Spinal genesis of Mayer waves
George Zaki Ghali , Michael George Zaki Ghali , Emil Zaki Ghali
1 United States Environmental Protection Agency, Arlington, VA, USA
2 Department of Toxicology, Purdue University, West Lafayette, IN, USA
3 Department of Neurological Surgery, Houston Methodist Hospital, Houston, TX, USA
4 Department of Neurobiology and Anatomy, Drexel University College of Medicine, Philadelphia, PA, USA
5 Department of Medicine, Inova Alexandria Hospital, Alexandria, VA, USA
6 Department of Cardiothoracic Surgery, El Gomhoureya General Hospital, Alexandria, Egypt
Abstract Variability in cardiovascular spectra was first described by Stephan Hales in 1733. Traube and Hering initially noted respirophasic variation of the arterial pressure waveform in 1865 and Sigmund Mayer noted a lower frequency oscillation of the same in anesthetized rabbits in 1876. Very low frequency oscillations were noted by Barcroft and Nisimaru in 1932, likely representing vasogenic autorhythmicity. While the origins of Traube Hering and very low frequency oscillatory variability in cardiovascular spectra are well described, genesis mechanisms and functional significance of Mayer waves remain in controversy. Various theories have posited baroreflex and central supraspinal mechanisms for genesis of Mayer waves. Several studies have demonstrated the persistence of Mayer waves following high cervical transection, indicating a spinal capacity for genesis of these oscillations. We suggest a general tendency for central sympathetic neurons to oscillate at the Mayer wave frequency, the presence of multiple Mayer wave oscillators throughout the brainstem and spinal cord, and possible contemporaneous genesis by baroreflex and vasomotor mechanisms.
Key Words: Mayer waves; genesis; origins; central; sympathogenesis; spinal cord; cervical; transection
Introduction
Cardiovascular variability spectra of central neuronal and efferent nerve discharge include classic fast and slow sympathetic rhythms (Zhong et al., 1991, 1992, 1993a, b; Barman and Gebber, 1992; Barman et al., 1994, 1997, 2000, 2005;Ghali, 2017a, b), as well as lower frequency spectral bands classified as high, low, and very low frequency oscillations,believed to be generated by an elegant interaction of supraspinal and spinal sympathogenic, baroreceptor, and peripheral mechanisms (Figures 1 and 2) (Barcroft and Nisimaru,1932; Andersson et al., 1950; Preiss and Polosa, 1974; Nisimaru, 1984; Ursino et al., 1992; Montano et al., 1995, 1996,2000, 2009a, b; Seydnejad and Kitney, 2001; Julien et al.,2003; Julien, 2006; Morris et al., 2010). Traube (1865) and Hering (1869) first described respirophasic variation of the arterial pressure waveform in 1865, thus characterizing the high frequency band of oscillatory variability, with Sigmund Mayer describing a lower frequency oscillation of the arterial pressure waveform in anesthetized rabbits in 1876. A very low frequency band of activity was described by Barcroft and Nisimaru in 1932, likely reflecting vasogenic autorhythmicity (Siegel et al., 1976, 1984; Fujii et al., 1990; Ursino et al., 1992). Low and high frequency spectral components correspondent to Mayer and Traube Hering waves persist in sympathetic neural discharge, arterial pressure waveform,and cardiac interval following spinalization (Shirakawa and Uefuji, 1988; Montano et al., 2000). These findings clearly evidence the capacity for the spinal cord to generate these oscillations (Montano et al., 2000). In general, we will refer to the low frequency spectral band, as Mayer waves, and the high frequency spectral band, as Traube Hering waves throughout our discussion. We specifically evaluate the empirical basis for spinal mechanisms underlying the genesis of Mayer waves and the contribution of these oscillations to generation of sympathetic activity and amplification of power within the sympathetic nerve discharge (Montano et al., 2000), seeking to illumine a cohesive understanding of the interaction of supraspinal and spinal oscillators in generating low frequency oscillations in cardiovascular variability spectra (Barcroft and Nisimaru, 1932; Anderson et al., 1950;Preiss and Polosa, 1974; Nisimaru, 1984; Ursino et al., 1992;Montano et al., 1995, 1996; van de Borne et al., 2001; Seydnejad and Kitney, 2001; Julien et al., 2003; Barres et al., 2004;Julien, 2006; Morris et al., 2010).
