Reversibility of visual field defects through induction of brain plasticity: vision restoration, recovery and rehabilitation using alternating current stimulation
Bernhard A. Sabel , Ying Gao Andrea Antal
1 Institute of Medical Psychology, Medical Faculty, Otto-von-Guericke University of Magdeburg, Magdeburg, Germany
2 Center for Behavioral and Brain Sciences (CBBS), Magdeburg, Germany
3 Sabel Vision Restoration Center, Magdeburg, Germany
4 Department of Clinical Neurophysiology, University Medical Center Göttingen, Göttingen, Germany
Abstract For decades visual field defects were considered irreversible because it was thought that in the visual system the regeneration potential of the neuronal tissues is low. Nevertheless, there is always some potential for partial recovery of the visual field defect that can be achieved through induction of neuroplasticity.Neuroplasticity refers to the ability of the brain to change its own functional architecture by modulating synaptic efficacy. It is maintained throughout life and just as neurological rehabilitation can improve motor coordination, visual field defects in glaucoma, diabetic retinopathy or optic neuropathy can be improved by inducing neuroplasticity. In ophthalmology many new treatment paradigms have been tested that can induce neuroplastic changes, including non-invasive alternating current stimulation. Treatment with alternating current stimulation (e.g., 30 minutes, daily for 10 days using transorbital electrodes and ~10 Hz) activates the entire retina and parts of the brain. Electroencephalography and functional magnetic resonance imaging studies revealed local activation of the visual cortex, global reorganization of functional brain networks, and enhanced blood flow, which together activate neurons and their networks. The future of low vision is optimistic because vision loss is indeed, partially reversible.
Key Words: alternating current stimulation; glaucoma; low vision; optic nerve; rehabilitation; recovery; stress;vision; vision restoration therapy; visual field
Introduction
Visual field defects (VFDs) due to damage of the retina, optic nerve, or brain is a growing problem in our aging society.In Germany alone, about 10,000 people are diagnosed with visual impairment annually, with an estimated prevalence of 1.2 million (Bertram, 2005), possibly even more. The overwhelming majority of patients are not completely (“blackblind”) but partially blind (partially sighted). For many decades VFDs are considered to be irreversible, without a chance of recovery. Recent researches and clinical findings,however, warrant a more optimistic view that partial recovery of visual functions is possible. Among the proposed mechanisms of recovery is the activation of residual vision(Sabel et al., 2011) and the modulation of the brain’s functional connectivity networks (Bola et al., 2014), which requires healthy vascular functioning (Sabel et al., 2018). We suggest that vision recovery is more related to the improved functioning of the eye-brain-vascular system triade, i.e. not just to the production of just one specific molecule or cell type, but a more “holistic” response where the brain and the vascular systems play a central role (Sabel et al., 2018).
In any event, uncovering the mechanisms of action of vision recovery and repair will pave the way for a better understanding of neuroplasticity in the visual sciences. Fundamentally, here are the possible mechanisms currently under study: (i) the first approach is the replacement (augmentation) of the lost nervous tissue itself (in particular the retina or optic nerve) by means of axonal regeneration, stem cell transplantation or retina chip implants (“bionic eye”) (Auvray, 2019; Huang et al., 2019; Vaucher et al., 2019; Yin et al.,2019). (ii) The second approach aims to make more efficient use of the remaining brain tissue by “reprogramming” or“reorganizing” activities of nerve cells and their connections with the goal of activating residual vision. If the retina and/or the optic nerve are completely damaged (e.g., complete loss of the retina) then recovery is, of course, impossible(Figure 1). However, if the optic nerve and brain tissues are only partially damaged, with at least a small amount of residual vision, almost all patients can achieve some degree of improvement. The aim of this review is to summarize recent knowledge related to the second approach, concentrating on the application of alternating current stimulation (ACS).Electronic search strategy included PubMed until November 2019 covering the English language literature, original papers, using the keywords “alternating current stimulation”and “vision” (30 of 48 papers), “alternating current stimulation” and “visual disorders” (14 of 21 papers), “transorbital alternating current stimulation” (10 of 17 papers).
