Controlling Self-Assembly of 3D In2O3 Nanostructures for Boosting Photocatalytic Hydrogen Production
Ruijie Chen , Di Li , Zhenyuan Fang , Yuanyong Huang , Bifu Luo , Weidong Shi ,*
1 School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, Jiangsu Province, P. R. China.2 Institute for Energy Research, Jiangsu University, Zhenjiang 212013, Jiangsu Province, P. R. China.
Abstract: Exploring economical and efficient photocatalysts for hydrogen production is of great significance for alleviating the energy and environmental crisis. In this study, 3D In2O3 nanostructures with appropriate self-assembly degrees were obtained using a facile hydrothermal strategy. To study the significance of 3D In2O3 nanostructures with appropriate self-assembly degrees in photocatalytic hydrogen production, the photocatalytic performances of samples were evaluated based on the amount of hydrogen gas release under visible-light irradiation (λ > 400 nm) and simulated solar light illumination. Interestingly, the 3D In2O3-150 nanostructured photocatalyst(hydrothermal temperature was 150 °C, denoted as In2O3-150) exhibited extremely superior photocatalytic hydrogen evolution activity, which may have been caused by their unique structure to improve light reflection and gas evolution. The special structure can enhance light harvesting and induce more carriers to participate in photocatalytic hydrogen production. Despite possessing similar 3D nanostructures, the In2O3-180 photocatalyst exhibited poor photocatalytic activity. This may have been caused by the high self-assembly degree, which can hinder light irradiation and isolate a portion of the water. In addition, the 3D nanostructures could effectively make uniform the carrier migration direction, which is from the interior to the rod end. However, the direction of carrier migration of the In2O3-110 photocatalyst could transfer in various directions, whereas the In2O3-130 photocatalyst could transfer to both ends of the rod. This might cause partial migration to counteract each other. The compact cluster rod-like structure of In2O3-180 might prevent the light from exciting the carrier effectively. Through a photocatalytic recycling test, the 3D In2O3-150 nanostructured photocatalyst exhibited outstanding photochemical stability. This work highlights the importance of controlling the self-assembly degree of 3D In2O3 nanostructures and explores the performances of 3D In2O3 nanostructured photocatalysts in hydrogen production under visible light and simulated solar light.
Key Words: In2O3; 3D nanostructures; Self-assembly; Photocatalytic activity; Hydrogen production
1 Introduction
Hydrogen energy, as a green source converted from solar, is expected to mitigate the energy crisis by virtue of its sustainability and powerful energy density1-5. Since the pioneering of work by Fujishimaet al., great efforts have been devoted to hunt for more economical and efficient photocatalysts for hydrogen production6-10. Up to now, the technology of sunlight-driven splitting water to bubble hydrogen has been widely considered as a promising approach which will extremely accelerate the process of human development11-14. In addition to TiO2, a host of photocatalytic semiconductors rush into the sight of scholars. Yet to date, numerous semiconductors with photocatalytic activity have been studied, such as Cu2O, ZnO,Ta2O5, CdS and ZnIn2S415-23. It is important to note that its representative shortcomings include the marginal response of optical range, the low separation rate of photoexcited electronhole and the poor chemical stability24-28. To overcome these drawbacks, it is highly urgent to develop a state-of-the-art photocatalyst with eminent photocatalytic efficiency and stability.
Presently, indium oxide (In2O3) has spurred considerable attentions on account of its suitable bandgap (2.8 eV), stable photochemical properties and low toxicity29-32. So far, In2O3has been synthesized to one-dimensional (1D) or two-dimensional(2D) structures for application in different fields. Ramos-Ramonet al.manifested that Ga loaded on 1D In2O3nanostructures can ameliorate the photocatalytic degradation performance on methylene blue dye33. Wanget al.demonstrated that 2D ultrathin In2O3nanosheets with uniform mesopores can effectively detect nitric oxide at a quite low detection limit34. In comparison with 1D and 2D, three-dimensional (3D) structures possess unique merits in adsorption, reflection, and carrier separation35,36. Currently, 3D hierarchical In2O3structures,assembled by nanosheets and nanoneedles, have been reported on sensor and degradation37,38. Based on these above reports, 3D hierarchical In2O3structures exhibit high self-assembly degree which may influence the reflection of light and the evolution of gas. However, to the best of our knowledge, the controlling selfassembly degree of 3D In2O3nanostructures for promoting visible-light photocatalytic hydrogen production has not yet been reported.
