A New Epi-neoverrucosane-type Diterpenoid from the Liverwort Pleurozia subinflata in Borneo
Takashi Kamada ·Mary Lyn Johanis ·Shean-Yeaw Ng ·Chin-Soon Phan ·Monica Suleiman ·Charles S. Vairappan
Abstract
KeywordsEpi-neoverrucosane·Diterpenoid·Pleurozia subinflata ·Liverwort·Borneo
1 Introduction
Liverworts are the largest group of pioneering land plants which arose during the adaptation of plants from marine to terrestrial environment [1].They produced terpenoids and/or aromatic compounds as their major lipophilic constituents [2-5].Many types of sesquiterpenoids from liverworts are the enantiomer to those metabolites from higher plants [6].While,diterpenoids such as clerodanes,dolabellanes,fusicoccanes,kauranes,labdanes,pimaranes and others were found in numerous liverworts [6].Recently several bioactive cyathane diterpenoids were discovered [7,8].Cyathane is precursor structure for biosynthesis of the verrucosanetype diterpenoid,a fused 3,6,6,5-tetracyclic carbon skeleton [9].First verrucosane diterpenoid was isolated fromMylia verrucosa[10].Later,some analogs such as neoverrucosane-,homoverrucosane-,epi-neoverrucosane-andepi-homoverrucosane-type were reported [11-13].The latestepi-neoverrucosane analog was reported in 2013 [14].Hereby,we report yet another newepi-neoverrucosane diterpenoid,5β-acetoxy-13-epi-neoverrucosanic acid (1) was isolated,along with three known secondary metabolites,13-epi-neoverrucosan-5β-ol (2),chelodane (3) and (E)-βfarnesene (4) from east Malaysia’s liverwortPleurozia subinflata(Fig.1).
Besides,liverworts have long been used as traditional medicine by the indigenous people in some parts of China.In the past half-century,Prof.Yoshinori Asakawa (Tokushima Bunri University,Japan) has begun to study the chemical composition of liverworts collected from Asia,Europe and South America,many of which have reported to have diverse chemical structures and exhibited numerous biological activities [2,6].Thus,research focusing on the biological activity of liverwort and industrial use are significant.Our research examined the antifungal effects of the four isolated compounds against selected fungi separated marine organisms.
2 Results,Discussion and Conclusion
Compound 1 was isolated as colorless oil and analyzed for the molecular formula C22H34O4by HR-ESI-MS [M-H]-ion atm/z361.2391.The13C and1H NMR data (Table 1) indicated the presence of an isopropyl unit atδC31.1,23.2 and 22.3;δH1.51-1.55,1.00 and 0.78,one carboxylic carbon atδC183.0,an acetoxy unit atδC171.5,21.5;δH2.04,one oxygenated methine atδC75.1;δH5.28,two tertiary methyls atδC25.5 and 16.4;δH1.12 and 0.87,six methylenes,four methines,and three quaternary carbons which corresponding well to HSQC spectrum.Based on these findings,six degrees of unsaturation wascalculated from HR-ESI-MS,and it attributed to two carbonyl units and one tetracyclic ring for 1.
