Six prepared Rumex extracts were subjected to liquid chromatography-tandem mass spectrometry. Antimicrobial activity of extracts and pure compounds (catechin, quercetin, isoquercitrin, emodin, and gallic acid) was examined by a microtiter plate method, while for determination of compound binary combinations activity a checkerboard method was applied. Active fractions of extracts were detected by agar-overlay high-performance thinlayer chromatography-bioautography assay followed by liquid chromatography - diode array detection - mass spectrometry analysis. Results: A total of 28 compounds were detected in two extracts of R. crispus and 26 compounds in four different R. sanguineus extracts, with catechin as a dominant component. Anti-A. baumannii activity was confirmed for all six R. sanguineus and R. crispus extracts at the concentration range from 1 to 4 mg/mL. Neither examined single compounds nor their binary combinations exhibited an anti-A.baumannii activity (MIC>256 μg/mL). The bioautography showed that fractions with the most prominent anti-A. baumannii activity tended to contain more polar compounds, predominantly flavonol (quercetin and kaempherol) glycosides; but also fractions containing flavanone (eriodictyol) glycosides and anthraquinone (emodin) glycosides; and less polar eriodictyol aglycone. Conclusions: The results justify and elucidate the traditional application of R. sanguineus and R. crispus extracts for wound healing, indicating the necessity for their further examination in combat against multidrug-resistant A. baumannii isolates from wounds.
KEYWORDS: Acinetobacter baumannii; Isolates; Wound; Multidrug resistance; Rumex extracts; High-performance thin-layer chromatography-bioautography
1. Introduction
Acinetobacter baumannii (A. baumannii) is a Gram-negative coccobacillus that colonizes the oral cavity, respiratory and gastrointestinal tract, but is also a recognized opportunistic pathogen that causes various severe infections. A. baumannii is a frequent cause of wound infections, particularly war-related injuries, because of easy wound/burn contamination. On the other hand, it is a clinically dominant species with a pronounced tendency to induce nosocomial infections, especially in intensive care units[1,2]. The infections with the etiological agent as A. baumannii have a high mortality risk[3,4].
This genomic species is the most troublesome member of Acinetobacter calcocaceticus-A. baumannii complex (Acb-complex), showing pronounced resistance to conventional antibiotics. In the early 1970s, infections caused by this bacterium were treated with gentamicin, minocycline, nalidixic acid, ampicillin, and carbenicillin. However, between 1971 and 1974, this bacterium became resistant to the aforementioned antimicrobials. During the early 1990s, the bacterium has exhibited resistance to betalactams[5], aminoglycosides[6], chloramphenicol, tetracycline, and fluoroquinolones[7]. At the end of the 1990s, carbapenems were the only treatment choice[5], and soon rifampicin was introduced in combination with carbapenems. To combat multi-drug resistant strains, tigecycline, polymyxin B, and colistin are used nowadays. However, resistance to these antibiotics has also been demonstrated recently, which makes this bacterium one of the greatest threats to human health today. The emergence of A. baumannii strains resistant to all known antibiotics, i.e. pandrug resistant strains indicates urgent necessity to discover novel antimicrobial agents or therapeutic strategies[8]. Natural plant products, particularly those used in traditional medicine have become the solutions to overcome this problem[9-11]. Many studies have proved the effectiveness of nature-derived antimicrobial agents in the treatment of various diseases and support their usage to a great extent nowadays.
Besides the increasing emergence and spread of multi-drug resistant and pandrug resistant microorganisms, environmental awareness among the population is the reason for the current application of natural products. The use of natural antimicrobial agents as phytopharmaceuticals and food preservatives is an increasingly accepted alternative to the synthetic and usually toxic, teratogenic or mutagenic chemicals. From 2000 to 2006, about 50% of new molecules were extracted from natural products, indicating their importance in the development of new drugs for the treatment of infectious diseases[12]. Thus, in comparison to conventional antibiotics, natural antimicrobial agents have many advantages, such as less harm, broad acceptance because of their traditional use, better biodegradation, less bacterial resistance, etc.
Rumex crispus (R. crispus) L. and Rumex sanguineus (R. sanguineus) L. from the Polygonaceae family have been used in ethnopharmacology for various medical purposes, and some of the applications indicate their antimicrobial activity and/or wound healing potential[13]. For instance, R. crispus has been used in Hungary and in Romania for diarrhea, rashes, sores and wounds treatments[14,15], while the dried underground plant parts have been used in traditional Turkish medicine as a blood cleanser[16]. In other parts of the world, it has also been used against skin diseases and against dysentery[17]. R. crispus has been applied in India and Pakistan for treating a wide range of skin problems (sores, ulcers, and wounds) and the underground parts have been used in diarrhea treatment[18,19]. Similarly, R. crispus was used by American Indian tribes for the treatment of diarrhea, dysentery and skin problems[20], including fungal infections[21]. The infusion or decoction of R. crispus has been commonly used in folk medicines by natives of Africa for the treatment of helminths, wounds, internal bleeding and vascular diseases[22]. In Italy, warm leaves of R. crispus and R. sanguineus are applied to treat abscesses. A compress made of halfpeeled leaves along with other components has been applied to wounds and sores as the cicatrizing agent. Leaves crushed in mortar were used against abscesses, burns and insect bites[23]. In addition, young leaves of R. crispus that appear in the spring have been used for consumption, while the seeds are collected during the summer and used as an Asian national remedy. It has been reported that Rumex extracts possess antioxidant, antimicrobial and antifungal properties[20], but their activity against A. baumannii has not been examined.