Supraspinal Sympathogenesis
Supraspinal brainstem oscillators generate basal sympathoexcitation (Ghali, 2017a, b, 2019a; Guyenet, 2006) and the respiratory rhythm and pattern (Marchenko et al., 2012,2015, 2016; Ghali and Marchenko, 2013, 2015, 2016a, b; Zaki Ghali, 2013; Ghali, 2015, 2017c, d, e, 2019b, c; Ghali and Beshay, 2019; Zaki Ghali et al., 2019). Dittmar demonstrated precipitous falls in arterial pressure following spinal transection in 1873, indicating a critical importance for supraspinal mechanisms in supporting the sympathetic activity. Later studies evidenced the critical importance of presympathetic units located within the rostral ventrolateral medulla as critically important in providing basal, and mediating reflexive changes of, sympathetic activity and arterial pressure (Barman and Gebber, 1981a, b, 1983, 1985, 1992, 1997; Gebber and Barman, 1981, 1982; Sun et al., 1988; Guyenet et al.,1990, 2010; Guyenet, 2006), with contemporaneously performed studies evidencing the medullary lateral tegmental field as originating the sympathetic activity relayed to the rostral ventrolateral medulla, a generally underappreciated contribution, extensively and thoroughly evaluated and elucidated by the works of Gebber and Barman (Zhong et al., 1991, 1992, 1993a, b; Gebber and Barman, 1982, 1985;Pilowsky et al., 1986; Sun et al., 1988; Strack et al., 1989;Guyenet et al., 1990; Dempesy et al., 1995, 2000; Barman and Gebber, 1997; Marchenko and Sapru, 2003; Guyenet, 2006;Ghali, 2017a, b). Presympathetic neurons, including cells with pacemaker properties, provide excitatory drive to preganglionic sympathetic neurons in the intermediolateral cell column located within the thoracolumbar spinal cord (Lipski et al., 1996; Schreihofer and Guyenet, 1997; Guyenet, 2006;Stornetta, 2009; Accorsi-Mendonça et al., 2016; Ghali, 2017a,b), which project to postganglionic sympathetic neurons in the paravertebral chain ganglia, supplying vasoconstrictor tone to the venous capacitance vessels and arteriolar resistance vessels, as well as the sinoatrial and atrioventricular nodes and atrial and ventricular myocardium (Guyenet et al., 1990; Guyenet, 2006; Ghali, 2017a, b). Vagal output principally modulates sinoatrial and atrioventricular nodal tone and rate of depolarization, as well as atrial contractility (Corley et al., 1973; Slenter et al., 1984; Zarse et al., 2002), with sparse ventricular innervation (Hodges et al., 1989; Brack et al., 2009).
Spinal Sympathogenesis
The spinal cord has an empirically validated powerful capacity to generate sympathetic discharge (Goltz, 1874;Sherrington, 1906; Langley, 1919a, b; Yates, 1921; Meckler and Weaver, 1985; Weaver and Stein, 1989; Lahlou and Demenge, 1993; Kimura et al., 1995; Baldridge et al., 2002;Laird et al., 2006; Goodchild et al., 2008; Hou et al., 2013;Ghali, 2019a) and respiratory output (Coglianese et al., 1977;Viala et al., 1979; Aoki et al., 1980; Viala, 1986; Perségol and Viala, 1994; Reinoso et al., 1996; Dubayle and Viala, 1996a,b, 1998; Morin et al., 2000; Kobayashi et al., 2010; Ghali and Marchenko, 2016a). Several studies have demonstrated spontaneous (Coglianese et al., 1977; Aoki et al., 1980; Reinoso et al., 1996; Kobayashi et al., 2010; Ghali and Marchenko, 2016a), as well as pharmacologically (Viala et al., 1979;Viala, 1986; Perségol and Viala, 1994; Dubayle and Viala,1996 a, b, 1998; Morin et al., 2000; Ghali and Marchenko,2016a) and asphyxia induced (Ghali and Marchenko, 2016a)rhythmic (Coglianese et al., 1977; Viala et al., 1979; Aoki et al., 1980; Viala, 1986; Perségol and Viala, 1994; Dubayle and Viala, 1996 a, b, 1998; Reinoso et al., 1996; Morin et al.,2000; Kobayashi et al., 2010; Ghali and Marchenko, 2016a)and patterned (Reinoso et al., 1996) activity in respiratory neural output and electromyogram recordings following spinalization. We have previously demonstrated various patterns of rhythmic activity and slow oscillations in phrenic nerve discharge (Ghali and Marchenko, 2016a), as well as acute recovery of arterial pressure (Ghali, 2019a) following high cervical transection in unanesthetized decerebrate rats(Figure 3).
