不同耕作方式下黑土微生物群落对干湿交替的响应*
刘 奎,葛 壮,徐英德,刘 磊,叶 超,李 明,赵 搏,梁爱珍,张彬†,汪景宽
不同耕作方式下黑土微生物群落对干湿交替的响应*
刘 奎1,葛 壮1,徐英德1,刘 磊1,叶 超1,李 明1,赵 搏1,梁爱珍2,张彬1†,汪景宽1
(1. 沈阳农业大学土地与环境学院,沈阳 110866;2. 中国科学院东北地理与农业生态研究所,长春 130102;3. 南京信息工程大学应用气象学院,南京 210044)
以吉林德惠市黑土长期田间定位实验地土壤为研究对象,通过室内模拟培养,采用高通量测序方法(16S rRNA)研究免耕和垄作土壤微生物群落对不同频率和强度的干湿交替处理的响应。结果表明:干湿交替显著降低免耕土壤中微生物群落的多样性,且频率越高干旱强度越大多样性降低越显著;但干湿交替对垄作土壤的微生物多样性影响不显著。与对照相比,干湿交替显著增加免耕土壤中浮霉菌门(Planctomycetes)和疣微菌门(Verrucomicrobia)的相对丰度,显著降低免耕和垄作土壤中Saccharibacteria菌门和Parcubacteria菌门的相对丰度。无论是免耕还是垄作条件下,干湿交替频率的不同导致土壤微生物群落结构产生显著差异,而干湿交替强度的不同对土壤微生物群落结构没有显著影响。研究结果为预测干旱气候对黑土生态功能的影响提供了理论基础。
耕作方式;干湿交替;土壤微生物群落;黑土;高通量测序
微生物是土壤生态系统的重要组成部分,在土壤有机质形成和转化、土壤养分循环等方面发挥着至关重要的作用[1]。土壤微生物群落组成及活性变化是衡量土壤质量和肥力的一个重要指标[2]。影响土壤微生物群落组成发生变化的因素很多,如耕作方式、水分管理和施肥措施等。其中,干湿交替是土壤微生物群落经常面临的环境变化。近年来,由于全球气候变化导致干旱和降水的模式会不断发生改变[3],气候变化模型预测未来土壤干湿交替的频率和强度将更大[4],因此探讨干湿交替对土壤微生物群落的影响具有重要意义。目前关于干湿交替对土壤微生物群落的影响已有一些报道,例如包丽君和贾仲君[5]研究了干湿交替对水稻土中古菌群落结构的影响,发现反复的干湿交替并未显著影响水稻土古菌的主要类群组成,但古菌的绝对数量和相对丰度发生了一定程度的变化;王苑等[6]研究发现多次干湿交替显著影响土壤微生物群落结构,大大降低土壤真菌与细菌的比例,使细菌成为优势种群。已有研究大部分仅关注某些特定土壤微生物类群对干湿交替的响应,也有一部分研究采用磷脂脂肪酸法(PLFA)或变性梯度凝胶电泳(PCR-DGGE)方法考察干湿交替对整个土壤微生物群落的影响,然而这些方法在对数量少但具有重要功能的土壤微生物类群的检测上有较大的局限性,极有可能低估土壤微生物的物种组成并高估其丰度[7]。而高通量测序技术则能在整体微生物群落水平分析微生物遗传多样性,并能较为客观地反映其中低丰度的重要功能微生物。
东北黑土区是我国的粮食主产区,然而长期的传统耕作导致农田黑土退化严重[8-10]。由于传统耕作方式频繁地耕翻,又加之缺少有机物料归还,使土壤微生物的群落多样性和数量显著下降[11-12]。近年来免耕秸秆还田作为一项提高土壤有机质含量、改善土壤结构和生物特性的有力措施受到了广泛关注。长期不同耕作方式使土壤的水气热和养分状况等理化性质发生变化[13-16],从而对土壤微生物群落的多样性和结构有着显著的影响[17-19]。路怡青等[20]对免耕和常规耕作的土壤微生物群落的数量进行了比较研究,发现免耕土壤微生物群落的数量显著大于常规耕作。Zhang等[21]研究了传统耕作与免耕对黑土微生物群落的影响,发现免耕秸秆还田显著增加了土壤微生物群落中真菌与细菌比例。然而目前关于不同耕作方式下农田土壤微生物群落对干湿交替响应的研究还鲜见报道。本文通过室内培养实验,利用高通量测序的方法,研究免耕和垄作土壤在经过不同频率和强度的干湿交替后,其土壤微生物群落的多样性、丰度和结构的变化规律,对预测干旱对不同耕作方式土壤功能的影响具有重要意义。