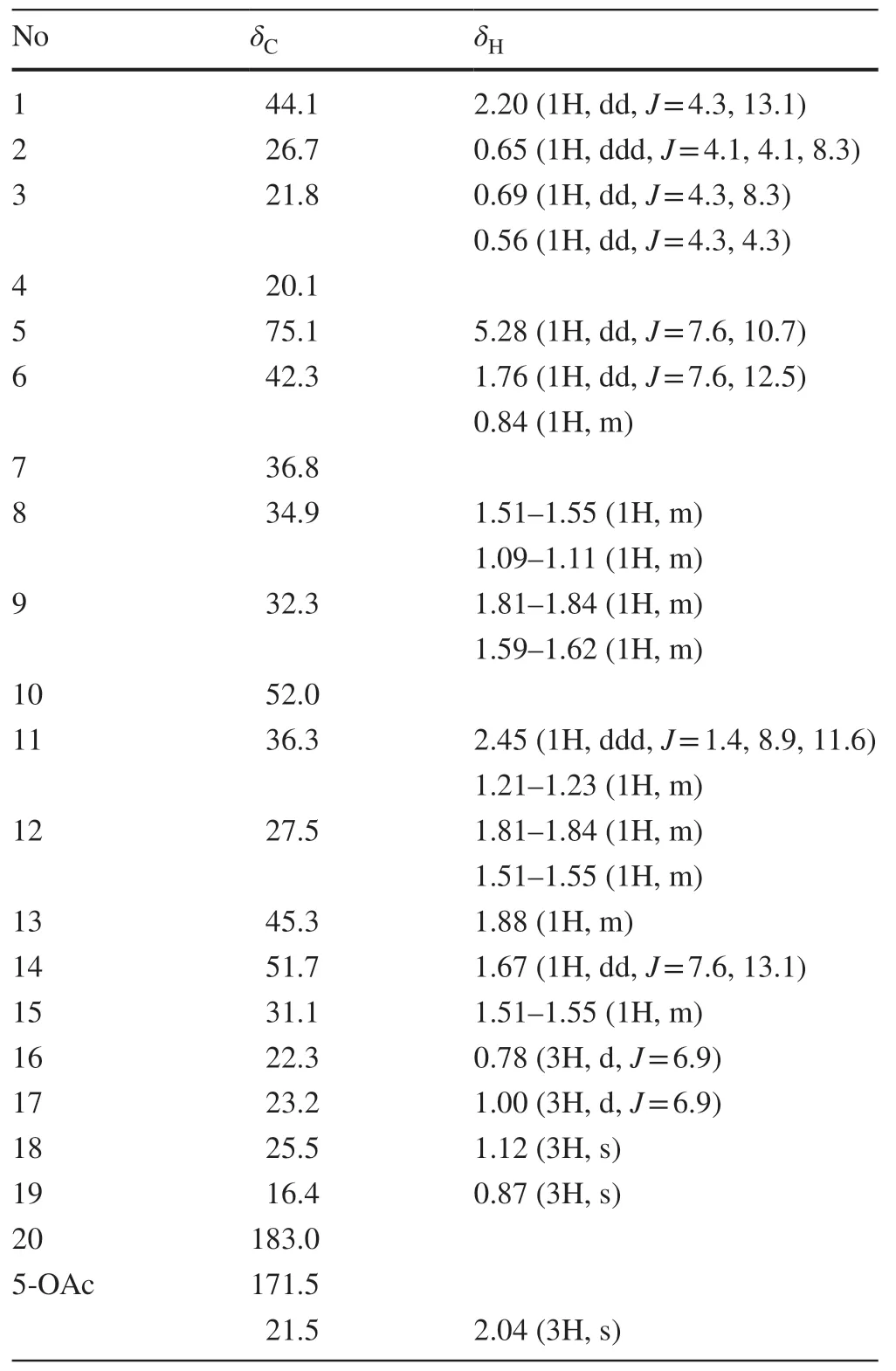
Table 1 1H and 13C NMR (600 and 150 MHz) data of 1 (in CDCl 3,δin ppm,J in Hz).
The 1 H-1H COSY experiment revealed the spin systems as depicted by the bold lines in Fig.2.In the HMBC spectrum,the three-bond correlations of H3-C(16) and H3-C(17) to the opposite carbons C(17) and C(16),and to C(13) and C(15),allowed the placement of isopropyl unit at C(13) which was further confirmed by 1 H-1H COSY.The acetoxy unit at C(5) was confirmed by HMBC correlations between H-C(5) to 5-OAc.The downfield shifted of13C and1H NMR at C(5) further supported this deduction.The HMBC correlations of H2-C(11) to C(20);and H-C(14) to C(20) suggested the carboxylic carbon at C(10).These findings together with HMBC correlations of H3-C(18) to C(2),C(3),C(4) and C(5);and H3-C(19) to C(1),C(6),C(7) and C(8) permitted establishment for the planar structure of 1 (Fig.2).