Recovery of arterial pressure (Goltz, 1874; Sherrington,1906; Langley, 1919a, b; Yates, 1921; Meckler and Weaver,1985; Weaver and Stein, 1989; Lahlou and Demenge, 1993;Kimura et al., 1995; Baldridge et al., 2002; Laird et al., 2006;Goodchild et al., 2008; Hou et al., 2013; Ghali, 2019a) and persistence of cardiovascular rhythmic oscillatory variability and Mayer waves in arterial pressure waveform and sympathetic neural discharge following high cervical transection in unanesthetized midcollicular decerebrate vagotomized cats (Montano et al., 2000) evidences spinal mechanisms mediating cardiovascular regulation (Fernandez de Molina and Perl, 1965; Kaminski et al., 1970) and capacity for generation of these waves and sympathetic activity (Figures 4 and 5). Though Mayer and Traube Hering waves were shown to persist following spinalization by Montano and colleagues(2000), spinal transection was shown to eliminate the 0.4 Hz arterial pressure Mayer wave oscillations by Randall and colleagues (2005) in unanesthetized rats, possibly the consequence of spinal shock.
Oscillations of much slower frequency in arterial pressure waveform, with periodicity varying from 25 to 60 seconds,persist in animals following spinalization (Fernandez de Molina and Perl, 1965; Kaminski et al., 1970). Low frequency oscillations, exhibiting a periodicity of 47 seconds,were noted in a patient with brain death. Spinal rhythmicity contemporaneously synchronous with vasomotor waves is detectable in the variability spectra of cardiac interval and arterial pressure in paralyzed patients (Guzzetti et al., 1994;Koh et al., 1994) and acute spinal cats (Montano et al., 1993).These findings, in constellation, support the hypothesis spinal mechanisms contribute to genesis of multiple types of oscillatory variabilities in sympathetic neural discharge and arterial pressure waveform (Shirakawa and Uefuji, 1988).Further studies are necessary in order to characterize the origins of these ultralow frequency oscillations in the arterial pressure waveform in the spinalized condition.
Cerebral compression or increased cerebrospinal fluid pressure generate large amplitude Mayer waves, unaffected by carotid and aortic denervation, in the spinal intact condition (Guyton and Satterfield, 1952), indicating a sympathetic centrogenic supraspinal or spinal vasomotor oscillator generating this rhythm, with modulability by changes in cerebrospinal fluid pressure. Covariance of Mayer wave amplitude with cerebrospinal fluid pressure may also lend credence to a possible mechanism for genesis of these oscillations by vasogenic autorhythmicity, or a harmonic thereof, with mechanotransduction to central neurons through the interstitium.The effects of cerebrospinal fluid pressure on Mayer wave amplitude generated by spinal mechanisms or vasogenic autorhythmicity may thus become more pronounced in the absence of descending excitatory and inhibitory inputs to the spinal cord following cervicomedullary transection.
The discussed findings thus provide robust evidence for and lend credence to a spinal origin for cardiovascular oscillatory variability and genesis of low frequency oscillations at the Mayer and Traube Hering wave spectral bands (Montano et al., 2000). We suggest these data indicate an originate spinal source for Mayer waves (Montano et al., 2000), as well as a possible general tendency for supraspinal and spinal sympathetic neurons to oscillate at this frequency. Such mechanisms may effectively compensate for the loss of bulbospinal presympathetic neuronal drive to preganglionic sympathetic neurons in patients with spinal cord injury and further elucidating the mechanistic underpinnings contributing to the genesis of these spectral components may improve our capacity to develop novel methods to augment arterial pressure in these patients (Goltz, 1874; Sherrington, 1906; Langley,1919a, b; Yates, 1921; Meckler and Weaver, 1985; Weaver and Stein, 1989; Lahlou and Demenge, 1993; Kimura et al., 1995;Baldridge et al., 2002; Laird et al., 2006; Goodchild et al.,2008; Hou et al., 2013; Ghali, 2019a) and respiratory output(Coglianese et al., 1977; Viala et al., 1979; Aoki et al., 1980;Viala, 1986; Perségol and Viala, 1994; Dubayle and Viala,1996a, b, 1998; Reinoso et al., 1996; Morin et al., 2000; Kobayashi et al., 2010; Ghali and Marchenko, 2016a).