Herein, we firstly report a simple method to tailor the selfassembly degree of 3D In2O3nanostructures. The photocatalytic activity is evaluated by photocatalytic hydrogen production under visible-light irradiation (λ >400 nm) and simulated solar light. As a consequence, the as-obtained 3D In2O3-150 nanostructured photocatalyst can dramatically enhance photocatalytic performance in comparison with other morphologies. Moreover, the 3D In2O3-150 nanostructured photocatalyst exhibits the optimal photocatalytic performance and superior robustness after four times recycling photocatalytic reactions. The excellent photocatalytic performance of 3D In2O3-150 nanostructured photocatalyst is mainly assign to the efficient dissociation of photogenerated charge carriers. To validate the association between microstructure and carrier migration, a series of characterizations are carried out, and in the light of these results, the possible photocatalytic water splitting mechanism over 3D In2O3nanostructures is proposed. This work will shed light on a facile strategy for controlling self-assembly degree of 3D In2O3nanostructures to optimize the photoelectric properties and photocatalytic activity.
2 Experimental section
2.1 Sample preparation
All of the chemical reagents were analytical grade, and purchased from Guoyao Fine Chemical Co., Shanghai, China,which were used directly without further purification. The In2O3was synthesized exploiting a precursor-calcining tactic. In a typical synthesis, 0.5 mmol InCl3·4.5H2O (≥ 99.0%) and 5 mmol urea (≥ 99.0%) were dispersed in 20 mL deionized water,respectively. Before InCl3·4.5H2O solution dropped into urea solution dropwise, InCl3solution and urea solution need to agitate continuously by vigorous magnetic at room temperature for 5 min. After vigorous magnetic agitating for 15 min, the mixed solution was transferred into a 50 mL Teflon-lined stainless steel autoclave, and heated at a different temperature(110 °C, 130 °C, 150 °C, and 180 °C) for 12 h. After the reaction completed, autoclave temperature cooled down to normal atmospheric temperature. The white precipitates were collected by centrifugation and washed with distilled water and ethanol for three times, then dried at 60 °C for 12 h in a vacuum oven. At last, the precursors were annealed in air at 500 °C for 2 h and the heating rate was set at 5 °C·min-1. To make a distinction, the obtained yellowish solid products are referred to as In2O3-110,In2O3-130, In2O3-150, and In2O3-180, respectively.
2.2 Characterization
All of the crystal phases and crystal structures of the asobtained samples were analyzed by X-ray diffraction (XRD) on a D/MAX-2500 diffractometer (Rigaku, Japan). The structure and morphology of as-prepared samples were examined by scanning electronic microscopy (SEM) on an S-4800 field emission SEM (SEM, Hitachi, Japan). The UV-Vis diffused reflectance spectra (DRS) of the samples were analyzed by an UV-Vis spectrophotometer (UV-2450, Shimadzu, Japan). The functional groups were detected by Fourier transform infrared(FT-IR, Nexus 470, Thermo Electron Corporation). Exploiting a NOVA 3000e (America) nitrogen adsorption instrument to characterize the nitrogen (N2) adsorption and desorption isotherms of the as-obtained samples. The Mott-Schottky plots and electrochemical impedance spectra (EIS) were collected by exploiting a CHI 760E (Chenhua, China) electrochemical workstation at room temperature. The photocurrent measurements were performed by using the CHI 852C electrochemical workstation (Chenhua, China). In detail, the transient photocurrent and electrochemical impedance spectroscopy were tested by the CHI-852C (Chenhua, China)electrochemical workstation. The electrochemical workstation used Pt wire and Ag/AgCl electrode as counter and reference electrode which were immersed in a Na2SO4electrolyte solution.In the preparation of working electrode, 300 mg of samples were dispersed in a 3 mL ethanol solution containing of 0.03 mL of oleic acid and 0.01 g of PVP (polyvinyl pyrrolidone). The Mott-Schottky was analyzed under the same conditions expect potassium ferricyanide and potassium ferrocyanide electrolyte solution.