Taking into account the traditional application of R. crispus and R. sanguineus plant extracts in treating wound healing and the necessity to find alternatives to combat multiple drug resistant (MDR) bacteria, the activity of these extracts, their pure compounds individually and in binary combinations against isolates of A. baumannii from wounds was examined. Also, high-performance thin-layer chromatography (HPTLC)-bioautography assay and chemical characterization of the potent extract were performed to elucidate compound(s) responsible for anti-A. baumannii activity.
2. Materials and methods
2.1. Standards and reagents
Reference standards of the phenolic compounds were obtained from Sigma—Aldrich Chem (Steinheim, Germany), Fluka Chemie Gmbh (Buchs, Switzerland), Chromadex (Santa Ana, USA), or from Extrasythese (Genay Cedex, France). HPLC gradient grade methanol was purchased from J. T. Baker (Deventer, The Netherlands), and p.a. formic acid and DMSO from Merck (Darmstadt, Germany). Naturstoff reagent A (diphenylboric acid 2-amino-ethyl ester) was purchased from Roth (Carl Roth Cmbh + Co Karlsruhe, Germany), polyethylene glycol 4000 from Sigma—Aldrich (Germany), HPLC grade ethanol from J. T. Baker (Deventer, The Netherlands), toluene, analytical grade from Centrohem (Stara Pazova, Serbia) and ethylacetate, analytical grade, from Fischer Company (Fisher Scientific UK Ltd).
2.2. Plant extract preparation
Extracts were prepared using R. crispus (voucher number 2-1721) and R. sanguineus (voucher numbers 2-1735 and 2-1736) from family Polygonaceae. Taxonomic determination of plant material, voucher specimens preparation, and plants samples deposition (at BUNS Herbarium) were done by Goran Ana kov Ph.D., University of Novi Sad Faculty of Sciences, Department of Biology and Ecology. Six extracts in total were prepared for testing the anti-A. baumanni activity (Supplementary Table 1). All the extracts were prepared by maceration of dry, grounded plant material (above ground plant parts — for the herb extracts; and the underground parts — for the rhizome extracts) with 80% ethanol at constant shaking for 48 h. Filtered extracts were evaporated in vacuum and re-dissolved in 70% ethanol (for microbiological analysis) or dimethyl sulfoxide (DMSO) (for chemical analysis), giving a final concentration of 200 mg/mL or 300 mg/mL. Herb extracts of Rumex species were washed (liquid-liquid extraction) with petroleum ether to remove lipids and pigments. Washed extracts were concentrated in vacuum and redissolved in DMSO or 70% ethanol giving a final concentration of around 200 mg/mL or 300 mg/mL.
2.3. Bacterial strains
A total of 25 bacterial strains were used in the study. Three reference strains of A. baumannii were used, two from American Type Culture Collection (ATCC 19606 and ATCC BAA747, Rockville, MD, USA) and one from the National Collection of Type Cultures (NCTC 13420, Public Health England, UK). In addition, two reference strains, Escherichia coli (E. coli) ATCC 25922 and Staphylococcus aureus (S. aureus) ATCC 25923, were used as quality controls. The twenty remaining strains were MDR A. baumannii isolates from outpatient and clinical wounds, which have been characterized previously[16]. All the bacterial strains were stored in Luria Bertani broth supplemented with glycerol (10% v/v) at —70 ℃. All antibacterial tests were performed using Mueller Hinton agar (MHA) and Mueller Hinton broth (MHB).
2.4. Antibacterial activity determination
2.4.1. Effect of plant extracts
In order to find the alternative solution(s) for the eradication of A. baumannii, the minimal inhibitory concentrations (MICs) of extracts were examined by a slightly modified microtitre plate method[24]. In the 96-well microtiter plates, double dilutions of extracts were prepared in sterile distilled water and the final concentrations of each extract in the microtiter plate ranged from 0.25 to 8 mg/mL. The final concentrations of the plant extract solvents did not exceed 1.9% for ethanol extracts and 1% for the extracts prepared in DMSO. All experiments included the control of the maximum solvent concentration in the final volume to confirm the absence of inhibition. The final bacterial count in the test was approximate 1×106CFU/mL. Reference strains E. coli ATCC 25922, S. aureus ATCC 25923 and gentamicin were used as method quality controls. Microtiter plates were incubated overnight at 37 ℃, after which 10 μL of 1% triphenyl-tetrazolium chloride (TTC) solution was added to each well and the microtiter plates were additionally incubated for 2 h at 37 ℃ until red color of formazan appeared. This modification was made in order to make MIC values determination more precise. The minimum concentration of extracts that prevented the appearance of red color, i.e. formation of formazan was considered as a MIC value.
Minimal bactericidal concentration (MBC) was determined by spreading 10 μL of the suspension from wells without obvious bacterial growth onto MHA, in order to determine if the type of the bacterial inhibition was permanent or reversible. Plates were incubated for 24 h at 37 ℃. After the incubation, the presence or absence of growth was recorded, where the lowest plant extract concentration at which the bacterial cell count was reduced by 99.9% compared to the initial number, was considered as MBC.