Figure 1 Effects of gravity upon cardiovascular variabilities in a healthy subject.

Figure 2 MAP and RSNA power spectra and coherence in conscious rats.
Spinal Cord Generates Sympathetic Mayer Waves
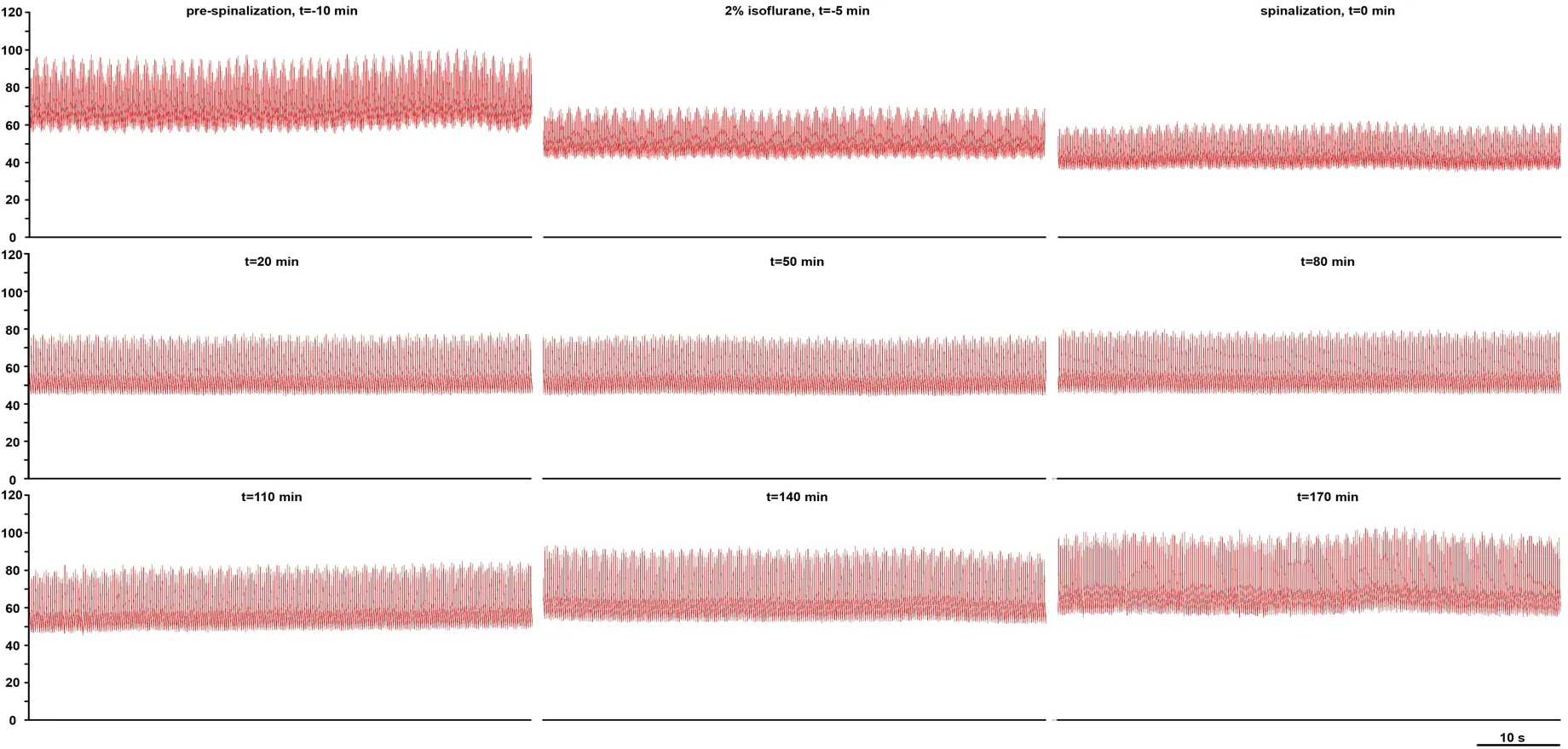
Figure 3 Recovery of arterial pressure recovery following high cervical (C1) transection.