1 材料与方法
1.1 供试材料
试验土壤为黑土,取自吉林省德惠市米沙子乡黑土农田试验基地(44°12′N,25°33′E)长期定位试验样地。该区属于中温带大陆性季风气候,年均气温4.4 ℃,年均降雨量520 mm。长期试验开始于2001年秋,布置不同的耕作与作物轮作处理,每种处理包含3个重复小区,随机区组设计。本研究选取其中的免耕和垄作处理,种植作物均为玉米(L.)连作。免耕对土壤的扰动小,主要是播种和施肥时免耕播种机切刀开沟的扰动;垄作除播种、中耕和锄草外,其他时期不进行土壤扰动,中耕时在作物种植行顶端起垄。两种耕作方式下秸秆全部还田,每年在播种时施用N 100 kg·hm–2、P 45.5 kg·hm–2、K 78 kg·hm–2,在玉米第6叶时期追施N 50 kg·hm–2[22]。
土壤样品采集于2014年春季播种前,用土钻采集0~10 cm 表层土壤。每个小区散点取样后混合成为一个样品。新鲜土样装入冷藏箱带回实验室,剔除可见根系和石块并混匀后过4 mm筛,在冰箱中保存。
1.2 培养试验设计
设计五个不同频率和强度的干湿交替处理:(1)对照处理(CK),土壤含水量保持在田间持水量的 60%;(2)1次中等干旱—浇水循环(MDW1),使土壤含水量从田间持水量的 60%逐渐下降至萎焉点的120%并保持该水平12 d后浇水,使土壤含水量恢复至田间持水量的60%并保持2 d;(3)1次极干旱—浇水循环(VDW1),使土壤含水量从田间持水量的60%逐渐下降至萎焉点的80%并保持在该水平,12 d后进行浇水使土壤含水量恢复至田间持水量的60%并保持2 d;(4)3次中等干旱—浇水循环(MDW3),使土壤含水量从田间持水量的60%逐渐下降至萎焉点的120%并保持在该水平,12 d后进行浇水使土壤含水量恢复至田间持水量的60%并保持2 d,设置3次循环,每次周期为14 d;(5)3 次极干旱—浇水循环(VDW3),使土壤含水量从田间持水量的60%逐渐下降至萎焉点的80%并保持在该水平,12 d后进行浇水使土壤含水量恢复至田间持水量的60%并保持2 d,设置3次循环,每次周期为14 d。其中干旱—浇水循环的周期是根据预试验的结果设置的,预试验发现土壤含水量从田间持水量的60%逐渐下降至不同目标含水量所需的最长时间为12 d。
将低温保存的土样装入底部用封口膜挡住的 PVC 管(高5 cm、直径4.8 cm),使得管中的土壤容重达到田间对应耕作处理下的土壤容重。不同耕作方式下土壤的装入量(按干土重计)为免耕115.75 g、垄作113.04 g。两种耕作处理一共装30个PVC管(2种耕作×5种干湿交替×3个重复)。用注射器向装好的 PVC 管中均匀地加入蒸馏水,使得土壤含水量达到田间持水量的60%,然后将PVC管竖直放入250 mL的培养瓶中,用封口膜封口以减少水分蒸发而同时不造成厌氧条件。然后放入培养箱中,在20 °C条件下预培养2周,然后按照上述干湿交替试验设计进行。土样的逐渐变干是通过在培养瓶中放入装有硅胶干燥剂(50 g)的小烧杯来实现的。在样品变干的过程中不定期称量PVC管以了解水分的损失情况,同时更换硅胶干燥剂,当土壤含水量下降至目标含水量后将小烧杯移除。对土样进行浇水是通过用注射剂向土壤表面均匀加入蒸馏水来实现的,通过称量PVC管确定水分加入量。当干湿交替实验结束后立即采集土壤进行土壤理化性质和微生物群落分析。土壤有效磷采用0.5 mol·L–1NaHCO3溶液浸提—钼锑抗比色法测定,土壤速效钾采用1 mol·L–1NH4OAc浸提(1︰10土液比)、火焰光度法(型号AP1200)测定[23]。土壤pH使用复合电极测定,土水比为1︰5。土壤全碳和全氮含量采用元素分析仪测定(Elementar CN Analyzer,德国)。
