Effects of neoadjuvant chemotherapy on respiratory function in patients with breast cancer
Lei Ding,Liping Wang,Jian Yin,Zhiyi Fan,Zijing He
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Anesthesiology,Peking University Cancer Hospital &Institute,Beijing 100142,China
Abstract Objective:To evaluate changes in chest X-rays,pulmonary function tests (PFTs) and quality of life in female breast cancer patients who had been treated with four cycles of neoadjuvant chemotherapy consisting of a regimen of cyclophosphamide,epirubicin and 5-fluorouracil (CEF regimen),and to determine the correlation between pulmonary function parameters and declined quality of life.Methods:Twenty-nine eligible female patients diagnosed with breast cancer at the first visit who were 20−60 years old,were classified as the American Society of Anesthesiologists (ASA) I−II and patients whose body mass index (BMI) <30 kg/m2 were recruited and subjected to chest X-ray examinations,PFTs and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30)questionnaire before and after receiving 4 cycles of the CEF regimen.Results:In this study,chest X-rays showed no abnormal changes after chemotherapy,but significant decreases in carbon monoxide diffusing capacity (DLCO) and percentage of the DLCO predicted value (DLCO%) (P<0.001).A significant increase in maximal ventilatory volume (MVV) (P=0.004) was observed,and most patients experienced dyspnea (P=0.031) and fatigue (P<0.001).However,there was no significant correlation between the changes in these PFTs parameters and the results of the EORTC QLQ-C30 (P>0.05).Conclusions:Neoadjuvant chemotherapy can reduce lung diffusion function and quality of life in females with breast cancer.
Keywords:Breast cancer;neoadjuvant chemotherapy;pulmonary function tests;quality of life
Introduction
Breast cancer is now the most frequently diagnosed cancer in Chinese women,and the number of new cases and deaths accounted for 12.2% of global cases and 9.6% of related deaths (1).In recent years,neoadjuvant chemotherapy has been widely used for the treatment of breast cancer because of its efficacy rate of 70%−90% in early breast cancer and improvements in the success rate of breast conservative surgery as well as in the long-term prognosis in patients with complete pathological remission(2,3).However,it also has obvious side effects in various organs in patients,including the heart,lung,liver,kidney,muscles and nerves.Patients with breast cancer often experience dyspnea after chemotherapy,which might be associated with the chemotherapy-induced injury of respiratory muscles and peripheral nerves (4).However,the correlation between dyspnea and lung injury after chemotherapy remains unclear.The main objective of this study was to observe the effects of neoadjuvant chemotherapy [4 cycles of a cyclophosphamide,epirubicin and 5-fluorouracil (CEF) regimen]on pulmonary function,lung tissue structure and the quality of life in patients with breast cancer.The secondary objective was to determine whether the decline in pulmonary function was related to dyspnea,fatigue and a decrease in quality of life after chemotherapy.
Materials and methods
Patient selection
We undertook this prospective cohort study after obtaining the approval of the local ethics committee (Ethics Committee of Peking University Cancer Hospital,June 23,2014;No 2014KT33).All participants signed an informed consent form before enrollment.The study was conducted in the Department of Anesthesiology at Beijing Cancer Hospital.The eligibility criteria included:1) a diagnosis of breast cancer during the first visit that resulted in being scheduled for surgery;2) female gender;3) an age of 27−60 years old;4) the American Society of Anesthesiologists(ASA) I−II classification;and 5) a body mass index (BMI)<30 kg/m2.Thirty-two recruited patients received CEF regimens [cyclophosphamide (CTX) 600 mg/m2+epirubicin (EPI) 100 mg/m2+5-fluorouracil (5-FU) 600 mg/m2,28 d per cycle]for 4 cycles before surgery.Three patients were excluded because they refused to undergo the second PFTs.
The exclusion criteria included:1) a history of mental illness or depression;2) neuromuscular disorders,diabetes mellitus or the use of drugs with effects on neuromuscular transmission;3) history of other malignancies and/or previous chemotherapy or radiotherapy;4) history of thoracic surgery;5) BMI≥30 kg/m2;4) history of smoking;5) hypoproteinemia or electrolyte disturbances;6)concomitant respiratory diseases (bronchial asthma,chronic obstructive pulmonary disease,restrictive lung disease,bronchiectasia or pulmonary bullae);7) pregnancy;or 8) refusal to participate in the study.