2.3 Photocatalytic activity test
All The photocatalytic activity of all In2O3photocatalysts for water-splitting hydrogen evolution was appraised in a glass gas circulation system which is connected with gas chromatography.Exploiting a 300 W Xenon arc lamp as a light source to simulate solar condition and exploiting the same lamp equipped with a UV cut-off filter (λ> 400 nm) to simulate visible light condition.Typically, 50 mg of synthetic photocatalysts were used to disperse in 100 mL of experimental aqueous solution. In the photocatalytic reaction system, the 10% (volume fraction)triethanolamine was used as a hole scavenger. After that, a given mass of H2PtCl6·6H2O aqueous solution was suspended for thein situloading of 3% (mass fraction) Pt as a promoter during the process of photocatalytic hydrogen evolution. In order to remove the dissolved oxygen in solution, the whole photocatalytic reaction system was cautiously vacuumed prior to the photocatalytic reaction. Meanwhile, exploiting a circulating condensing system to control the temperature continuously kept at 5 °C. During the process of photocatalytic experiment, the released gas was gathered at a certain time interval.Subsequently, a gas chromatography (GC-7920, China Education Au-Light) was exploited to analyze the evolved gas.
3 Results and discussion
The crystalline phases of all as-obtained samples were identified by XRD analysis. The XRD pattern of In2O3in Fig. 1a shows six main peaks around 21.5°, 30.5°, 35.3°, 45.4°, 50.9°,and 60°. which could be matched well with the (122), (222),(400), (431), (440) and (622) planes of the standard card of In2O3(JCPDS 71-2194)33,34,39. No superfluous peaks were detected at the sensitivity limit of the instrument, suggesting that temperature did not alter the crystalline structure of the In2O3photocatalyst. In the FT-IR spectra (Fig. 1b), three obvious sharp band peaks at 420, 565, and 602 cm-1are earmarked to the phonon vibrations of In―O bonds30,36,40. The evident photon modes at 131, 305, 366, 493, and 627 cm-1were viewed in the Raman spectra (Fig. 1c), which are accord with the vibrational modes of In2O336,41. These results indicate that the yellowish powder synthesized by the precursor-calcination method are In2O3photocatalysts we wanted. The optical absorption of the as-obtained samples was confirmed by UV-Vis diffuse reflectance spectroscopy. As shown in Fig. 1d, the samples absorb light with wavelength around 440 nm, suggesting that all as-prepared photocatalysts have visible light response ability. In other words, the as-prepared photocatalysts can be used as visible-light-driven photocatalytic materials42. Moreover, the band gap energy (Eg) of the as-obtained photocatalytic materials can be counted by the following formula:

Fig. 1 (a) XRD patterns of as-prepared samples; (b) FT-IR, (c) Raman and (d) DRS spectra and Tauc plots of as-obtained samples.
Eg= 1240/λ
whereλrefers to the absorption edge of the photocatalyst. As observed in the Tauc plots, the band gap energy of In2O3samples was measured to be about 2.8 eV, which is similar to the previous reports43,44. Appropriate band gaps underline the In2O3photocatalysts could be a promising visible-light-driven material applied into photocatalytic hydrogen production sphere.
In order to further acquaintance, the morphologies of asobtained photocatalysts In2O3-110, In2O3-130, In2O3-150, and In2O3-180 were analyzed by SEM. As shown in Fig. 2a, it could be obviously observed that the In2O3-110 exhibited irregular nanosheet morphology with large size. The rod structure of In2O3-130 with length around 2 μm and good dispersion distribution, could be observed in Fig. 2b. 3D rod-like of In2O3-150 photocatalysts with lengths about 1 μm and diameters around 200 nm could be seen in Fig. 2c. With the hydrothermal temperature increasing, the In2O3-180, shown in Fig. 2d, were gradually aggregated into bulk-like microstructure.