The relative stereochemistry of 1 was deduced from the NOESY correlations (Fig.2) and comparison of its chemical shift,coupling constants and NOE correlations with those of known analogs [10-14].The NOE correlations of H-C(5) to H2-C(6α) (δH1.76),H3-C(18) and H3-C(19);and H-C(14) to H3-C(19) have suggested H-C(5),H-C(14),H3-C(18) and H3-C(19) on α relative configuration.While,NOE correlations of H-C(1) to H2-C(3β) (δH0.56) and H2-C(6β) (δH0.84) showed H-C(1) must be on another face,β relative configuration.The earlier NOE cross peak of H-C(1) to H2-C(3) (δH0.56) has led to the assignment of H2-C(3) (δH0.56) on β configuration,therefore H2-C(3) (δH0.69) must be on α configuration.With this finding,the configuration at H-C(2) can be assigned based on vicinal coupling constants of cyclopropane unit between H-C(2) and H2-C(3α) (3J2-3α= 8.3 Hz) and between H-C(2) and H2-C(3β) (3J2-3β= 4.3 Hz).These coupling constant values suggested H-C(2) has acisrelationship with H2-C(3α) within the cyclopropane unit,therefore α configuration was assigned for H-C(2).While,the carboxyl unit at C(10) was assigned on β configuration due to atrans-fused at C/D ring junction.Thus,the relative configurations of 1R*,2S*,4S*,5R*,7S*,10S* and 14R* were determined as identical to those of known analogs of neoverrucosane andepi-neoverrucosane [10-14].To distinguishepi-neoverrucosane from neoverrucosane,the NOE correlations of H-C(1) to H-C(15) and H3-C(17);and H-C(13) to H-C(14),showed 13-isopropyl unit to H-C(14) has atransconfiguration,suggested aepi-neoverrucosane.Furthermore,similar NOE correlations of H-C(1) to H-C(15);and H-C(13) to H-C(14) were observed in 12-acetoxy-13-epi-neoverrucosann-5-one [14].On the contrary,these NOE were not observed in neoverrucosane-type,neoverrucosan-5β,9β-diol,instead H-C(13) to H-C(20) was detected [15].Thus,the structure 1 was established without confusion.The configuration of isopropyl unit at C(13) generated during formation of tricyclic system (Fig.3) determined the biosynthesis of neoverrucosane orepi-neoverrucosane [9].To the best of our knowledge,compound 1 was considered as first discovery of 13-epi-neoverrucosane that containing a carboxyl moiety or even among related skeletons such as verrucosane and neoverrucosane.The methyl at C(20) of 1 might have followed a three-step oxidation,via a hydroxyl and carbonyl,to the corresponding carboxylic acid [16],as shown in the purposed biosynthetic pathway (Fig.3).
The known compounds were identified as 13-epineoverrucosan-5β-ol (2) [11],chelodane (3) [17],and (E)-β-farnesene (4) [18],after compared its spectroscopic data with published literatures.The tetracyclic diterpenes are relatively rare in nature,and mainly found in the species ofPlagiochila,JamesoniellaandFossombronia[6].However,we foundepi-neoverrucosane-type diterpene derivatives (1 and 2) from east Malaysia’s liverwort,Pleurozia subinflata.These secondary metabolites seem to be the good chemosystematic markers forP.subinflatain Borneo.Compound 3 was widespread in marine sponges such asChelonaplysilla erecta,Raspailiasp.and even inSigmosceptrellasp.[17,19,20].However,this is the first record for 3 found in liverwort.Compound 4 was the most common farnesane-type sesquiterpene in liverworts.It was distributed throughout more than 20 Jungermanniales and Pleuroziales species includingPleurozia[6].