Vision and the Brain
The overriding importance of the brain in normal vision is easily understood when one compares the weight of the retina (~ 1 g) with the estimated total weight of visual system tissue in the brain (~ 300 g). Indeed, more than half of the human cerebral cortex is involved in visual processing. It analyses and interprets the visual information coming from the retina and travelling to the cortex, where it is translated to conscious visual experience. Then, the question arises:what functions these 300 g tissue have when the retina or optic nerve are partially damaged (as in glaucoma or optic neuropathy). Whether a patient can see something objectively well (e.g., visual responses measured with perimetry) is, in fact, not only a matter of retinal “sensory” stimulus processing but also a result of the interpretation of the visual information by the brain. Therefore, when we talk about vision loss such as non-correctable acuity loss and foggy vision, we have to consider that it might not be just an “eye problem”.
Perfect synchronization of neuronal oscillations in the brain is a prerequisite for optimal sight. Spatial networking and temporal organization (“coherence”) are key elements to coordinate the neuronal activities in the brain (Uhlhaas and Singer, 2006). In other words, the better the coherence of neural processing in the brain, the better is the visual performance. This coherence can be measured by functional magnetic resonance imaging (fMRI) or electroencephalography(EEG). The fundamental principle of this synchronization can be visualized with hypothetical “brainwaves” (Figure 2).Synchronization manifests a functional signal, while in the case of desynchronization, because of the conditions have suboptimal timing, there is no signal or low signal. In a similar vein, normal vision is the product of optimized coordination and synchronization between different brain regions,both temporally and spatially. Whether this synchronization functions well or not, will make a difference between optimal or low vision.
Brain Desynchronization in Visual Field Defects
Depending on the location and the size of the lesion, visual system damage will lead to smaller or larger VFDs. Because there is a strong interconnectivity of 100 billion of nerve cells in the human brain, even localized lesions of the peripheral visual system (optic nerve) can affect distant brain areas on a global level (Bola et al., 2013), a principle long known as “diaschisis” (Carrera and Tononi, 2014). This complex interaction during a simple visual task — pushing the button when the dot flashes during visual field test require interactions of different neuronal circuits. During the task, the patient is instructed to press a button whenever he or she detects a target stimulus. To complete this very simple task, the brain needs to ascertain optimal interactions of different sub-functions: sufficient sensory pre-processing by the retina, undisturbed transmission of visual impulses via the optic nerve to the brain’s visual cortex, a sufficient level of vigilance (“Sufficiently awake”) and motivation (“I want to press the button, to perform the test”), attention to the task (“Always press the button when the light appears,otherwise do not”), retrieving visual memory (“What does a dot look like?”) and the interpretation (“Is it a dot?”),while the eyes remain stable by fixating a fixation spot in the center of the screen (“Do not move your eyes”). Finally, the coordinated execution of a motor response (“press the button”) is required, including the feeling that the button was indeed pushed. Each of these neuropsychological sub-functions need their own neural circuits, which, in the complex world of the brain, requires a coordination of all circuits in time and space in a precise and synchronized manner within the short reaction time period of 200-500 ms (Bola and Sabel, 2015). However, if the brain network is desynchronized, e.g., by distraction, fatigue, or by tissue damage (e.g.,glaucoma or optic neuropathy), then the retinal signal is not(optimally) amplified (or recognized) by the brain. Especially in the areas of relative visual defects, the disturbance of this synchronization is particularly important.