2.4.2. Effect of selected extract compounds
In order to determine anti-A. baumannii activity of components from plant extracts, some dominant compounds from extracts and those detected in the R. crispus herb extract after chemical characterization were tested. The following five phenolic compounds were tested: quercetin, quercetin-3-O-glucoside, catechin, emodin and gallic acid.
Anti-A. baumannii effect of standard compounds was tested using the same method as described for herbal extracts. Compounds were diluted depending on their solubility in water, methanol or DMSO (not exceeding 1%) to the final tested concentrations ranging from 0.125 to 256 μg/mL.
2.4.3. Effect of extract compounds in binary combinations
The anti-A. baumanni effect of selected compounds in the examined extracts was tested after the preparation of different binary combinations (1:1, v/v). This method was used due to high MIC values (>256 μg/mL) and poor antibacterial activity of a single compound. For each combination, double dilutions were prepared so that the concentrations of bioactive compounds varied from 16 to 128 μg/mL. The incubation period, data interpretation and presentation of obtained results were performed as described for herbal extracts and individual bioactive components.
2.5. Agar-overlay HPTLC-bioautography assay
The method that combines microbiological assay with the thinlayer chromatography[14,15] was used for further analysis of anti-A. baumannii active substances. In this test, the focus went towards Rumex species which showed the best activity (i.e. lowest MIC and MBC values).
Selected extract separation was performed on HPTLC silica gel 60 F254 aluminum plates (Merck, Germany), measuring 10 cm×20 cm. R. crispus 171_H extract was applied to the plate in the form of a narrow band (5 μL, i.e. 1 mg/mL). Four such bands were applied on one piece of plate. After drying the samples, the plate was developed with the previously optimised mobile phase (ethyl acetate-tolueneformic acid-water 80:10:5:5, v/v/v/v). The development mode was ascendant, in a saturated chamber. After chromatographic separation, the adsorbent layers were dried in an oven at 90 ℃ for 5 min to remove the solvent completely. Methanolic Naturstoff reagent A, NA (1.0%) and ethanolic polyethylene glycol 4000 solution (5.0%) were used to visualize the separated compounds. The sprayed plates were observed under VIS and UV light (366 nm) and documented.
Separation of the extract components was done in quadruplicate, with two bands used for the bioautographic analysis of plant extract, one band used for visualization of separated components, and one for the extraction of spotted fractions (zones) and further liquid chromatography with diode array detection and mass spectrometry (LC/DAD/MS) analysis (as described below). Fractions on the HPTLC plates were spotted in accordance with the results of the bioautography test.
For the agar-overlay bioautography assay, two unsprayed HPTLC plate bands were cut out after extract separation. One strip was used as a whole, but the other was chopped into squares based on the difference in colour obtained on the third strip. The prepared parts of the HPTLC plates were positioned on the surface of the MHA and then topped with an inoculated semisolid medium (a bacterial suspension approximate 1.5×108CFU/mL) supplied with 1% TTC solution (final ratio 30:1:1, v/v/v). The plates were incubated for 24 h at 37 ℃. After incubation, the absence of bacterial growth indicated the zones with antimicrobial activities. The TTC was used to facilitate the visualization of the presence/absence of bacterial growth[25]. Inhibition zones are visible as transparent zones against red-colored bacterial growth. The HPTLC plate strips were photographed using the Canon EOS 100D camera.
2.6. Extracts' chemical composition analysis
All the measurements were done using Agilent Technologies 1200 Series High-performance liquid chromatography coupled with Agilent Technologies 6410A Triple Quad tandem mass spectrometer with electrospray ion source and controlled by Agilent Technologies MassHunter Workstation software (ver. B.03.01).
2.6.1. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of plant extracts
For the quantitative LC-MS/MS analysis of the selected 45 compounds, extracts were diluted with 0.05% aqueous formic acid and methanol (1:1) to a final concentration of 2 mg/mL. The method used in this study for the Rumex extracts analysis was previously developed, validated and published by Orčiċ et al[26].
2.6.2. LC/DAD/MS analysis of R. crispus herb extract HPTLC-fractions
Following the results of the bioautography test, eight different zones were spotted on the HPTLC plate. Every zone was scraped from aluminum sheet, and compounds were extracted from the silica gel with 80% methanol (400 μL), and filtered through syringe filters, regenerated cellulose, 0.45 μm into vials. A total of 5 μL of these samples were injected into the system, with Zorbax Eclipse XDB-C18 (50 mm×4.6 mm×1.8 μm) rapid resolution column held at 50 ℃. The mobile phase was delivered at a flow rate of 0.8 mL/min in a gradient mode (0 min 20% B, 6.67 min 60% B, 8.33 min 100% B, 12.5 min 100% B, re-equilibration time 4 min). Eluted components were firstly recorded on diode array detector, full spectra in 190-700 nm range, but also detected by MS, using the ion source parameters as follows: nebulization gas (N2) pressure 40 psi, drying gas (N2) flow 9 L/min and temperature 350 ℃, capillary voltage 4 kV; and using MS2Scan run mode (negative and positive ionization, NI/PI, m/z range of 120—1 000 and fragmentor voltage of 80 V). For the one dominant compound in fraction 7 (F7), a product ion scan experiment was conducted using collisionally induced dissociation (high-purity N2as the collision gas, collision energies ranging 10—40 V in 10-V increments).