Data collection
Chest X-ray findings were recorded before and after 4 cycles of chemotherapy.PFTs were performed by the same physician at the same laboratory using the same device(Jaeger MasterScreen,CareFusion,CO,Germany) before and after chemotherapy.PFTs measurements include forced vital capacity (FVC),forced expiratory volume in 1 second (FEV1),FEV1% (FEV1/FVC),maximum midexpiratory flow (MMEF25−75),maximal ventilatory volume(MVV),total lung capacity (TLC),residual volume (RV),ratio of RV to TLC (RV/TLC),vital capacity (VC),tidal volume (VT),carbon monoxide diffusing capacity (DLCO)(adjusted to hemoglobin levels) and DLCO%.Quality of life was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) before and after chemotherapy.
Statistical analysis
Continuous variables were expressed as the.Categorical and counted variables were expressed as the number of cases (percentage).The pre-chemotherapy and post-chemotherapy comparison was performed with a pairedt-test.The Wilcoxon signed-rank test was used when the data did not conform to a normal distribution.The correlations between the changed parameters in the PFTs and EORTC QLQ-C30 were analyzed by Pearson correlation analysis.The significance level was set at P<0.05.The data were analyzed using IBM SPSS Statistics(Version 19.0;IBM Corp.,New York,USA).
Results
Patient characteristics
The demographic characteristics and clinical features of the recruited patients are summarized inTable 1.The mean age was 46±8 years old,and the mean BMI of the patients was 25±3 kg/m2.Four patients (13.8%) had received only primary education,fifteen patients (51.7%) had received only secondary education,and ten patients (34.5%) hadreceived a college education.Twenty-three patients(79.3%) were in clinical stage II,and six patients (20.7%)were in clinical stage III.
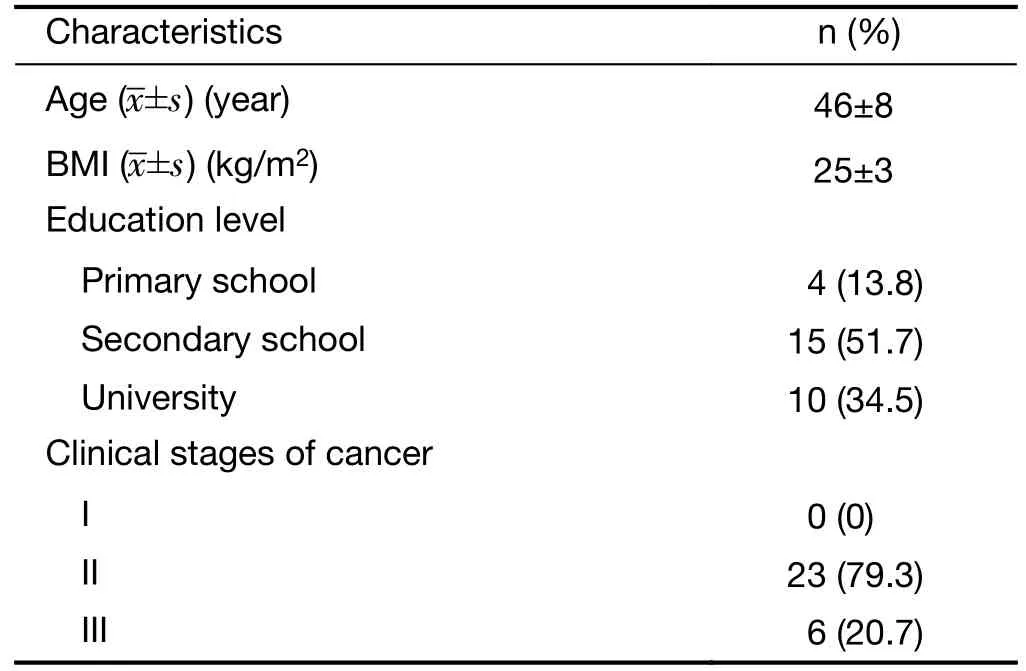
Table 1 Demographics and clinical characteristics (n=29)
Chest X-ray examination
The chest X-rays revealed no abnormal changes before or after chemotherapy.