Residual Vision
Many patients have partial damages with well-defined “areas of residual vision”, also known as “relative defects” (Figure 1A).These areas of residual vision are characterized by reduced vision and they are not blind (Kasten et al., 1998b). It was proposed that residual vision is the basis of the reversibility of VFDs (Sabel et al., 2011). In perimetric tests it is displayed as gray regions of the visual field maps which are distinctly different from the intact areas (white) and visual field sectors of absolute defects (black). These grey regions are not the result of perimetric measurement errors, but they rather represent partially surviving neuronal assemblies or photoreceptors. The typical forms of residual vision are the classic “relative defects”with increased perception threshold, reduced reaction speed and/or detection accuracy (Herrmann et al., 2013). Another form of residual vision is “blindsight,” in which react correctly to stimuli that appear in the blind hemifield (e.g. in hemianopia or optic nerve damage), without patient being aware of having seen anything (Pöppel et al., 1973). The cellular basis of this partial vision is thought to be partial survival of normally functioning healthy neurons as well as unhealthy (hypometabolic) neurons that managed to survive (“silent survivors”;Figure 1C) (Henrich-Noack et al., 2013a, b, 2017).
Residual vision can be quite variable in repeated sessions,because, in contrast to the healthy visual system, they are very susceptible to environmental and physiological influences, such as the circadian rhythm, daily fluctuations in attention, blood oxygen and glucose levels, blood pressure and vascular regulation, intracranial pressure and perfusion changes, and external influences such as stress, changes of temperature and atmospheric pressure (“weather sensitivity”) (Sabel et al., 2018). These intervening variables can induce fluctuations in the perimetry test, because the partially damaged areas work at their metabolic limit. Furthermore,residual vision is sometimes quite subtle and often cannot be captured with standard perimetry. Therefore, in this case brighter or larger (= more easily recognizable) stimuli should be used for testing. Figure 3 shows a case of a patient who failed to respond to any of the many stimuli presented in standard perimetry, but when an “easier” (larger) stimulus was used, the patient´s residual vision became apparent.This can be explained by the greater number of neurons or their increased firing rate which is provoked by the larger (or brighter) stimulus.
Brain’s Role in Residual Vision
As mentioned above, the brain, can be viewed as a kind of“amplifier” that processes neuronal impulses of the retina that creates conscious (or unconscious) visual experience.This brain-“amplifier” is particularly important for partial blindness, because here the optimal processing of reduced residual vision is needed. Just like a night vision device,where the light source is reduced, the brain is challenged to perceive despite lowered level of neural signals as is the case,for example, after optic nerve damage, when the amount of sensory input is decreased. The “amplification effect” can theoretically be achieved by the entrainment of functional oscillatory neuronal networks as decribed above (Polat et al.,2004). When input from the periphery is reduced, though the brain may still receive subtle visual impulses, the sum of this activity is insufficient for normal conscious vision.Therefore, patients with VFDs have two problems: on one hand, less information is reaching the brain, and, on the other hand, due to an impairment in the network synchronization, the brain cannot process the visual impulses properly. Nevertheless, the brain network synchronization can be modified, normalized and maintained for extended periods of time by external means, e.g., by inducing plasticity in the normal visual system (“perceptual learning”) (Chung et al.,2004) or after visual system damage (Freund et al., 1997;Pascual-Leone et al., 2005).
Neuroplasticity
Very broadly speaking, plasticity is the ability of the brain to adapt and adjust to changes. Such brain adaptations happen daily in the normal brain, when we learn something new or memorize new experiences and when we adapt to neuronal damage (Freund et al., 1997; Pascual-Leone et al., 2005). The biological basis of plasticity are thought to be subtle changes at the synaptic level, which alter (strengthen or weaken)neural circuits: on one hand, synapses are constantly changing their strength, which, in turn, alters local levels of neurotransmitters and neurotrophic factors (Zuccato and Cattaneo, 2009). On the other hand, unused synapses may weaken or withdraw their contact with post-synaptic neurons. According to the slogan “use it or lose it”, in the long term only those brain networks that are in regular use function optimally and are maintained for long periods of time. While the structure of the human brain is rather stable in adulthood on a macro-level, on a micro-level it is not rigid but constantly adjusting to bodily and environmental demands. Functional network changes, depending on the current needs, can occur within milliseconds (Bola and Sabel, 2015).