2.7. Statistical analysis
The MICs were logarithmically transformed and data were tested for normality of distribution. Because of the lack of normal distribution, differences in extract activity and differences in MIC and MBC among different plant species and plant parts were estimated by the Wilcoxon signed-rank test. The level of significance for all analyses was set as α=0.05.
All the experiments were performed in triplicates and on three independent occasions and the results are represented as geometric means of replications.
3. Results
3.1. Anti-A. baumannii effect of R. sanguineus and R. crispus
The extracts of R. sanguineus and R. crispus showed significant bacteriostatic and bactericidal activity against MDR A. baumannii isolates from wounds (Table 1).
The extracts of R. sanguineus from the Zmajevac on Fruška Gora Mountain (4NZ_H_p and 4NZ_R) exhibited bacteriostatic activity with the MIC ranging 1.0-2.8 mg/mL for the extract of plant aerial parts (herb) and 1.4-4.0 mg/mL for the extract of the underground plant parts (rhizome). The bactericidal effect of these extracts showed the same MBC values (2.0-5.7 mg/mL) regardless of the part of the plant. Extracts of the same plant species originating from the Iriški venac on Fruška Gora Mountain (4Z_H_p and 4Z_R) also exhibited a bacteriostatic and bactericidal effect, with MIC values of 1.0-2.0 mg/mL for herb extract, or 1.4-2.8 mg/mL for the extract of rhizomes, while MBC values varied in the range 1.0-4.0 mg/mL for herb extract, or 1.4-5.7 mg/mL for rhizomes extract. The differences in MIC and MBC values among different R. sanguineus extracts were significant (P<0.001). Considering the different parts of R. sanguineus, herb extracts exhibited a better antibacterial effect, with lower MICs compared to rhizome extracts (P=0.002 for 4NZ_

H and P<0.001 for 4NZ). Similarly, the plant origin, i.e. locality, also influenced antimicrobial activity, since both rhizome and herb extracts from Iriški venac showed better anti-A. baumannii activity than those obtained from Fruška Gora (P<0.001 and P=0.027, respectively).
The extracts of R. crispus also exhibited significant antimicrobial activity against A. baumannii isolates from wounds. Bacteriostatic activity against MDR A. baumannii was recorded with MIC values as 1.0-2.0 mg/mL for both extracts (Table 1), while the bactericidal effect was observed at higher concentrations of 1.4-4.0 mg/mL for extract 171_H and 2.0-5.6 mg/mL for extract 179_R, with significant difference in MICs and MBCs (P<0.001). Herb extracts showed lower MICs in comparison to rhizome extracts (P=0.041).
3.2. Compounds of Rumex plant extracts by LC-MS/MS quantitative analysis
The preliminary results of LC-MS/MS analysis showed that R. sanguineus and R. crispus plant extracts consisted of various secondary metabolites which indicate that these plants are a potentially rich source of the biologically active phenolic compounds (Table 2). A total of 30 compounds were detected in the extracts of both Rumex species and the presence of 26 compounds in four different R. sanguineus extracts was confirmed. For extracts of R. sanguineus plants, the present phenolic compounds can be classified in different groups as follows: phenyl-carboxylic acids (p-hydroxybenzoic acid, protocatechuic acid, p-coumaric acid, gallic acid, caffeic acid, ferulic acid, syringic acid, and 5-O-caffeoylquinic acid); flavones (apigenin); flavonols and their glycosides (kaempferol, kaempferol-3-O-glucoside, quercetin, quercitrin, quercetin-3-O-glucoside, rutin); flavanones (naringenin); flavane-3-ols (catechin and epicatechin); and quinic acid. Of the compounds in the R. sanguineus extracts, catechin was dominant in amounts 12.31-16.63 mg/g of dry extract in the herb, and 3.49-3.60 mg/g of dry extract in the rhizome. In the herb extracts, it was followed by naringenin (0.63-0.68 mg/g of dry extract) and gallic acid (0.57-0.66 mg/g of dry extract in the herb extracts), and followed by epicatechin in the rhizome extracts (0.66-0.77 mg/g of dry extract),
In R. crispus extracts, 28 compounds was confirmed (Table 2), and can be classified in different groups as follows: flavonoid aglycones (apigenin, epicatechin, catechin, luteolin, myricetin, quercetin, naringenin) and methylated derivatives (isorhamnetin and chrysoephanol); flavonoid glycosides: 3-O-glycoside (quercetin-3-Oglucoside, kaempferol-3-O-glucoside, and quercitrin), 7-O-glucoside (luteolin-7-O-glucoside and apigenin-7-O-glucoside), 8-C-glycoside (vitexin) and 3-O-ester (epigallocatechin gallate); hydroxybenzoic acids (gallic acid, p-hydroxybenzoic acid, protocatechuic acid, and syringic acid); quinic acid, phenylpropene acids (ferulic acid, caffeic acid, p-coumaric acid) and 5-O-caffeoylquinic acid and anthraquinones (aloe-emodin, emodin, chrysophanol). The most dominant compounds in both R. crispus extracts were catechin (41.96 mg/g of dry herb extract and 13.25 mg/g of dry rhizome extract) and emodin (2.60 mg/g of dry herb extract and 2.27 mg/g of dry rhizome extract).