1.3 土壤微生物群落分析
土壤DNA的提取采用Power Soil@DNA Isolation Kit试剂盒(Mo Bio公司,美国),采用1%琼脂糖凝胶电泳和Nanodrop 2000分光光度计检测所提取DNA的质量,然后于–20℃低温保存。
对细菌16s rRNA的V3-V4区域进行PCR扩增,所用引物是338F(5’-ACTCCTACGGGAGGCAGC AG-3’)和806R(5’-GGACTACHVGGG TWTCTAAT- 3’)。PCR反应体系(25 μL)包括2.5 μL 10×Pyrobest Buffer,2 μL dNTPs(5 mmol·L–1),1 μL 338F/806R(10 μmol·L–1),0.4 U Pyrobest DNA聚合酶,15 ng DNA模板。PCR反应程序:95℃预变性5 min;95℃变性30 s,55℃退火30 s,72℃延伸60 s,25个循环;72℃延伸10 min。
用2%琼脂糖凝胶提取扩增子,用试剂盒(Axygen Biosciences,美国)对扩增子进行纯化后上Illumina Miseq测序平台进行测序。首先使用QIIME软件对原始序列进行质量控制,和过滤,移除低质量的原始序列,用过滤后的序列进行后续分析。对每个样品的reads数进行统计,并用UCLUST软件根据序列相似性进行聚类,选择97%作为相似性阈值,得到可操作分类单元表(OTU)。
1.4 数据处理
利用单因素方差分析(ANOVA)和图基检验(Tukey’s HSD)考察干湿交替对不同土壤理化性质、多样性指数及相对丰度的影响。应用R软件(version 3.4.4)进行主成分分析(PCA)和冗余分析(RDA)。用OringinPro 2015和R软件作图。
2 结 果
2.1 干湿交替对免耕和垄作土壤理化性质和微生物多样性的影响
免耕和垄作土壤的pH、全氮、全碳和有效磷变化趋势一致,均不受干湿交替处理的影响;而速效钾随着干湿交替的频率和强度的加强呈显著降低趋势(表1)。与对照处理相比,不同频率和强度的干湿交替显著降低了免耕土壤微生物的Chao1指数和香农指数,尤其是在3次极干旱—浇水循环条件下降低最显著(表2)。干湿交替对垄作土壤微生物Chao1指数和香农指数的影响相对较小,仅3次极干旱—浇水循环处理土壤Chao1指数显著低于对照。两种耕作处理相比,垄作土壤中的两种微生物多样性指数均显著高于免耕土壤(表2)。
2.2 干湿交替后不同土壤微生物群落的相对丰度
两种耕作方式的土壤中均检测到变形菌门(Proteobacteria)、酸杆菌门(Acidobacteria)、放线菌门(Actinobacteria)、绿弯菌门(Chloroflexi)、拟杆菌门(Bacteroidetes)、Saccharibacteria、芽单胞菌门(Gemmatimonadetes)、厚壁菌门(Firmicutes)、浮霉菌门(Planctomycete)、疣微菌门(Verrucomicrobia)和Parcubacteria等11个菌门,将相对丰度低于1%的微生物合并为Other。