Many researchers, including the Nobel laureate Torsten Wiesel (Rockefeller University, New York) (Gilbert and Wiesel, 1992) have demonstrated plasticity in the visual system (Sabel et al., 2011), giving new perspectives for ophthalmological interventions and new hope to patients that suffer vision impairments wrongly claimed to be irreversible.Groundbreaking results about the visual system’s plasticity was already published in the 1980s by Ulf Eysel (University of Bochum) (Eysel et al., 1980) who studied adult cats to determine their visual receptive fields in the visual cortex.Following a binocular retinal lesion and after a certain time of recovery, neurons which had lost their retinotopic organization and functions could react to visual stimuli again.However, they reacted now to other areas of the visual field to which they had never reacted to before. This was a first proof that receptive fields can spontaneously reorganize.Most recently, our own research showed that even function brain networks far beyond the lesion site, could be reorganized (Bola et al., 2014).
Numerous independent researchers have extensively studied and confirmed the plasticity of the visual system (Sabel et al., 2011), e.g., Gilbert and Wiesel (1992). They examined retinal and brain lesions in adult cats and monkeys and reported a massive reorganization of receptive fields. Other evidence of plasticity came from rat studies: after lesions of the optic nerve an amazing spontaneous recovery was observed shortly after the damage, although the number of neurons was declining (Sautter and Sabel, 1993). Mild, moderate or severe crush of the optic nerve produced partial or complete loss of the ability to perform a brightness discrimination task. At postoperative day 14, the number of morphologically ‘intact’ retinal ganglion cells (RGCs) declined even further to 11% in the mild injury group, despite nearly complete spontaneous behavioral recovery. This can be explained by the increased excitability of RGCs (Prilloff et al., 2007).Spontaneous recovery after the damage of visual system is also often observed by experienced clinicians who witness sometimes remarkable (and unexplained) spontaneous recovery in acute cases of vision loss in the first few weeks after stroke or traumatic optic neuropathy (Zhang et al., 2006). In a retrospective study of 254 patients spontaneous improvement of homonymous hemianopia was detected in ~50% of patients which was first seen within 1 month after the injury.In most cases, the improvement occurred within the first three months from injury.
Activation of Residual Vision with Neuroplasticity
After partial damage of the visual system neurons in areas of residual vision there are not only dead or surviving, normally functioning neurons, there are also cells, which are inactive or “silent” due to hypometabolism (Sabel et al., 2018)(Figure 1). Here the combination of the number of visual signals, their firing strength and their synchronization in cell assemblies are not sufficient to lead to conscious vision. The signal is subthreshold, too weak or surrounded by a noisy neuronal environment. To activate the potentials of residual vision, two mechanisms could be considered: reducing the hypometabolic state and improving the coherence of retinal signal transmission, greater number of active neurons and a better synchronization state might lead to better function(Pascual-Leone et al., 2005; Sabel et al., 2011). It is the greater strength and a better “timing” of these neural, electrical impulses (action potentials) from the retina to the brain,which neurophysiologically explains the improvement of vision, i.e. the partial reversibility of VFDs (Figure 2).

Figure 1 Mechanisms of vision recovery.

Figure 2 Schematic of neuronal synchronization.Assuming that retinal signals are normal and identical, it depends on the synchronization state of the brain if the sum of all neuronal circuits(retina + brain) adds up to a signal that is strong enough to produce a sensory (visual) perception (visual perceptual threshold is represented with the dotted line).
How can residual vision be activated or strengthened?Here, two mechanisms are suggested: (i) normalization of vascular dysregulation with improved blood flow in the microcapillaries of the eye and brain, and (ii) the synchronization of brain functional connectivity networks. In the normal state the brain is not only well prepared to select, amplify,evaluate and save visual information, but also the functional networks of the brain are constantly adapting to new conditions, e.g., to bodily (internal) and to environmental (external) changes. Through vision training or neuromodulation by means of weak ACS the damaged visual functions can partially be strengthened - even when there is only a limited amount of neuronal regeneration.