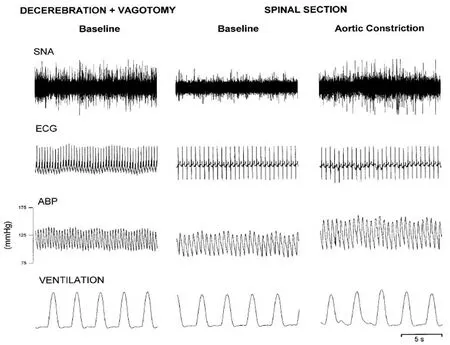
Figure 4 Cardiovascular and respiratory variabilities in a vagotomized unanesthetized midcollicular decerebrate cat.
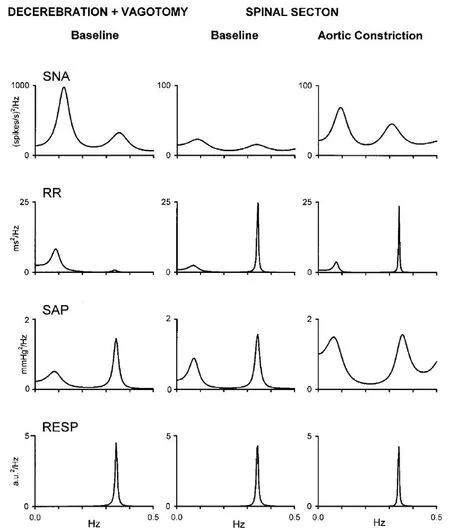
Figure 5 Cervical transection and aortic constriction mediated changes in cardiovascular spectral variabilities in a vagotomized unanesthetized midcollicular decerebrate cat.
Mayer Wave and Cardiovascular Variability Coherence Following Cervicomedullary Transection
The persistence of Mayer and Traube Hering waves in cardiac sympathetic nerve activity, cardiac interval, and systolic arterial pressure following C1 spinal transection (Montano et al., 2000) confirmed spinal capacity for genesis of these waves. Spinal transection effected reduction of mean sympathetic nerve activity from 36.9 to 7.6 spikes/s and increased cardiac interval from 349 to 499 ms, though arterial pressure remained stable, increasingly from 119 to 122 mmHg (Montano et al., 2000).
Spectral components of sympathetic and cardiovascular variability in the spinal intact condition were similar to those previously demonstrated in vagus intact unanesthetized decerebrate cats (Montano et al., 1992). Sympathetic Mayer waves demonstrated coherence with cardiac interval Mayer waves in 7 cases, whereas coherence between sympathetic and cardiac interval variability, as well as sympathetic and ventilatory variability, at the Traube Hering frequency band,was noted in 9 cases. Spectra of sympathetic nerve discharge,cardiac interval, and systolic arterial pressure continued to demonstrate Mayer and Traube Hering waves, though amplitudes were reduced in absolute values, with a significant reduction of variance, though the normalized ratio between the power contributed by the Mayer and Traube Hering wave spectral components was not significantly altered following spinal transection. A significant decrease in Mayer wave central frequency of sympathetic, cardiac interval, and systolic arterial pressure variabilities followed spinal transection, evidencing a critical role for descending excitatory mechanisms in supporting a higher frequency of this spectral component.Coherence was significantly correlated between the sympathetic and cardiac interval variability at the Mayer wave correspondent low frequency components in 5 cases and at the Traube Hering frequency band in 4 animals, evidencing common spinal originate genesis.
Effects of Spinospinal Sympathetic Reflex on Mayer Wave Spectral Power and Coherence
Spinospinal sympathetic reflexes
Sympathetic afferent fibers mediate positive feedback excitatory sympathetic reflexes, as described in cats (Lioy et al., 1974) and dogs (Pagani et al., 1982), classically termed the spinospinal sympathetic reflex and mediating an augmentation of the arterial pressure and sympathetic activity(Brown, 1965; Sato, 1973; Malliani and Pagani, 1976; Pagani et al., 1982; Hainsworth et al., 1994; Kimura et al., 1995).These reflexes are elicited by a variety of maneuvers inclusive of aortic constriction (Montano et al., 2000), volume transfusion increasing stretch on the aortic wall (Sato et al., 1973), or increases in coronary vessel pressure (Brown,1965; Hainsworth et al., 1994). Aortic constriction mediated augmentation of sympathetic discharge results from activation of the positive feedback spinospinal sympathetic reflex(Corbett et al., 1971; Malliani et al., 1971; Pagani et al., 1974;Malliani and Pagani, 1976, 1983; Malliani, 1982; Montano et al., 2000; Ghali, 2019a), contemporaneously augmenting the low and high frequency components of sympathetic nerve activity spectral variability.