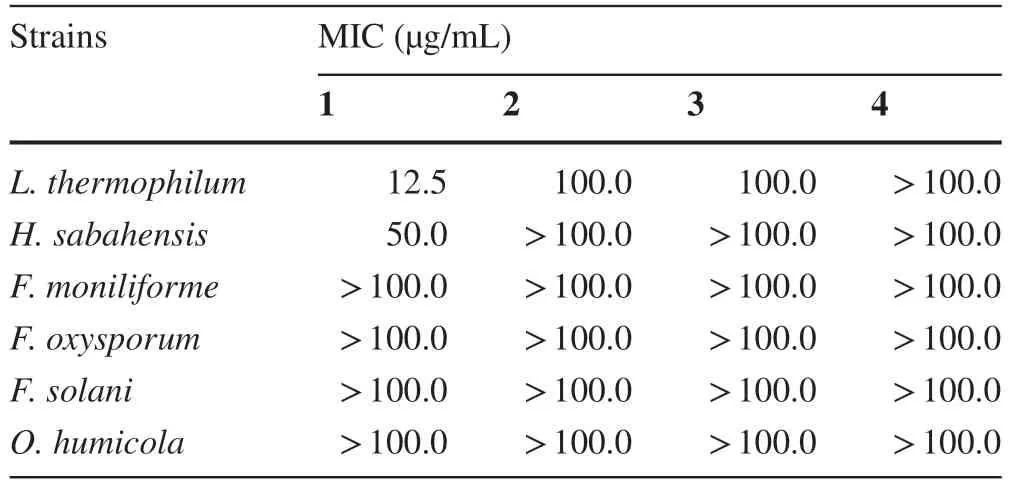
Table 2 The MIC (μg/mL) of 1-4 against six fungal strains
Compounds 1-4 were evaluated its biological potentials against fungal strains isolated from the Bornean ocean,Lagenidium thermophilumIPMB 1401,Haliphthoros sabahensisIPMB 1402,Fusarium moniliformeNJM 8995,Fusarium oxysporumNJM 0179,Fusarium solaniNJM 8996 andOchroconis humicolaNJM 1503 (Table 2).The minimum inhibition concentration (MIC) values of compound 1 againstL.thermophilumandH.sabahensiswere 12.5 and 50 μg/mL,respectively.While,compounds 2 and 3 showed MIC values of 100 μg/mL againstL.thermophilum.Compound 4 was inactive (> 100 μg/mL) against the tested fungi.
In conclusion,this is the first time ever since 2013 of the lastepi-neoverrucosane being discovered from nature.A new 13-epi-neoverrucosane diterpenoid,5β-acetoxy-13-epi-neoverrucosanic acid (1) along with three known secondary metabolites,13-epi-neoverrucosan-5β-ol (2),chelodane (3) and (E)-β-farnesene (4) were found in east Malaysia’s liverwortPleurozia subinflata.Compound 1 exhibited effective antifungal activity (MIC values of 12.5 μg/mL) againstLagenidium thermophilum.
3 Experimental Section
3.1 General Experimental Procedures
Optical rotation was taken on the automatic polarimeter (AUTOPOL IV automatic polarimeter) in chloroform solutions at 28 °C.IR spectrum was recorded on the FTIR spectroscopy (Perkin Elmer).NMR spectra were recorded on the 600 MHz FT-NMR (Jeol) using deuterated chloroform (CDCl3) with tetramethylsilane (TMS) as the internal standard.MS spectra were obtained using LC-ESIIT-TOF-MS (Shimadzu).For preparative TLC,Merck Kieselgel 60 F254was used and Kieselgel 60 was used for column chromatography.Purification was performed using high performance liquid chromatography (LC-10 AT,Shimadzu) equipped with UV detector.
3.2 Biological Materials
Specimens ofP.subinflata(M.Suleiman &S.-Y.Ng 5946)were collected from Mount Trus Madi (5 ° 33′ 13.1″ N,116 ° 30′ 41.9″ E),Sabah,Malaysia in August 2015.The specimens were identified based on external morphology by the fifth author.A voucher specimen (BORHB0026) is deposited in the BORNEENSIS Herbarium at Institute for Tropical Biology and Conservation (ITBC),Univeristi Malaysia Sabah (UMS).
3.3 Extraction and Isolation
The air-dried liverwort specimens (42 g) were extracted using 100% methanol (MeOH) (1.0 L × 3 each for two days).The crude extract was partitioned between distilled water (150 mL) and ethyl acetate (EtOAc) (50 mL × 3).After removal of the organic solvent,the EtOAc fraction (500 mg) was chromatographed on a Si gel column using hexane (Hex) and EtOAc system as eluent with increasing polarity (Hex/EtOAc: 9:1,8:2,7:3,5:5,100% EtOAc) to yield five fractions,1-5.Fraction 2 (76 mg) was subjected to repeated preparative TLC with toluene to yield 2 (8.8 mg),3 (7.4 mg) and 4 (15.4 mg).Fraction 3 (60 mg) was subjected to repeated preparative TLC with hexane/EtOAc: 7:3,and the resulted sub-fraction was further purified by semi-preparative high performance liquid chromatography (HPLC) to yield 1 (12.8 mg).The isolation was operated using a reverse phase C 18 column (5 μm,10 mm × 250 mm) measured at UV wavelength of 210 nm under gradient elution with the following conditions: 40-100% acetonitrile (MeCN)/H2O.