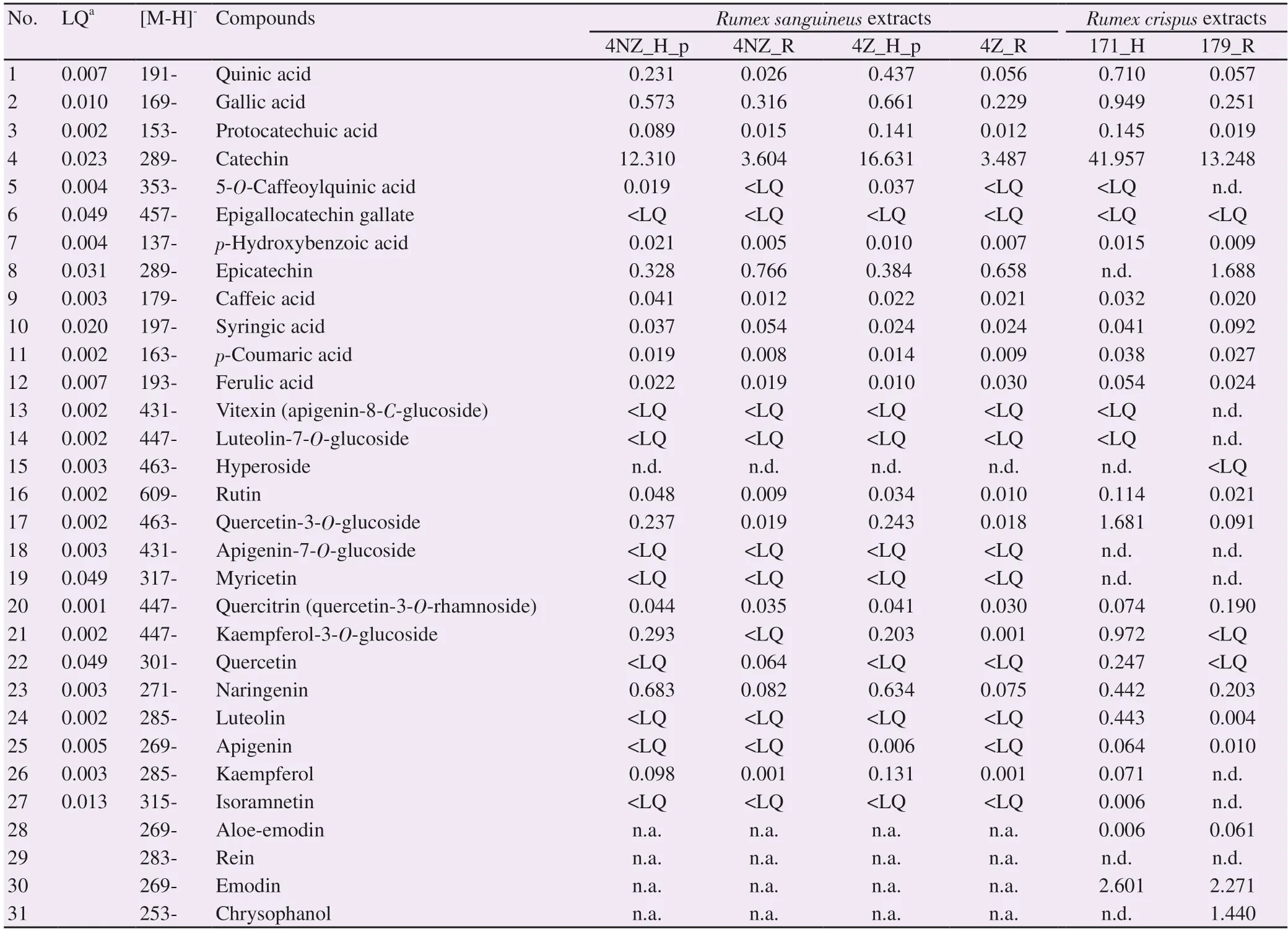
Table 2. Results of quantitative (LC-MS/MS) analysis of the phenolic compounds in Rumex plant extracts (mg of compound/g of dry extract).
3.3. Effect of extract compounds alone and in binary combinations
In order to identify the biologically active components responsible for the antimicrobial activity of plant extracts, five standard bioactive components: catechin, quercetin, quercitrin, gallic acid, and emodin were tested. These components were selected according to the criteria for their presence in plant extracts in which antimicrobial activity was detected against the MDR A. baumannii, as well as on the basis of data previously published in the literature.
In all R. sanguineus extracts, catechin was the most dominant component with the amount of 3.487 to 16.631 mg per gram of dry extract (Table 2). The extract of R. sanguineus (4Z_H_p) with the highest catechin content (16.631 mg per gram of dry extract) exhibited the best anti-A. baumannii activity. However, catechin alone did not exhibit antibacterial activity against MDR A. baumannii isolates from wounds, even at the highest tested concentration (256 μg/mL) (Table 1).

Table 3. Results of LC/DAD/MS analysis of 8 different HPTLC-fractions of Rumex crispus herb extract.
The antibacterial activities of quercetin and its derivative quercetin-3-O-glucoside (isoquercitrin) as the bioactive components of the flavonoid class were also tested. Quercetin was 0.064 mg/g in R. sanguineus (4NZ_R) dry extract (Table 2). Methanol and DMSO solutions of quercetin and water solution of isoquercitrin did not exhibit considerable antibacterial activity against MDR A. baumannii isolates since MIC values were greater than 256 μg/mL (Table 1).