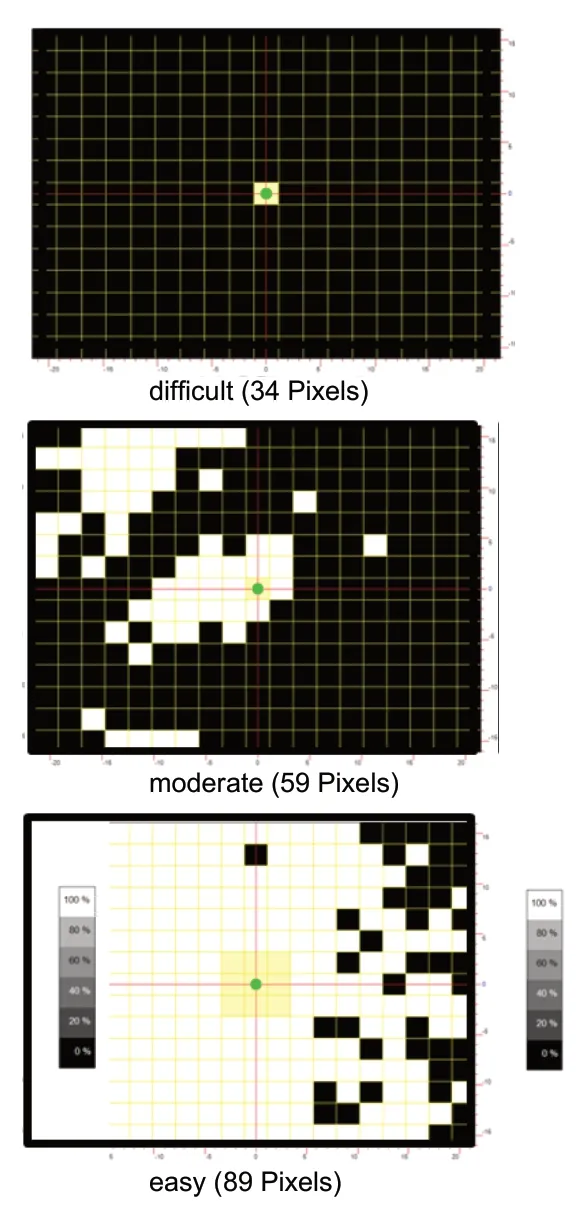
Figure 3 Residual vision detection.
Treatment of Vision Loss with Non-Invasive Alternating Current Stimulation
During the last decade, several methods were developed and clinically tested to improve vision in partially blind patients.This includes vision training exercises (Zihl and Cramon,1979; Kasten et al., 1998a; Poggel et al., 2004; Sahraie et al.,2006; Mueller et al., 2007; Jung et al., 2008; Romano et al.,2008; Otto and Michelson, 2014), retinal implants (Bloch et al., 2019), and non-invasive brain current stimulation of the eye and brain (Shandurina and Panin, 1990; Fujikado et al.,2006; Gall et al., 2011, 2013; Schatz et al., 2011; Anastassiou et al., 2013). Here, either direct current or ACS were used to enhance brain excitability or resynchronize neuronal oscillations by means of defined electric pulses. One of them, ACS,uses weak current pulses, which are delivered through electrodes on the forehead for 20-40 minutes daily for a period of 10 days externally (Figure 4) (see video: www. youtube.com/watch?v=g8p3mWsLvAI). Here, the current flowing between the electrodes, reaches the eye, where it stimulates RGCs to fire at predetermined frequencies. Current flows also through the skull to frontal cortex and through the optic foramen along the base of the brain (Gall et al., 2016), where it stimulates several areas of the midbrain, brainstem and cerebellum (but not visual cortex!).
