Myelin positron emission tomography(PET) imaging in multiple sclerosis
Multiple sclerosis (MS) is a neurodegenerative disease characterized by inflammation and demyelination. Studies are focused on encountering remyelination therapies that can be applied to delaying, or even decreasing, the motor and cognitive disabilities caused by the disease.
The cellular/molecular changes in MS are dynamic, with inflammation, demyelination and remyelination inducing progressive neurodegenerative changes in the central nervous system, as a whole. Moreover, the imaging methods for accompanying the process are, as yet, shortcoming.
Magnetic resonance imaging (MRI) is the usual imaging method applied in MS diagnosis. However, due to limitations in specificity, it is difficult to discriminate between inflammation and demyelination in conventional MRI sequences, due to evident water tissue signaling. The routine MRI sequences comprise T1-weighted, T2-weighted and post-gadolinium T2-weighted images, which, although effective for monitoring disease activities, are inefficient for quantifying myelin content.When applying MRI in the diagnosis and monitoring of disease progression during patient-status evaluation, specific neurological assessment is essential. Furthermore, a specific tool for monitoring progressive myelin changes is also required.
Positron emission tomography (PET) is an imaging technique capable of quantifying processes at the cell/molecule level, depending on the radiotracer used (molecule with affinity for the target labeled with a positron emitter radioisotope). PET imaging has already been used in MS, especially in neuroinflammation studies. Over the past decade, stilbene-derivative molecules have been developed and tested forin vivoevaluation of myelin content (Wang et al., 2009; Wu et al., 2010), thereby facilitating the identification of demyelination processes in MS animal models.
In 2011, the widely used amyloid PET tracer,11C-Pittsburgh Compound B (PIB) was first employed for monitoring myelin in MS patients (Stankoff et al., 2011). Since then, studies have been focused on evaluating amyloid PET tracers forin vivomyelin PET imaging, with both MS animal models (Faria Dde et al., 2014; Carvalho et al., 2019) and MS human patients (Glodzik et al., 2015; Veronese et al., 2015; Grecchi et al., 2017; Auvity et al., 2020). The first successful use of amyloid PET tracers specific for β-amyloid plaque detection in humans, was in 2004 (Klunk et al., 2004). Until 2011, white-matter uptake was considered as non-specific binding, when studies indicated special application to MS as a myelin marker (Stankoff et al., 2011).
Radiotracer with application to various targets can be interpreted as non-specific. In the case of amyloid PET tracers, the theory is that the target site in tracer binding is the β-structure in amyloid plaques, whereas in myelin sheath binding it is this same structure in myelin basic proteins. The analysis of dementia through PET imaging with an amyloid tracer, is completely different from that of myelin content in MS. Whereas in normal white matter uptake, binding occurs with intact myelin fibers,uptake is reduced during demyelination through the partial or total loss of myelin fibers. On the other hand, in the analysis of PET tracers in dementia, the presence of β-amyloid plaques indicates cortical uptake, which does not occur in normal brain tissue. Briefly, in Alzheimer’s disease image analysis is focused on increased uptake in cortical areas of the brain, whereas in the case of MS this is on decreased uptake in white matter.
11C-PIB and11C-MeDAS are the most promising carbon-11 PET tracers for myelin imaging. A study comparing these when applied to animal models (Faria Dde et al., 2014) indicated certain advantages of11C-MeDAS over11C-PIB, one of which by indicating higher uptake in the spinal cord, an affected area in MS. However, to date this tracer has not been used in humans,contrary to11C-PIB which has been for more than a decade,hence making it a possible candidate for use in MS patients.
The short half-life of carbon-11 (20.4 minutes) limits the use of PET tracers labeled with this radioisotope, to sites where there is a cyclotron (particle accelerator where positron emitters are produced), thus making it a complex and expensive radiopharmaceutical structure. As an alternative, amyloid PET tracers are labeled with fluorine-18 (18F), which has a longer half-life (110 minutes), thereby facilitating tracer-shift to centers that have a PET scanner, but not a cyclotron/radiopharmacy structure, an obvious advantage. These tracers (18F-florbetaben,18F-florbetapir,18F-flutemetamol) have already been validated for detecting β-amyloid plaques. A recent comparison with11C-PIB and11C-MeDAS in myelin imaging (Auvity et al.,2020) presented promising results. The disadvantage of these tracers is the number of studies using18F labeled tracers,which are scarcer than carbon-11 labeled, in myelin PET imaging. However, it is probably only a question of time before new studies are available.
Currently, studies using amyloid PET tracers for myelin imaging are limited to validating demyelination detection, reaching,in some cases, proof-of-concept for applying myelin PET imaging to monitoring myelin-change progression. Consequently,higher efficiency in technique validation to arrive at proof-oftool propriety becomes a prime necessity.
Studies of imaging-tool validation and proof-of-concept always start with well-designed animal-model research. In MS,although several animal models are available, none mimic the human disease completely. Thus, choice of the appropriate model is based on research questions: is there progressive demyelination and remyelination; is the demyelinated area in the brain, focal or widespread; is there an inflammatory compound together with myelin changes; is the animal a model of relapsing-remitting (RRMS) or progressive (PMS) MS.
Figure 1 presents11C-PIB PET imaging in various species.Starting from left to right: 1) the brain of a lysolecithin inoculated rat with spot-focal demyelination at the points of stereotaxic injection (corpus callosum and striatum), where decreased tracer uptake is apparent; 2) marmoset brain with experimental autoimmune encephalitis, presenting a wide-spread demyelinated area after peripheral myelin-antigen immunization; 3) the brain of an RRMS patient with a clear focal-demyelination area. These are clear examples of the possibilities available when studying myelin PET imaging in MS.