Gallic acid, as a representative of hydroxybenzoic acid derivates, was present in Rumex extracts, especially in R. sanguineus 4Z_H_p (0.661 mg per gram of dry extract) (Table 2). The results show no activity against MDR A. baumannii (Table 1). The lack of activity was also recorded for emodin in the herb and rhizome extracts in the amount of 2.3-2.6 mg per gram of dry extract (Table 2).
In addition, similar to the results of the individual bioactive components of herbal extracts, their binary combinations did not exhibit an antibacterial effect (Table 1).
3.4. Agar-overlay HPTLC-bioautography detection coupled with LC/DAD/MS analysis
After the HPTLC separation of R. crispus herb extract (sample 171_H), eight different zones of compounds similar in colour were detected (Figure 1B). Some of these fractions (F1-F5 and F7) showed considerable anti-A. baumannii activity according to bioautography (Figure 1A). The quantitative LC-MS/MS indicated that this plant is a potentially rich source of biologically active phenolic compounds (Table 3). Using LC/DAD/MS analysis of different fractions, additional compounds were detected: quercetin-3-O-glucuronide (F2), eriodictyol (F7) and its derivatives -hexoside (F4) and -rhamnoside (F5), emodin derivatives -8-O-glucoside (F4 and F5), and -8-O-malonyl hexoside (F4); but also some of the compounds confirmed by LC-MS/MS analysis (e.g. rutin, 5-Ocaffeoylquinic acid) were not detected in the fractions due to their small amount present on HPTLC or wastage during the process of compounds extraction from the silica gel.
According to the LC/DAD/MS analysis of each fraction, a total of 36 different compounds was detected in R. crispus 171_H extract, among which 25 were identified (Table 3). The obtained spectral data were not sufficient for more detailed identification of the remaining 11 compounds. The most abundant compounds were catechin (~41.800 μg) in the zone F6, emodin (~2.590 μg) in F7, and quercetin-3-O-glucoside (~1.680 μg) in F2.
The strips were cut according to eight fractions spotted under the UV illumination and bioautography was carried out (Figure 1). The bacterial growth inhibition was obvious on the start of the strip, i.e. in the zone with fractions F1-F5 (Rf<0.4) on the HPTLC strip (Figure 1A). In these zones mostly the flavonoid glycosides were detected while their aglycones had higher Rfvalues (less polar compounds), located in the upper fractions, near the front of the chromatogram (Figure 1B and C). According to the bioautography assay coupled with LC/MS analysis, the detected compounds with anti-A. baumannii activity were mostly in the fractions of quercetin derivatives: quercetin-3-O-glucuronide (F2), isoquercitrin (quercetin-3-O-glucoside) (F2 and F3), quercetin-3-O-derivative (F3), quercitrin (quercetin-3-O-rhamnoside) (F3) and quercetin-3-O-glucoside (F2 and F3) (Figure 1 and Table 3). The other polyphenols glycosides were present in A. baumannii inhibiting fractions: kaempferol-3-O-glucoside (F4), emodin-8-O-glucoside (F4 and F5), emodin-8-O-malonyl-hexoside and eriodictyol-hexoside in F4, eriodictyolrhamnoside and galloyl-catechin in F5. However, the water solution of pure quercetin-3-O-glucoside tested alone did not show antibacterial activity. The predicted amount of quercetin-3-O-glucoside on the HPTLC was 1.681 μg (F3 and F4) (Table 3). Other phenolic glycosides present were not as affordable as the pure compounds for further testing in this study. Similarly, in the fraction F7, which showed bacterial inhibition in bioautography, the quercetin (0.247 μg) and emodin (2.590 μg) were detected and they did not show anti-A. baumannii activity when tested alone. The approximate amount of gallic acid, emodin, and catechin in F6 or F8 was 0.946 μg (F6), 2.590 μg (F8), and 41.800 μg (F6), respectively.