The restoration of retinal structure and function following trancorneal electrical stimulation using alternating currents(TcES) has been extensively investigated in animal models.In a rat model of retinitis pigmentosa, whole-eye electrical stimulation (4 μA at 5 Hz) significantly improved retinal function as measured by electroretinogram and optomotor response (Hanif et al., 2016). Preservation of photoreceptors and retinal function in a rat model of retinitis pigmentosa was also observed (Morimoto et al., 2007) after TcES treatment (50-100 μA at 20 Hz). ACS has been shown to exert neuroprotective and pro-regenerative effects in rodent models of optic neuropathies, including acute nerve injury(Morimoto et al., 2005; Tagami et al., 2009; Henrich-Noack et al., 2013a, b, 2017), ischemia (Inomata et al., 2007) and glaucoma (Fu et al., 2018). Fu et al. (2018) showed that the stimulation applied immediately after intraocular pressure(IOP) promoted RGC survival following acute ocular hypertensive injury in an acute glaucoma model in gerbils. Other effects of ACS are related to the vascular system. For example, vascular changes were noted in the cat retina during and after stimulation (Mihashi et al., 2011; Morimoto et al.,2014).
In human subjects one of the effects of the ACS treatment is probably the resynchronization of brain functional connectivity networks (Bola et al., 2014), which were disorganized after optic nerve damage (Bola et al., 2013, 2015). Here, the function of subcortical areas (e.g., pulvinar, mesencephalic structures) is rarely investigated. The other presumed mechanisms are enhanced blood flow in the eye and brain, which was observed in normal subjects and in patients with retinal damage (Fujikado et al., 2006) or stroke (Sabel et al., 2019).The clinical value of ACS was tested in several controlled trials in which patients with glaucoma, optic nerve damage or retinitis pigmentosa received ACS which was either applied to the eyes or to the forehead (Gall et al., 2011, 2013; Schatz et al., 2011; Anastassiou et al., 2013). In an open-label, clinical observational study 446 patients affected by optic nerve damage due to traumatic brain injury, inflammation, brain tumor or vascular lesions were treated with transorbital ACS(Fedorov et al., 2011). The results after ten days of stimulations (25-40 minutes each, frequencies < 20 Hz, current <1000 μA) showed long-lasting improvements in acuity and VF size. VF enlargement was present in about 40% of the patients. A second ten-day course conducted six months later in a subset of 62 patients resulted in additional significant improvements of visual acuity (VA).
In subsequent studies, a total of about 150 patients with optic neuropathy were tested in prospective, double-blind,randomized, placebo-controlled trials (Gall et al., 2011,2016; Sabel et al., 2011). In a clinical trial (Sabel et al., 2011)patients with chronic partial optic nerve lesions were treated with ACS or placebo-stimulation. ACS was delivered for 40 minutes on 10 days, applied by four stimulation electrodes placed at or near the eyeball with eyes closed. Stimulation frequencies were between the individual alpha-range and the flicker fusion frequency. ACS, but not placebo, led to significant improvement of a VF detection deficit by 69%.ACS has also significantly improved temporal processing of visual stimuli and VA. These changes were associated with alpha-band changes in the EEG power spectra. Visual improvements were stable for at least 2 months (Figure 5).