PFTs
Compared with pre-chemotherapy values,DLCO values significantly decreased after 4 cycles of neoadjuvant chemotherapy (6.93±0.81 mmoL/kPa/minvs.5.86±1.08 mmoL/kPa/min,P<0.001),while DLCO% decreased by 11.4% [(90.2±11.8)%vs.(78.8±14.2)%,P<0.001].A significant increase in the MVV value was observed after chemotherapy (89.85±19.81 L/svs.97.89±20.15 L/s,P=0.004) (Table 2).
EORTC QLQ-C30
The four subscales in the EORTC QLQ-C30 related to respiratory function were observed with attention:physical functioning,dyspnea,fatigue,global health status.There were significant increases in the dyspnea (2.8±9.4vs.11.1±16.1,P=0.031) and fatigue scores (3.4±10.3vs.18.4±17.6,P<0.001) after chemotherapy compared with the scores obtained pre-chemotherapy (Table 3).
Correlations between changed parameters in PFTs and EORTC QLQ-C30
The results indicated no significant correlation between the changed parameters in the PFTs and the subscales related to respiratory function in the EORTC QLQ-C30 (P>0.05,Table 4).
Discussion
In recent years,neoadjuvant chemotherapy followed by resection has been widely used for the treatment of breast cancer.It has been noted by clinicians that neoadjuvant chemotherapy not only improves the success rate of surgery and the prognosis but also has side effects on various body systems and often results in lung injury.Previous studies have confirmed that CTX and taxanes have pulmonary toxicity.In addition,there have been some reports of lung injuries in patients after combination chemotherapy,highdose sequential chemotherapy regimens,or high-dose chemotherapy combined with autologous bone marrow transplants (5-8).Possible risk factors for the development of pulmonary toxicity in patients receiving chemotherapyhave been identified and include pre-existing lung disease,a history of smoking,industrial exposures,and thoracic irradiation (8).In Peking University Cancer Hospital,the CEF regimen (combined use of CTX,EPI and 5-FU) is a common neoadjuvant