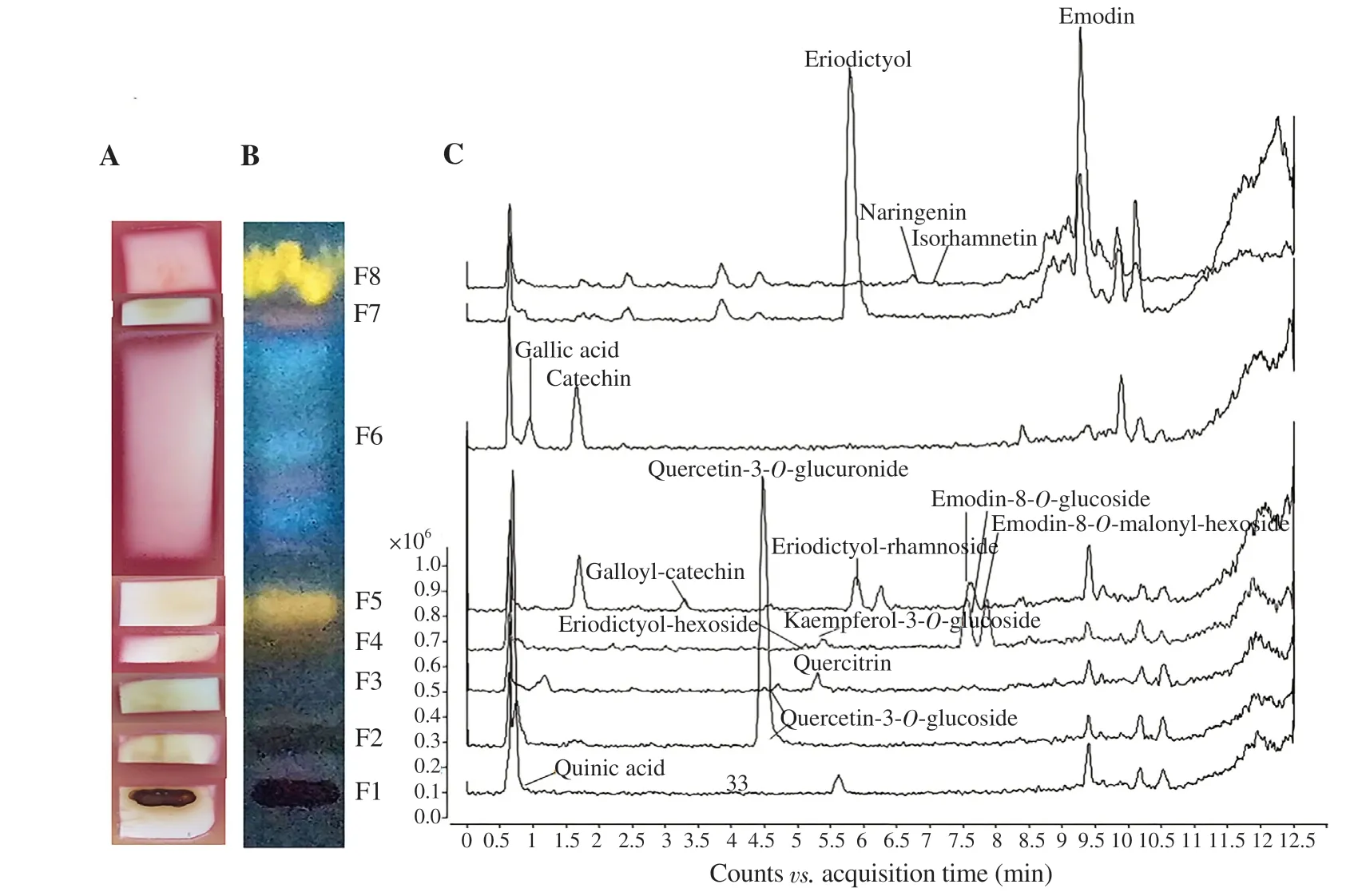
Figure 1. Bioautogram (A), high-performance thin-layer chromatography chromatogram,detection with NP/PEG reagent under 366 nm (B), and liquid chromatography-diode array-mass spectrometry detection of the compounds in each zone (fraction) (C) of Rumex crispus herb extract agaist Acinetobacter baumannii ATCC 19606 strain.
4. Discussion
Extracts of R. sanguineus and R. crispus plants have not been examined previously against A. baumannii, despite their application as traditional wound remedies. In the present study, we confirmed anti-A. baumannii activity of the ethanol extracts. Yildirim et al.[27] also proved that ether and ethanol extracts of R. crispus leaves and seeds showed antibacterial activity in contrast to aqueous extracts. However, they have demonstrated antibacterial activity by disc diffusion method only against bacterial strains S. aureus and Bacillus subtilis with an inhibition zone of 0.8-1.1 cm, whereas strains of Pseudomonas aeruginosa, E. coli, and Candida albicans were resistant to these extracts. In our study, the antibacterial activity of R. crispus extracts was detected against Gram-negative MDR isolates by the microdilution method. It seems that the antibacterial activity of ethanol extracts of herb and rhizomes is better compared with the activity of ether and aqueous extracts tested by Yildirim et al.[27]. Ethanol extracts of R. crispus rhizome showed similar activity against Pseudomonas aeruginosa and E. coli and it was superior to water, acetone, and methanol extracts[28].
The variation in the composition of extracts had an impact on anti-A. baumannii activity, depending on species, plant part, and geographical origin. The effect of geographical variations on the composition of the extract can be overcome by plant growth in a greenhouse under strictly controlled conditions.
All examined extracts showed significant anti-A. baumannii activity against MDR isolates from wounds. These MDR strains could be eradicated by tested Rumex extracts avoiding last line defense antibiotics, such as polymyxins. For these reasons, the extracts should be further examined for its potential topical application in the treatment of A. baumannii infected wounds in vivo.
The selected components of extracts did not show considerable activity against A. baumannii when they were applied as single agents. Catechins are generally considered as efficient antimicrobial agents that exhibit antibacterial activity by inhibiting the N-terminal fragment of DNA gyrase or interacting with its ATP binding site[28]. Catechin is the dominant component of the genus Rumex plants extracts, and its level was significantly higher in herb than in rhizome extracts, which indicates that this component may be responsible for antibacterial activity. However, this activity is not confirmed by the study with catechin activity alone. The antibacterial activity of catechin derivatives, that were not examined in the present study, seems to be more active
against Gram-negative bacteria, with MICs in range of 32-512 μg/mL, although some are inactive (MIC>800 μg/mL)[29-31]. Quercetin and quercetin-3-O-glucoside were also inactive against A. baumanni, which is in accordance with the previous report, which found that quercetin-3-O-glucoside was inactive against various bacteria even in extremely high concentration (100 mg/mL)[32,33]. Gallic acid was inactive against A. baumannii at higher concentrations, although it was reported that gallic acid showed MICs against A. baumannii transposon mutant strains as 128-256 μg/mL[34]. The discrepancy is probably due to the difference in the degree of sensitivity/resistance, compared to MDR isolates from wounds in the present study. Similarly, after 24 h of treatment with 200 μg/mL catechin and gallic acid, the number of clinical isolates of MDR A. baumannii belonging to European clones Ⅰand Ⅱ was generally low with 1.2%-9.7% and 4.3%-8.7% of reduction, respectively[35]. When emodin was administered alone, it did not exhibit considerable anti-A. baumannii activity neither, which is in accordance with the previous report, which showed no activity against Gram-negative bacteria Klebsiella pneumoniae and E. coli in emodin (MIC>500 μg/mL)[36].