Several studies have demonstrated spinospinal sympathetic, somatosympathetic, and viscoerosympathetic reflex mediated increases in arterial pressure in animal preparations(Goltz, 1874, Yates et al., 1921; Trostel et al., 1991; Ghali,2019a) and human patients (Corbett et al., 1971). Saline infusion in cats, chronically spinalized at low cervical levels,induces reflex tachycardia and sympathosympathetic neural circuit activation (Malliani and Pagani, 1983). Hindlimb pinch was shown to elicit increases in arterial pressure in vagus intact high cervically transected unanesthetized decerebrate rats (Ghali, 2019a). In patients with cervical spinal cord injury, mechanical stimuli applied below the level of injury elicit increases in sympathetic tone and arterial pressure, though no changes in heart rate or pulse pressure (Corbett et al., 1971). Deep inspiration similarly elicits increases in arterial pressure, which could be mediated by spinospinal sympathetic reflex by chest wall afferents, and bladder percussion elicits increases in mean arterial pressure, pulse pressure, vasoconstriction, and a reduction in blood flow,likely mediated by sensory fibers in the bladder wall.
Aortic constriction, effected utilizing a screw clamp, was demonstrated to augment Mayer wave amplitude in chloralose anesthetized cats (Killip, 1962). Aortic constriction was also shown to augment spectral power of Mayer and Traube Hering waves in vagotomized unanesthetized midcollicular decerebrate cats (Montano et al., 2000), as well as mediate increases of systolic arterial pressure to 151 mmHg and mean sympathetic nerve activity to 33.6 spikes/s, though the cardiac interval remained stable (Montano et al., 2000),with an increase in both variance and spectral power of low and high frequency components in sympathetic nerve discharge. Fractional distribution of spectral power between the low frequency spectral band correspondent to Mayer waves and the high frequency spectral band correspondent to Traube Hering waves was analogous following aortic constriction, with spectral power ratio remaining unchanged(Pagani et al., 1986; Malliani et al., 1991).
Aortic constriction mediated spinospinal sympathetic reflex in bilaterally vagotomized unanesthetized midcollicularly decerebrate cervically transected cats caused coherence of sympathetic and cardiac interval Mayer waves to become significant in all animals (Montano et al., 2000). Mayer wave components of sympathetic and cardiac interval variabilities were not correlated at baseline, though aortic constriction elicited significant coherence of these oscillations. Traube Hering component of sympathetic nerve and cardiac interval variabilities demonstrated coherence at baseline, becoming correlated with the high frequency component in cardiac interval variability, and with ventilation in 6 cases, following aortic constriction. Dorsal rhizotomy was performed in 3 animals between C7 and T7 levels, preventing aortic constriction mediated spinospinal sympathetic reflex and effecting a decrease in frequency of sympathetic nerve discharge from 16.3 to 9.2 spikes/s, though this was not significant.
The fractional distribution of Mayer and Traube Hering waves in sympathetic nerve activity, arterial pressure, and cardiac interval was not statistically significantly changed following aortic constriction mediated augmentation of spinospinal sympathetic activity in bilaterally vagotomized unanesthetized midcollicular decerebrate cats in the spinalized condition (Montano et al., 2000). Thus, while authors have utilized the low frequency high frequency ratio as a marker indicating relative sympathovagal balance, this may be valid in the spinal intact condition exclusively, with preserved supraspinal integrating mechanisms. If the low frequency high frequency power spectral density ratio reflected sympathovagal balance in the cervically transected preparation,spinospinal sympathetic reflex elicited by aortic constriction should effect a predictable change. Vagotomy utilized in this preparation may preclude this interpretation of the results.