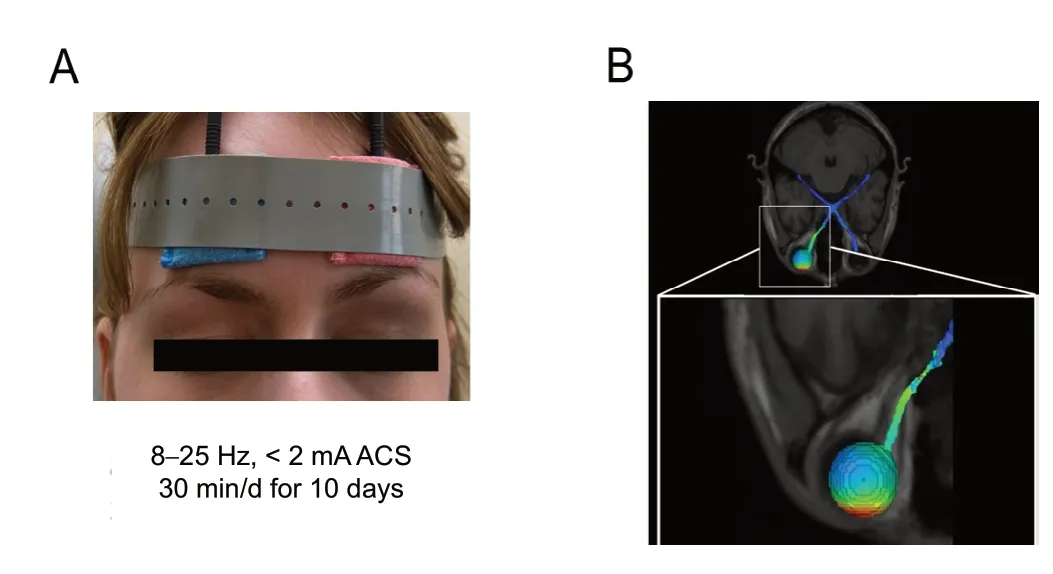
Figure 4 Schematic of alternating current stimulation (ACS).

Figure 5 Visual fields of two single patients as determined by perimetry (central 30 degrees of visual angle).
In another study (Gall et al., 2011), patients with chronic visual field impairments after optic nerve damage were randomly assigned to ACS or sham stimulation group. Current trains and current thresholds were automatically adjusted by the stimulation device to the individual phosphene thresholds for a 10-day treatment period at a frequency of 5-30 Hz.Detection ability increase in the defective visual field was significantly larger after ACS than after sham stimulation.Improvements in NEI-VFQ subscale ‘general vision’ were observed in both groups but were larger in the ACS group,than in the sham group.
The most comprehensive methodological study (multicenter, prospective, randomized, double-blind, sham-controlled trial) with the largest sample size (91 subjects) was carried out by Gall et al. (2016). In this study subjects suffering from various pathologies (glaucoma, AION, other causes of optic atrophy) underwent ten sessions of ACS (up to 40 minutes, frequency < 25 Hz, current < 1000 μA) which led to significant visual field improvements compared to that measured in the sham stimulation group. The results showed that the patients treated with transorbital ACS had a mean improvement of the visual fields of 24.0% above baseline,which was significantly greater than after sham-stimulation(2.5%). This improvement persisted for at least 2 months.About 60% of the patients treated with ACS were subjectively satisfied with the treatment and 30% were aware of vision improvements. The patients reported improved mobility and spatial orientation, an enlarged visual field, increased color saturation, less glare, and a clearing up of unexplained visual “fog” (Gall et al., 2016). Adverse effects were minimal;some patients experienced tingling under the stimulation electrode and very rarely, short-term blood pressure fluctuations or headaches. Serious adverse effects (SAEs) were not observed at any time.
However, it should be noted that recovery or restoration of vision is rather variable between patients, ranging from “no change” to “massive” improvements. We believe that clinical outcome depends on several factors, such as topography of the visual damage, the relative size of the residual vision areas, and the individual level of stress and tension (Sabel et al.,2018). Interestingly, especially patients with high stress levels show less improvement during and after the therapy, which probably relates to the high level of stress hormones that can induce vascular dysregulation in the eye and brain (Sabel et al., 2018). To counteract this negative impact of stress, ACS therapy should be supplemented with holistic methods that include psychological counselling and relaxation exercises(Sabel et al., 2018) such as meditation (Dada et al., 2018).
Outcome depends less on other factors such as the duration of the disease or the age of the patient or the cause of vision loss. Note, however, when the brain itself is damaged,as in stroke or traumatic brain injury, the recovery potential is lower. Similar to earlier observations of studies with vision restoration training (Kasten et al., 1998; Poggel et al., 2004;Sahraie et al., 2006; Mueller et al., 2007; Jung et al., 2008;Romano et al., 2008), the stability of improved vision varies between individuals, ranging from weeks to years. In any event, the presence of residual vision is essential; complete blindness has no chance for any improvement. In the clinical setting it is important to give the patient a realistic prognosis and to explain to him/her that the complete remedy of the visual loss is not to be expected and that some degree of vision loss will remain, despite noticeable improvements of vision.