chemotherapy regimen used for breast cancer treatment.Most patients receive four cycles of treatment with the CEF regimen and undergo surgery 2−3 weeks after they have finished the last cycle of chemotherapy.During our clinical work,we observed that patients with breast cancer often developed dyspnea and suffered from a reduced quality of life after neoadjuvant chemotherapy.However,there have been few reports about lung injuries after 4 cycles of CEF regimen treatment,and it remains unclear whether such symptoms are associated with lung injury induced by chemotherapy.Moreover,previous studies have often lacked a simultaneously comprehensive assessment of the effects of chemotherapy on quality of life and iconographic changes.The aim of this study was to analyze the effects of neoadjuvant chemotherapy on pulmonary function and quality of life in breast cancer patients through the evaluation of chest X-ray,PFTs and EORTC QLQ-C30 results before and after the same number of cycles of the same chemotherapy regimen (4 cycles of a CEF regimen)and to explore the correlation between the parameters of PFTs and the subscales in a quality of life questionnaire.
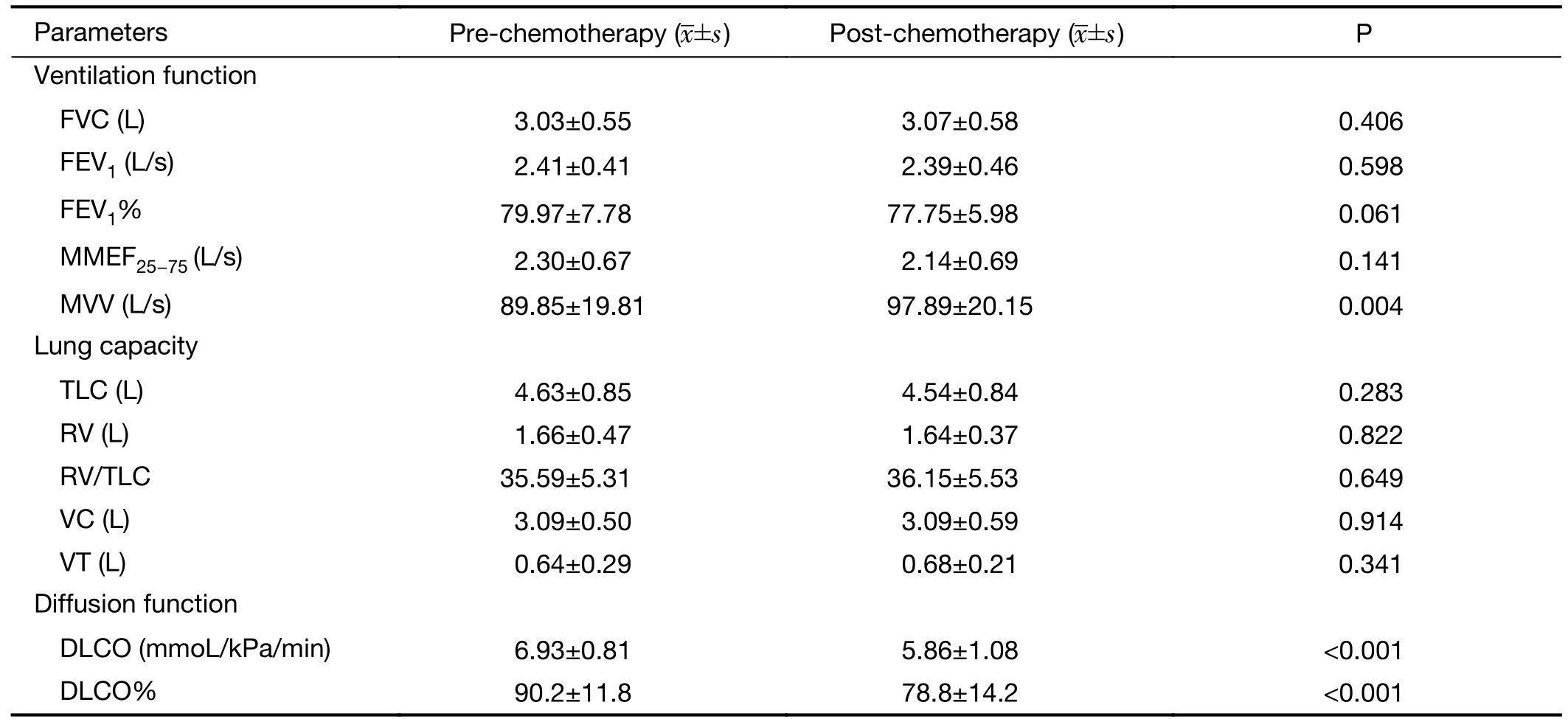
Table 2 PFTs pre-chemotherapy and post-chemotherapy
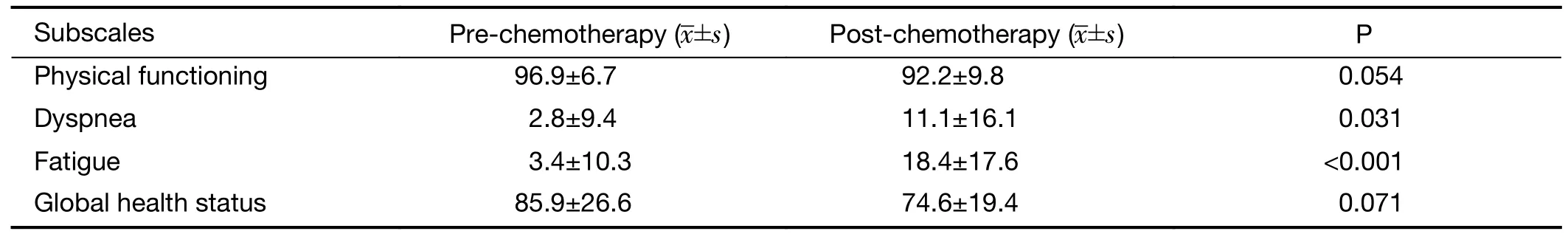
Table 3 EORTC QLQ-C30 pre-chemotherapy and post-chemotherapy

Table 4 Correlation between pulmonary function parameters and EORTC QLQ-C30 pre-chemotherapy and post-chemotherapy
PFTs have an important significance for guiding the early detection of diseases in lung tissues and airways,the evaluation of the severity and prognosis of lung injury,the identification of the causes of dyspnea,and the diagnosis of lesions.PFTs measure a number of parameters.Among these,DLCO represents the diffusion capacity of gas through the alveolar capillary barrier and is,in fact,one of the most sensitive variables that measures pulmonary function changes due to drug-induced toxicity (9).