Mayer waves in patients with spinal cord injury
Mayer waves have also been variably demonstrated in the arterial pressure waveform and cardiac interval variabilities of patients sustaining spinal cord injury, either described as a constant feature across all patients (Koh et al., 1994) or occurring only in some patients (Guzzetti et al., 1994). In another series of patients, cardiovascular spectral variabilities, including the high (0.27 Hz) and low (0.1 Hz) frequency spectral components, were demonstrated in normal subjects,though the low frequency component correspondent to Mayer waves was absent in patients with spinal cord injury(Inoue et al., 1991). Differences amongst these studies could putatively be explained by different patterns of spinal cord injury. Amplitude of cardiovascular spectral variability components was significantly increased by pressors in patients with high cervical spinal cord injury (Koh et al., 1994), likely by eliciting increased arterial pressure and thus stretch on the aortic wall activating and generating spinospinal sympathetic reflexes, well demonstrated to augment the spectral power of Mayer waves in animal preparations (Killip, 1962;Montano et al., 2000). Recovery of Mayer waves in cardiac interval and systolic arterial pressure variability spectra in patients with spinal cord injury (Guzzetti et al., 1994) may evidence genesis of these waves by spinal rhythmicity, enhanced by spinospinal sympathetic excitation. Analysis of cardiovascular variability spectra and Mayer wave characteristics in patients with spinal cord injury may thus represent a useful clinical metric of severity of injury and prognosticator for the development of dysautomonia. Patients with spinal cord injury may develop autonomic dysreflexia consequent to loss of descending modulatory inhibition of spinal sympathetic circuits (Ghali, 2019a; Jacob et al., 2001). This renders patients significantly sensitive to noxious and nonnoxious stimuli, such as bladder stretch, causing massive increases in sympathetic discharge and arterial pressure (Ghali, 2019a;Jacob et al., 2001).
Mayer wave amplitude is generally increased by postural tilt in healthy individuals, without a change in central frequency of the spectral band, as demonstrated in muscle sympathetic nerve activity, arterial pressure, and cardiac interval across several studies (Cooke et al., 1999; Furlan et al., 2000; Houtman et al., 2000; Kamiya et al., 2005). Spinal cord injury patients characteristically evidence paradoxical behavior of arterial pressure pulsations, most commonly demonstrating decreases in Mayer wave amplitude, following postural challenge during head up tilt (Guzzetti et al.,1994; Houtman et al., 2000; Munakata et al., 2001), likely occurring as a consequence of unloading of the aortic wall stretch receptors and decreasing spinospinal reflex mediated maintenance and support of sympathetic activity, converse to the consistent and characteristic augmentation which occurs in the upright position in normal subjects, likely occurring as a consequence of orthostatic decreases in arterial stretch placed on the baroreceptors and consequent disinhibition of sympathetic output and supraspinal Mayer wave oscillators (Cooke et al., 1999; Furlan et al., 2000; Kamiya et al.,2005), though occasionally Mayer wave amplitude increases with the upright position in patients with spinal cord injury(Guzzetti et al., 1994). Mayer wave amplitude also demonstrates differential responses to postural tilt in patients with high versus low levels of spinal cord injury, with increases in Mayer wave amplitude and decreases in baroreflex sensitivity in individuals with spinal cord injury below upper thoracic levels and insignificant decreases in Mayer wave amplitude and increases in baroreflex sensitivity in patients with lesions above the upper thoracic levels (Munakata et al., 2001). Differential effects of postural tilt on sympathetic nerve activity and Mayer wave amplitude in healthy individuals, compared to patients with spinal cord injury, could represent a useful clinical metric of, as well as inform mechanisms contributing to, dysautonomia in these patients.
27. King s son: In many fairy stories the sleeping heroine is woken by the kiss of a man (eg Briar Rose / Sleeping Beauty ). In this case it is his love and devotion that (indirectly) cause her awakening. In either case this symbolises the fact that a girl must be awakened to womanhood by a man. IRReturn to place in story.
Spinal Genesis of Mayer Waves: Perspectives and Significance
Persistence of Mayer and Traube Hering waves in sympathetic nerve discharge, cardiac interval variability, and systolic arterial pressure in variability spectra following high cervical transection evidences spinal cord capacity to generate these oscillations independent of supraspinal inputs(Kaminski et al., 1970; Montano et al., 2000). Decreased Mayer wave spectral band central frequency following high cervical transection in vagotomized unanesthetized midcollicular decerebrate cats (Montano et al., 2000) may evidence a contribution from supraspinal oscillators and/or a contribution by descending bulbospinal tonic excitatory inputs to spinal oscillators (Passino et al., 1997; Montano et al., 2000).Aortic constriction mediated spinospinal sympathetic reflex effected amplification of low and high frequency spectral power correspondent to Mayer and Traube Hering waves in sympathetic discharge demonstrates peripheral modulatability of spinogenic centrally generated rhythms (Montano et al., 2000). Alternatively, rhythmic fluctuations in arterial pressure sensed by afferent sympathetic fibers may relay a peripherally generated rhythm or harmonic of vasogenic autorhythmicity centrally. However, persistence of Mayer and Traube Hering waves in arterial pressure variability spectra following C7 to T7 dorsal rhizotomy indicates centrogenic spinal genesis of these oscillations independent of afferent input (Montano et al., 2000).