The Brunauer-Emmett-Teller (BET) surface area of the asobtained In2O3photocatalysts was measured by N2adsorption/desorption isotherms. As shown in Fig. 3, in the range of 0.4-0.8P/P0, all of the isotherms exhibit a hysteresis loop,judged as type-IV on the basic of IUPAC (International Union of Pure and Applied Chemistry) classification. This result indicates the presence of mesopores inside the as-obtained samples42,45.The BET surface area of In2O3photocatalysts was calculated to be 19.03 m2·g-1, 19.54 m2·g-1, 22.55 m2·g-1and 18.01 m2·g-1.In addition, through the inserted pore size distribution curves,the vast majority of pore diameter of In2O3photocatalysts is about 9 nm. The larger BET surface area of as-obtained In2O3-150 with rich mesopores might effectively improve the photocatalytic reaction.

Fig. 2 SEM images of (a) In2O3-110; (b) In2O3-130; (c) In2O3-150 and (d) In2O3-180.
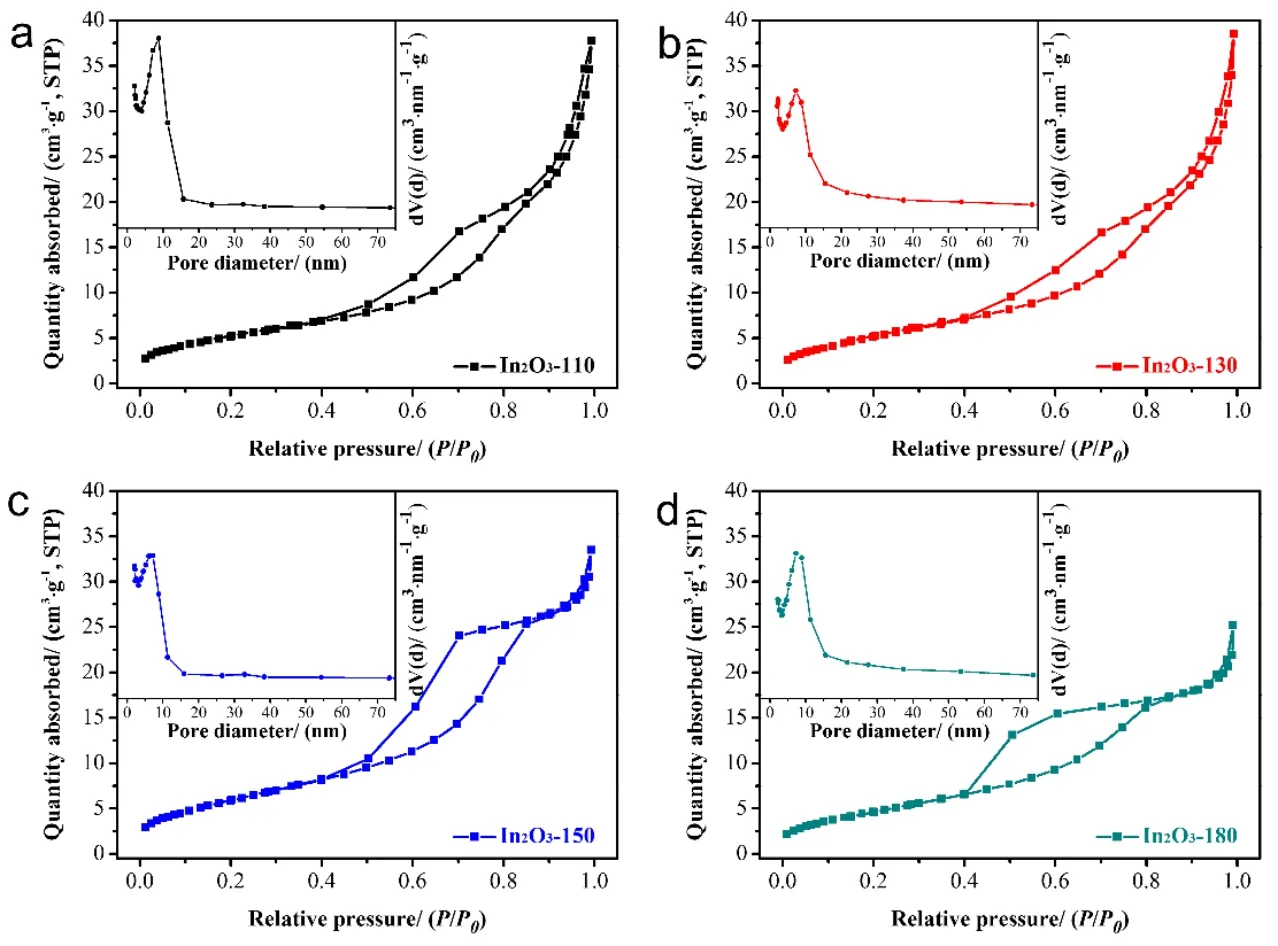
Fig. 3 N2 adsorption/desorption isotherms of as-obtained samples.