3.3.1 5β-Acetoxy-13-Epi-neoverrucosanic Acid (1)
Colorless oil;[α]D28.0+67.8 (c= 0.5,CHCl3);IR νmax3488,3060,1735 and 1712 cm-1;1H NMR (CDCl3,600 MHz)δ5.28 (1H,dd,J= 7.6,10.7 Hz,H-5),2.45 (1H,ddd,J= 1.4,8.9,11.6 Hz,H-11α),2.20 (1H,dd,J= 4.3,13.1 Hz,H-1),2.04 (3H,s,OAc),1.88 (1H,m,H-13),1.81-1.84 (1H,m,H-12α),1.81-1.84 (1H,m,H-9α),1.76 (1H,dd,J= 7.6,12.5 Hz,H-6α),1.67 (1H,dd,J= 7.6,13.1 Hz,H-14),1.59-1.62 (1H,m,H-9β),1.51-1.55 (1H,m,H-8α),1.51-1.55 (1H,m,H-12β),1.51-1.55 (1H,m,H-15),1.21-1.23 (1H,m,H-11β),1.12 (3H,s,H-18),1.09-1.11 (1H,m,H-8β),1.00 (3H,d,J= 6.9 Hz,H-17),0.87 (3H,s,H-19),0.84 (1H,m,H-6β),0.78 (3H,d,J= 6.9 Hz,H-16),0.69 (1H,dd,J= 4.3,8.3 Hz,H-3α),0.65 (1H,ddd,J= 4.1,4.1,8.3,H-2),0.56 (1H,dd,J= 4.3,4.3 Hz,H-3β);13C NMR (CDCl3,150 MHz)δ183.0 (C,C-20),171.5 (C,OAc),75.1 (CH,C-5),52.0 (C,C-10),51.7 (CH,C-14),45.3 (CH,C-13),44.1 (CH,C-1),42.3 (CH2,C-6),36.8 (C,C-7),36.3 (CH2,C-11),34.9 (CH2,C-8),32.3 (CH2,C-9),31.1 (CH,C-15),27.5 (CH2,C-12),26.7 (CH,C-2),25.5 (CH3,C-18),23.2 (CH3,C-17),22.3 (CH3,C-16),21.8 (CH2,C-3),21.5 (CH3,OAc),20.1 (C,C-4),16.4 (CH3,C-19);negative ion HRESIMS:m/z361.2391 (calcd for C22H33O4[M-H]-,361.2384).
3.4 Antifungal Assay
The minimum inhibitory concentration (MIC) of the fungistatic on hyphae was performed by incorporating the pure compound solutions (12.5,25.0,50.0,100.0 μg/mL) onto PYGS agar in a petri dish followed by inoculation of six tested fungal strains [21-23].The MIC was determined visually as the lowest concentration showing no hyphal growth after they were incubated at 25 °C for 7 days.
AcknowledgementsThis research was financially supported by the Sabah Biodiversity Centre Grant (SaBC) [No.GL0070] and Universiti Malaysia Sabah (UMS) Grant [SBK0258-SG-2016].The authors would like to thank Prof.Dr.Kishio Hatai (Borneo Marine Research Institute,UMS) for his kind guidance on bioassay.We are grateful to the Sabah Forestry Department for their support and assistance in the field.Finally,we were able to conduct research using literatures purchased through research project A provided by Shizuoka Institute of Science and Technology.
FundingThis research was financially supported by SaBC Grant [No.GL0070] and UMS Grant [SBK0258-SG-2016].
Compliance with Ethical Standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http://creat iveco mmons.org/licen ses/by/4.0/.
 Natural Products and Bioprospecting2020年1期
Natural Products and Bioprospecting2020年1期
- Natural Products and Bioprospecting的其它文章
- Natural Products and Bioprospecting
- Alkaloid Constituents of Ficus hispida and Their Antiinflammatory Activity
- Daphnane Diterpenoids from Trigonostemon lii and Inhibition Activities Against HIV-1
- UFLC-PDA-MS/MS Profiling of Seven Uncaria Species Integrated with Melatonin/5-Hydroxytryptamine Receptors Agonistic Assay
- Triterpenoidsfrom Ainsliaea latifolia and Their Cyclooxyenase-2(COX-2)Inhibitory Activities
- Dearomatized Isoprenylated Acylphloroglucinol Derivatives with Potential Antitumor Activities from Hypericum henryi