The ACS treatment approach was also tested in other disorders such as macular degeneration (Anastassiou et al.,2013) and retinitis pigmentosa (Schatz et al., 2011), (but they showed only rather modest success), and some older studies in optic neuropathy that were carried out in Germany (Erb,1882; Mann, 1904), Russia (Shandurina and Panin, 1990)and Japan (Fujikado et al., 2006).
Mechanisms of Visual Alternating CurrentTherapy
Understanding the dynamics of external current flow in the retina is very complex. It was found that rectangular waveforms activate both the bipolar cells and RGCs (Freeman et al., 2010). RGCs are more susceptible to short pulses(~150 ms), whereas the neurons of the inner retina respond more strongly to longer pulses (Freeman et al., 2010). In a rat model of retinitis pigmentosa TcES treatment induced stronger protection of the RGCs than the photoreceptors.Therefore, it was suggested that post-receptor neurons and the inner retina may be particularly responsive to whole-eye electrical stimulation (Hanif et al., 2016).
In the brain, similar to the normal learning process, transcranial stimulation induces plasticity by way of synchronous repetitive neuronal activation; the brain can also “learn” new synchronization patterns, which can be maintained even after the end of stimulation (Bola et al., 2014). This is a kind of“after-effect”, which also can be observed in normal subjects(Zaehle et al., 2010; Herrmann et al., 2013). The consequence is a physiological resynchronization of brain functional connectivity networks, which strengthens residual vision. If the network activity in the brain is well synchronized, then previous subthreshold impulses from the partially damaged visual fields can be amplified to raise above the threshold of perception. In this way, brain synchronization can improve residual vision, even if the retinal impulses are still weak (due to cell loss or hypometabolism).
fMRI results have shown that the synchronization of neural circuits can change the local and global activations in the brain. Probably already during stimulation and thereafter ACS increases neuronal activation in the visual cortex in normal subjects (Vosskuhl et al., 2016). After 10-day ACS treatment, a patient with hemianopia exhibited visual cortex reorganization, evidenced by significant blood-oxygen level dependent activity changes (Sabel et al., 2019). At first sight,this indicates greater local activation. However, it could also be an indication of a global activation of functional brain networks as it was measured by EEG in other studies. Here,following ACS, an increased alpha synchronization of functional networks was found between the visual and the frontal cortex (Bola et al., 2014). Therefore, ACS stimulation not only leads to local changes in the brain neural activity and blood flow, but also has a very important remote effect in areas distant from the lesion (Bola et al., 2014).
Conclusion
By using modern means of non-invasive ACS or other means, the prognosis is optimistic that partial recovery of vision can be achieved in patients with visual system damage.Even if the structural damage itself may remain irreversible and permanent and cannot be regenerated, the functional consequences of VFDs are - at least in part - reversible through induction of brain plasticity and resynchronization of the brain network (Sabel et al., 2011). Clinicians should give patients a more optimistic prognosis that some recovery is possible. They should not communicate that blindness is irreversible, so not to increase stress and thus accelerate the progression of vision impairment due to stress-induced vascular dysregulation. Vision loss is, at least in part, reversible.
Acknowledgments:We thank Sylvia Prilloff for her help with regard to the preparation of the manuscript.
Author contributions:All authors contributed to the design and implementation of the search strategy, to the analysis of the results and to the writing of the manuscript. All authors approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Transcriptional regulation of adult neural stem/progenitor cells: tales from the subventricular zone
- Targeting molecular pathways for the treatment of inherited retinal degeneration
- Green tea catechins inhibit microglial activation which prevents the development of neurological disorders
- Role of activin receptor-like kinase 1 in vascular development and cerebrovascular diseases
- Effects of durotomy versus myelotomy in the repair of spinal cord injury
- Spinal genesis of Mayer waves