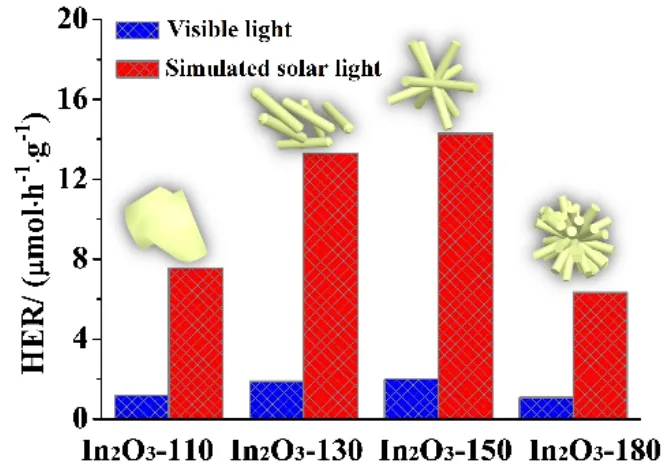
The photocatalytic hydrogen evolution performances of the various In2O3samples were evaluated by water splitting in 10%(volume fraction) triethanolamine under the visible-light irradiation (λ> 400 nm)46,47. As depicted in Fig. 4a, In2O3-180 exerts a relative inferable hydrogen production activity due to its less surface area and poor charge separation ability. Surprisingly,with improving the hydrothermal temperature from 110 °C to 150 °C, the photocatalytic activity of the as-prepared In2O3samples exhibits gradually enhanced hydrogen evolution performance. When the hydrothermal temperature increased to 180 °C, however, the performance of hydrogen evolution had greatly decreased. As shown in Fig. 4b, it can be evidently observed that the hydrogen evolution rates (HER) of as-obtained samples have great changes with the hydrothermal temperature.In comparison with the poorest hydrogen evolution performance of In2O3-180 under visible-light (HER = 1.08 μmol·h-1·g-1), the In2O3-150 sample presented a greatly enhanced HER of 1.99 μmol·h-1·g-1under visible-light. Furthermore, the stability of In2O3-150 was demonstrated by four consecutive runs with each run of 4 h. As exhibited in Fig. 4c, after the recycling stability experiment of In2O3-150 photocatalyst for photocatalytic hydrogen evolution, the photocatalytic activity of In2O3-150 had no noticeable deactivation. Moreover, the XRD analysis was conducted for contrasting the fresh In2O3-150 and the spent In2O3-150, which showed no obvious change between them (Fig.4d). All of above can be demonstrated that In2O3-150 has the good photocatalytic hydrogen production activity and excellent high photochemical stability under visible-light irradiation.

Fig. 4 The photocatalytic activities of as-obtained samples under visible-light irradiation (λ > 400 nm).

Fig. 5 The photocatalytic activities of as-obtained samples under the simulated solar light.
To satisfy the demand for the practical application, simulated solar light was further used as the optical source to evaluate the photocatalytic H2production activities of as-obtained In2O3photocatalysts. The experimental had been carried out under the same above condition except for the optical source with simulated solar light (Fig. 5). The HER of In2O3-150 photocatalyst presented a significant improvement, up to 14.29 μmol·h-1·g-1under the simulated solar light. As a result of recycling stability test, the In2O3photocatalysts has the value of the practical application to address the energy issues.