In our study,DLCO and DLCO% were normal before chemotherapy.However,DLCO significantly decreased after 4 cycles of neoadjuvant chemotherapy (6.93±0.81 mmoL/kPa/minvs.5.86±1.08 mmoL/kPa/min),while DLCO% decreased by 11.4% (90.2%vs.78.8%),which indicated the presence of mild disorders of pulmonary diffusion after chemotherapy (10) in accordance with the findings of Citron and of Yerushalmi.The former revealed that CEF regimens reduced DLCO by 12.6% in patients with breast cancer (11),while the latter proposed that pulmonary function in breast cancer patients significantly decreased after 4 cycles of an AC regimen (adriamycin +CTX),which resulted in a decrease in pulmonary diffusion function (12).Lung injury induced by chemotherapy may develop further into interstitial pneumonia or pulmonary fibrosis.Briasouliset al.reported that the incidence of interstitial pneumonia was 9% after combination chemotherapy with CTX,EPI and 5-FU in breast cancer(13).It has been known that chemotherapeutics can induce lung injury through multiple mechanisms,including direct toxicity in lung tissues and as a result of immune responses and increased capillary permeability (14).CTX can induce nonspecific inflammatory changes in the pulmonary interstitium and results in monocyte alveolitis,interstitial pneumonia or pulmonary fibrosis (15,16).Anthracyclines do not result in pulmonary toxicity by themselves but may increase the toxicity of other chemotherapeutics when used in combination (17).The pathological changes described above can increase the diffusion distance in the lung,reduce the pulmonary capillary bed and diffusion area,and lead to a decrease in DLCO (18-21).Clinical manifestations include interstitial pneumonia,pulmonary fibrosis,hypersensitivity syndrome,capillary leak syndrome and pulmonary vascular disease (5,6).Therefore,we speculate that the reason why DLCO and DLCO%decrease after treatment with CEF regimens in the early stage may be because of injuries resulting in interstitial restriction and increased diffusion distance that are attributable to chemotherapy-induced nonspecific inflammatory infiltration in alveoli and the interstitium.Consequently,inflammatory infiltration may damage the pulmonary capillary bed and cause a reduction in the diffusion area and the development of interstitial pneumonia or pulmonary fibrosis,which may lead to the further deterioration of pulmonary diffusion.
MVV represents the total amount of new air moved into the respiratory passages each minute through the deepest and fastest breathing allowed by the respiratory and resistance muscles,and it depends on lung volume,the compliance of the lung and thorax,and airway resistance.In this study,neither MVV nor MMEF25−75decreased after chemotherapy,which suggests that the mechanism of pulmonary toxicity induced by chemotherapeutics does not involve damage or obstruction of the trachea and bronchi.Because dyspnea often occurs after chemotherapy,we propose that the increase in MVV may be attributed to increased ventilation in patients to relieve dyspnea.
We also observed that the other parameters of PFTs showed no significant change after 4 cycles of chemotherapy.Thus,we suggested that DLCO is the most sensitive variable of PFTs that can indicate lung injury after CEF regimen chemotherapy.Lindet al.(22) studied the predictive value of serial DLCO and FEV1for toxicity in patients who have received high-dose chemotherapy(HDCT) for breast carcinoma,and suggested that the directional trend of DLCO after HDCT is a predictor for therapy-related pneumonitis.Moreover,in Schröder’s research about changes in pulmonary function and influencing factors after high-dose intrathoracic radiotherapy combined with chemotherapy,they discovered that DLCO seems to be the most reliable indicator for lung tissue damage after thoracic radiotherapy.Ventilation parameters appear to be less reliable (23).
Radiologic evaluation of the lung toxicity has usually been made by chest X-rays,which was also observed in this study but showed no significant changes after chemotherapy.This may be explained by the fact that the lung injuries were too minor to appear as obvious changes in the tissue structure after 4 cycles of chemotherapy.It may also be related to the relatively low sensitivity of chest X-ray examination.Our results indicate that PFTs especially DLCO and DLCO% are sensitive parameters which can reflect chemotherapy-induced lung injury.Compared with chest X-rays,PFTs can be applied in early diagnosis of lung injury.Dimopoulouet al.also observed no abnormalities in chest X-rays,even if the DLCO was reduced by more than 20% (24).Several other investigators have noted that radiographic abnormalities may often be delayed relative to changes in PFTs or may not develop at all (8).This suggests that PFTs are more sensitive than routine standard chest X-ray examination and even chest computed tomography assessment for the early detection of chemotherapy-induced lung injury (24).