Persistence of Traube Hering waves, which reflect respirophasic variation, following high cervical transection, in sympathetic discharge following spinal transection evidences spinal capacity for genesis of these oscillations (Montano et al., 2000). Theoretically, spinalization should eliminate the effects of ventilatory blood pressure changes on influencing sympathetic discharge through baroreflex mechanisms,though in the presence of intact vagus nerves, cardiac vagal premotoneurons could influence cardiac interval oscillatory variability as a result of changes in pulmonary stretch, which in turn could influence the arterial pressure variability. However, as a corollary, cardiac interval oscillations at the Mayer wave frequency band tend to decrease the amplitude of these oscillations in the arterial pressure waveform according to some studies (Cerutti et al., 1991, 1994). The mechanical ventilatory stimulus of ventilation could activate somatic and visceral afferents contemporaneously projecting to the spinal cord (Sica et al., 1997) and thus rhythmic discharge related to respiration could modulate sympathetic nerve activity. In unanesthetized decerebrate rats, spontaneous rhythmic activity in phrenic nerve discharge following high cervical transection occasionally demonstrates ventilatory entrainment (Ghali and Marchenko, 2016a). The interaction of sensory afferents with central spinal motoneuronal pools likely plays a critical role in the pathological repatterning of spinal circuitry and consequent dysautonomia following spinal cord injury. Further dissecting these mechanisms in the context of cardiovascular and respiratory rhythms and spectral variability will serve to enhance and improve our understanding of pathological synaptic plasticity in the spinal cord following various types of blunt, penetrating, contusive, or transective injury.
Conclusions
The persistence of Mayer waves in the sinoaortic denervated condition indicates the capacity for central genesis independent of baroreceptor pathways (Preiss and Polosa, 1974;Cerutti et al., 1991a, b, 1994; Di Rienzo et al., 1991; Jacob et al., 1995; Julien et al., 1995; Montano et al., 1995, 1996,2000; Mancia et al., 1999; Morris et al., 2010; Julien, 2006).Mayer wave persistence following high cervical transection in arterial pressure, peripheral resistance, blood flow, cardiac interval, and efferent sympathetic discharge indicates spinal capacity for genesis of these oscillations (Fernandez de Molina and Perl, 1965; Montano et al., 2000), though the decrease in central frequency of this spectral band following spinalization suggests a critical contribution of supraspinal mechanisms to provision of tonic excitatory inputs to spinal sympathetic oscillators generating Mayer waves (Montano et al., 2000). We suggest the presence of multiple sympathetic oscillators generating these waves, located in brainstem and spinal cord, as well as a general tendency for neurons to oscillate at the Mayer wave frequency (Barman and Gebber,1983; Gebber and Barman, 1985; Montano et al., 1995, 1996;Barman et al., 2000, 2005; Morris et al., 2010; Ghali, 2017a,b). Sympathetic excitatory reflexes mediated by the spinal cord augment Mayer wave amplitude, which may contribute to sympathetic overactivity in patients with myocardial ischemia (Malliani, 1982), heart failure (Malliani and Pagani,1983; Wang and Zuker, 1996), and dysautonomia following spinal cord injury (Corbett et al., 1971; Malliani, 1982).Further studies will certainly illumine our understanding of genesis mechanisms and functional significance of Mayer waves in the spinal intact condition and following spinal cord injury.
Author contributions:Study conception and design, data acquisition,analysis and interpretation, manuscript drafting and revising, final version of manuscript approval: GZG, MGZG, EZG.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Transcriptional regulation of adult neural stem/progenitor cells: tales from the subventricular zone
- Targeting molecular pathways for the treatment of inherited retinal degeneration
- Green tea catechins inhibit microglial activation which prevents the development of neurological disorders
- Reversibility of visual field defects through induction of brain plasticity: vision restoration, recovery and rehabilitation using alternating current stimulation
- Role of activin receptor-like kinase 1 in vascular development and cerebrovascular diseases
- Effects of durotomy versus myelotomy in the repair of spinal cord injury