To investigate the charge transfer capability of as-prepared samples, the electrochemical impedance spectra (EIS) were measured. As illuminated in Fig. 6a, the EIS Nyquist plot of In2O3-180 exhibits the largest semicircles, suggesting its weak photoinduced charge separation and migration efficiency48.Compared to the In2O3-180, the radius of semicircles was diminishing successively following with In2O3-110, In2O3-130,and In2O3-150. The In2O3-150 presents the smallest semicircle,indicating its best charge transfer capability. Fig. 6b showed the photocurrent response of as-synthesis samples with four intermittent switching cycles under the same light illumination.It can be markedly observed that all as-prepared samples owned the photocurrent intensities. Moreover, the In2O3-150 possessed the highest photocurrent intensity, indicating the pretreatment temperature can effectively improve the carrier separation efficiency.
To uncover the essence of the enhanced hydrogen evolution activity of In2O3, the conduction band (CB) positions of the In2O3materials are analyzed by Mott-Schottky plots (Fig. 7).The deduced flat-band potentials of In2O3-110, In2O3-130,In2O3-150, In2O3-180 are determined to be -0.41, -0.57, -0.65 and -0.30 V (vsAg/AgCl), which are converted to be -0.21,-0.37, -0.45 and -0.10 V (vsNHE)49. The In2O3-150 photocatalytic possess the most negative potential, which could provide the highest kinetics of the photocatalytic reaction, thus harvest a good photocatalytic activity.

Fig. 6 (a) EIS of as-obtained samples and (b) transient photocurrent response of as-obtained samples.

Fig. 7 Mott-Schottky plots of as-obtained samples (a) In2O3-110; (b) In2O3-130; (c) In2O3-150 and (d) In2O3-180.
On the basis of the above experiment results, a possible schematic of photocatalytic water splitting with controlling selfassembly degree of 3D In2O3nanostructured photocatalyst under the visible-light irradiation. Firstly, the 3D nanostructures can result in multiple reflections of light50-55. It can enhance light harvesting and induce more carriers to participate in the photocatalytic hydrogen production. In spite of possessing the similar 3D nanostructures, the In2O3-180 photocatalyst exhibits poor photocatalytic activity. It might be caused by the high selfassembly degree which can hinder the light irradiation and isolate a part of water. In addition, the 3D nanostructures could effectively uniform the carrier migration direction which is from the interior to the rod end. However, the direction of carrier migration of In2O3-110 photocatalyst could transfer in various directions and In2O3-130 photocatalyst could transfer to both ends of the rod. It might cause partial migration to counteract each other. The compact cluster rod-like structure of In2O3-180 might prevent the light from exciting the carrier effectively. It is exactly consistent with the charge separation efficiency measured by EIS and photocurrent response. Meanwhile, the 3D In2O3-150 nanostructured photocatalyst possesses the biggest specific surface area and the most negative potential which could provide more surface active sites and higher photocatalytic reaction kinetics. The reason of In2O3-150 with the optimal photocatalytic activity might consist the following elements: 1)the appropriate self-assembly degree; 2) the stronger ability of carriers transfer and separation; 3) the more negative conduction position and 4) the larger specific surface area.
4 Conclusions
In summary, the 3D In2O3nanostructured photocatalyst was successfully synthesized by hydrothermal strategy. By properly controlling the hydrothermal temperature, the photocatalytic activity of 3D In2O3nanostructured photocatalyst displays dramatically promoted. The hydrothermal temperature plays an essential role in controlling the distribution density of nanorods for improvement of the photocatalytic activity. The 3D In2O3nanostructured photocatalyst prepared under 150 °C, with appropriate self-assembly degree, exhibits the highest photocatalytic efficiency under visible-light irradiation and simulated solar light. Moreover, the as-obtained photocatalyst is cost-effective, highly stable, and visible-light driven, which is essential avail for its application.
- 物理化学学报的其它文章
- 魅力光催化剂
- Rod-Shaped Metal Organic Framework Structured PCN-222(Cu)/TiO2 Composites for Efficient Photocatalytic CO2 Reduction
- Cu2+ Modified g-C3N4 Photocatalysts for Visible Light Photocatalytic Properties
- 硫化镉反蛋白石光子晶体制备及光解水制氢
- Fabrication of Z-Scheme Heterojunction of SiC/Pt/Cds Nanorod for Efficient Photocatalytic H2 Evolution
- 光催化甲烷转化研究进展