We also evaluated the quality of life of breast cancer patients before and after chemotherapy.The results revealed a significant increase in dyspnea and fatigue after chemotherapy,which is consistent with the results of a study by Traceret al(25).Tracer regarded respiratory muscle weakness and skeletal muscle dysfunction as possible explanations for the increase in the severity of dyspnea.Although DLCO% correlated with dyspnea slopes,the underlying mechanism remains unclear.Thus,to clarify whether dyspnea can also attribute to chemotherapy-induced lung injury,we performed correlation analysis between the changed parameters in the PFTs and the subscales in the EORTC QLQ-C30 related to respiratory function.The results revealed no correlation between MVV,DLCO,DLCO% and physical functioning,dyspnea,fatigue,or global health status(P>0.05),which suggested that early interstitial lung injury resulting in a decline in diffusion function after chemotherapy was not the main reason for dyspnea and decreased quality of life.We hypothesize that the possible reason for dyspnea after chemotherapy may be fatigue and direct damage to skeletal muscle induced by chemotherapeutics (26-28) as well as changes due to the disuse of skeletal muscle caused by significant reduction of daily activities (29-31).
To identify the effects of neoadjuvant chemotherapy on lung injury and respiratory function in patients with breast cancer,we evaluated chest X-rays,pulmonary function tests and quality of life before and after chemotherapy.Consistent with previous studies,chemotherapy resulted in a significant decrease in DLCO and DLCO% in this study,which indicated the presence of interstitial lung injury.However,we found no abnormalities in chest X-rays after chemotherapy,which may be related to the low sensitivity of chest X-ray examination.More sensitive examination methods,such as high-resolution computed tomography,which is not used as part of routine follow-up,may contribute to the early discovery of pulmonary interstitial changes induced by chemotherapy.In addition,we found no correlation between dyspnea and significant decreases in DLCO after chemotherapy,which may be attributed to the mild nature of the lung injuries observed as well as the small sample size and the short observation time of this study.
Limitations of the study should be acknowledged.The current study was a self-controlled study,designing to evaluate the effect of neoadjuvant chemotherapy on lung function in breast cancer patients.Although previous studies have not confirmed that early breast cancer has an adverse effect on lung function in patients,the correlation between reduced lung function and breast cancer disease progression cannot be excluded since our study did not design a parallel control group for comparison.Future research should increase the detection of lung function in breast cancer patients with different clinical stages before and after receiving different treatment measures (including neoadjuvant chemotherapy,radiotherapy and surgical treatment),so as to further identify the risk factors associated with decreased lung function in breast cancer patients.
Conclusions
The results of this study indicate that breast cancer patients developed a significant reduction in pulmonary function after receiving 4 cycles of CEF regimen neoadjuvant chemotherapy,especially in terms of their diffusion function.The results of a conventional chest X-ray test correlated poorly with a decline in PFTs and lacked both sensitivity and specificity.Neoadjuvant chemotherapy leads to an increase in fatigue and dyspnea and results in a decline in quality of life in breast cancer patients.There was no significant correlation between dyspnea and pulmonary dysfunction after chemotherapy.The decrease in pulmonary function,especially in diffusion function,after chemotherapy in breast cancer patients may be related to the pulmonary toxicity of chemotherapeutics.For breast cancer patients with complications resulting from lung disease,the possibility of lung injury caused by chemotherapy should be considered.The pulmonary function test is more helpful for the early detection of lung injury than an ordinary examination by imaging.
Acknowledgements
This study was supported by Science Foundation of Peking University Cancer Hospital (No.2014-18).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2020年1期
Chinese Journal of Cancer Research2020年1期
- Chinese Journal of Cancer Research的其它文章
- Incidence and mortality of oral and oropharyngeal cancer in China,2015
- Incidence and mortality of laryngeal cancer in China,2015
- Updates on larynx cancer epidemiology
- An exploration for quantification of overdiagnosis and its effect for breast cancer screening
- Factors associated with metastasis in superior mesenteric vein lymph node in subtotal gastrectomy for gastric cancer:Retrospective case control study
- Prognostic impact of D2-plus lymphadenectomy and optimal extent of lymphadenectomy in advanced gastric antral carcinoma:Propensity score matching analysis