RRMS is characterized by periods of relapse and recovery,and PMS by progressive disablement. The identification of MS phenotypes is a difficult task, restricted to experts in the field of neuroimmune pathology.
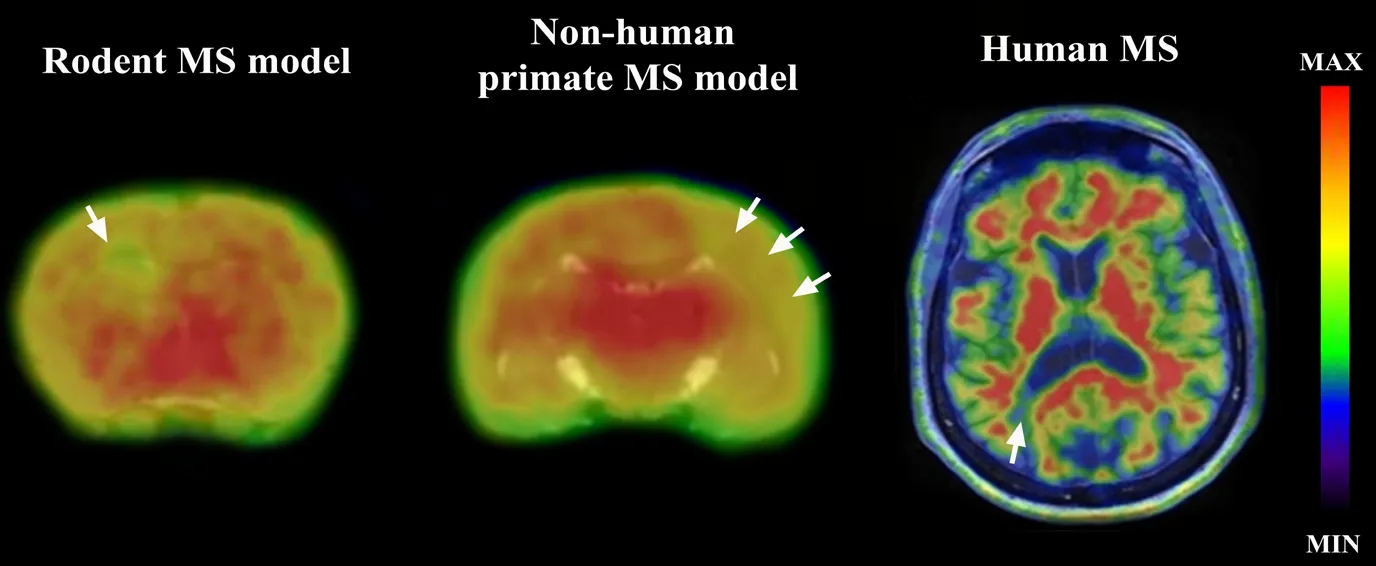
Figure 1 11C-PIB PET imaging fused to T1 MRI in different species showing demyelinated regions in the brain.
Two perspective lines of research in the field of myelin imaging in MS are progressive monitoring of myelin changes and identification of MS phenotypes.
Progressive monitoring is essential for evaluating the course of the disease and treatment response. As treatment could be pharmacological, non-pharmacological or both, anin vivoimaging tool that would facilitate evaluating sequential changes caused by treatment efficacy or otherwise, becomes a prime necessity. Disease progression monitoring is normally with MRI,where focus is on the number of lesions caused by either demyelination or inflammation. A specific tool for myelin imaging with the capacity to evaluate remyelination occurring either spontaneously in central nervous system or through novel remyelination therapy, is crucial.
The second line of research involves identifying MS phenotypes. Definition of the most appropriate MS type is essential for managing clinical decisions and determining the adequate treatment. Based on MRI and clinical signs, patients are classified as RRMS or progressive. However, as in some cases this can be complicated, this is the crucial moment when a specific imaging tool would be of great help in arriving at a plausible decision.
Briefly, myelin PET imaging in MS is a relatively new field of study, with promising results, and deserving of further study.Worthy of note: though studies of myelin imaging are focused on MS, other neurodegenerative diseases can also find benefit from the findings, since white matter changes are also known to occur elsewhere. This is also the case of other studies of brain development, where white matter fibers can be appraised during aging.
Daniele de Paula Faria*
Laboratory of Nuclear Medicine (LIM-43), Departamento de
Radiologia e Oncologia, Faculdade de Medicina, Universidade de
Sao Paulo, Sao Paulo, SP, Brazil
*Correspondence to:Daniele de Paula Faria, PhD,daniele.faria@hc.fm.usp.br.
orcid:0000-0002-1766-2786 (Daniele de Paula Faria)
Received:December 20, 2019
Peer review started:December 28, 2019
Accepted:February 12, 2020
Published online:April 3, 2020
doi:10.4103/1673-5374.280311
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Masaaki Hori, Juntendo University School of Medicine,Japan.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Transcriptional regulation of adult neural stem/progenitor cells: tales from the subventricular zone
- Targeting molecular pathways for the treatment of inherited retinal degeneration
- Green tea catechins inhibit microglial activation which prevents the development of neurological disorders
- Reversibility of visual field defects through induction of brain plasticity: vision restoration, recovery and rehabilitation using alternating current stimulation
- Role of activin receptor-like kinase 1 in vascular development and cerebrovascular diseases
- Effects of durotomy versus myelotomy in the repair of spinal cord injury