The activity of the components in binary combinations against A. baumannii was not observed. The synergy between quercetinepigallocatechin gallate against meticillin-resistant staphylococci has been previously proven[37], as well as between quercetin and gallic acid, p-anisic acid and cinnamic acid against Aeromonas salmonicida, but in all cases, MICs were very high (MIC> 256 μg/mL)[38]. It is a lack of data in the literature on the other combinations tested in this study (quercetin-isoquercitrin, quercetin-catechin, quercetin-emodin, quercetin-gallic acid, isoquercitrin-catechin, isoquercitrin-emodin, isoquercetin-gallic acid, catechin-emodin, catechin-gallic acid, and emodin-gallic acid), and the activities of these combinations against A. baumannii. The antibacterial activity was absent when standard compounds were administered alone and in binary combinations, while the activity was detected in the Rumex extracts by microdilution methods and bioautography assay. It suggests that interactions among these detected and other undetected compounds play a major role in the anti-A. baumannii activity. Further study should include higher concentrations of compounds (516 and 1 024 μg/mL) for a more precise estimation of their activity and interactions (additive or synergistic).
The bioautography showed that only fractions F6 and F8 did not express anti-A. baumannii activity and that active fractions/compounds are the most polar ones. The active fractions F1-F5 contained more polar compounds, flavonoid (quercetin-, kaempherol-, eriodictyol-) glycosides and/or anthraquinone (emodin-) glycosides that have not been examined in the present study as a single agent, indicating their potential anti-A. baumannii effect. In the inactive fractions, dominant compounds of F6 and F8 are gallic acid and catechin which had no considerable anti-A. baumannii activity when they were tested alone. The activity of F7 suggests that either compounds eriodictyol and/or luteolin is potentially responsible for the antibacterial activity and/or rather all the compounds of the fraction F7 act synergistically. This assumption is supported by the previous report of eriodicyol antimicrobial activity with MICs ranging from 250-800 μg/mL against Gram-negative bacteria E. coli, Salmonella enterica subsp. enterica serovar. Typhimurium, and Pseudomonas putida and its synergistic activity in binary combination with hesperetin and naringenin[39]. Similarly, luteolin was identified as a responsible component for antimicrobial activity of Rumex extracts during food preservation[40]. It is possible that, in general, synergism plays a major role in the extract activity when the MIC of the mixture was determined, and the individual compounds had weaker antimicrobial activity. Previously, it was reported that when all the active compounds based on the bioautography were isolated and characterized, they usually showed much lower activity than the expected, indicating the presence of the synergism[41]. Besides the better activity of extracts than single compounds of binary combinations and potential additive/synergistic interactions of the components, the extracts have other beneficial properties, such as antioxidant[42] and anti-inflammatory[43], which additionally can contribute to in vivo anti-A. baumannii effect. Thus, further experiments should focus on in vivo study on the effect of topical application on the healing of wounds infected with A. baumannii.
In this study, the antibacterial activity of the Rumex extracts against MDR A. baumannii isolates from wounds was confirmed, justifying their traditional application in the treatment of wound healing. Therapy options for wound infections due to MDR A. baumannii are limited and both R. sanguineus and R. crispus extracts are potential natural alternatives. The agar-overlay HPTLC-bioautography coupled with LC/DAD/MS analysis of spotted fractions gave more insight into the types of secondary biomolecules contributing to the extract activity. The fractions of R. crispus herb extract, containing flavonol (quercetin and kaempherol) glycosides, flavanon- (eriodictyol) and anthraquinone- (emodin) glycosides, and eriodictyol aglycone, have higher anti-A. baumannii activity. This study supports further in vitro and in vivo studies on these ethnopharmacological remedies as a valuable and promising source of antibacterial compounds against MDR A. baumannii.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, grant OI 172058.
Authors’ contributions
VAS, PK, and JN performed microbiological analyses, while ES, DO, and NMD performed chemical analyses. VAS and PK wrote the manuscript, and all authors discussed and analyzed the data. PK supervised the work.
 Asian Pacific Journal of Tropical Biomedicine2020年4期
Asian Pacific Journal of Tropical Biomedicine2020年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Identification and investigation of Calodium hepaticum in rodents and insectivores from Wuhan section of the Yangtze River in China
- Antibacterial activity of bacillomycin D-like compounds isolated from Bacillus amyloliquefaciens HAB-2 against Burkholderia pseudomallei
- Free and liposome form of gallic acid improves calvarial bone wound healing in Wistar rats
- A novel polyherbal formulation containing thymoquinone attenuates carbon tetrachloride-induced hepatorenal injury in a rat model