免耕土壤干湿交替处理后微生物在门水平上的相对丰度如图1a所示。其中变形菌门(Proteobacteria)在土壤各处理中是极其优势的门类,其占比最大(47.85%~54.1%),相对丰度在1次极干旱—浇水处理下最高,而在1次中等干旱—浇水处理下最低。与对照相比,干湿交替后免耕土壤中浮霉菌门(Planctomycetes)和疣微菌门(Verrucomicrobia)的相对丰度显著增加(<0.05),并且干湿交替的频率和强度越大,其相对丰度越高,尤其在3次极干旱—浇水处理中的相对丰度增幅分别为240.0%和176.4%。而免耕土壤中的Saccharibacteria菌门和Parcubacteria菌门的相对丰度与对照相比,随着干湿交替强度和频率的增大而降低,两类微生物相对丰度的降幅在3次极干旱—浇水处理中达到55.9%(Saccharibacteria)和89.3%(Parcubacteria)。免耕土壤中厚壁菌门(Firmicutes)及其他菌门的相对丰度变化不明显。

表1 不同干湿交替处理对免耕和垄作土壤理化性质的影响
注:CK,对照;MDW1,1次中等干旱—浇水循环;VDW1,1次极干旱—浇水循环;MDW3,3次中等干旱—浇水循环;VDW3,3次极干旱—浇水循环。同列不同小写字母表示该耕作方式向下各处理差异显著(<0.05),平均值后面的数字为标准差。下同。Note:CK,Control;MDW1,one round of moderate dry-wet alternation;VDW1,one round of extreme dry-wet alternation;M DW3,three rounds of moderate dry-wet alternation;VDW3,three rounds of extreme dry-wet alternation. Different lowercase letters in the same row indicate significant difference between treatments(<0.05),means ± standard deviations. The same below.

表2 不同干湿交替处理对免耕和垄作土壤微生物多样性的影响
垄作土壤干湿交替处理后微生物在门水平上的相对丰度如图1b所示。垄作土壤中变形菌门(Proteobacteria)和酸杆菌门(Acidobacteria)的相对丰度显著低于免耕土壤(<0.05),而放线菌门(Actinobacteria)的相对丰度显著高于免耕土壤(<0.05)。变形菌门(Proteobacteria)在垄作土壤中的相对丰度也最大,而且其相对丰度在对照处理时最低,在3次中等干旱—浇水处理中最高。干湿交替后垄作土壤中Saccharibacteria门和Parcubacteria门的相对丰度显著下降,而芽单胞菌门(Gemmatimonadetes)的相对丰度显著提高。厚壁菌门(Firmicutes)及其他菌门相对丰度的变化不明显。
免耕土壤中占比最大的菌属是鞘氨醇单胞菌属(),占比达18%~30%(图2a)。与对照相比,免耕土壤中乳杆菌属()、根霉菌属()、硝基菌属()和Groundwatermetagenome菌属随着干湿交替的频率和强度的增加而降低。芽单胞菌属()等其他的菌属变化不显著。垄作土壤的鞘氨醇单胞菌属()、乳杆菌属()、假橄榄属()、根霉属()和颗粒菌属()的相对丰度低于免耕土壤,而垄作土壤的未分类()和其他(Other)的菌属的相对丰度高于免耕土壤(图2b)。鞘脂单胞菌属()在垄作土壤中相对丰度占比最高(14%~21%)。与对照相比,垄作土壤中鞘氨醇单胞菌属()和Groundwater metagenome菌属随着干湿交替的频率和强度的增强其相对丰度降低,而芽单胞菌属()随着干湿交替的频率和强度的增强其相对丰度增加。根霉菌属()等其他菌属变化不显著。
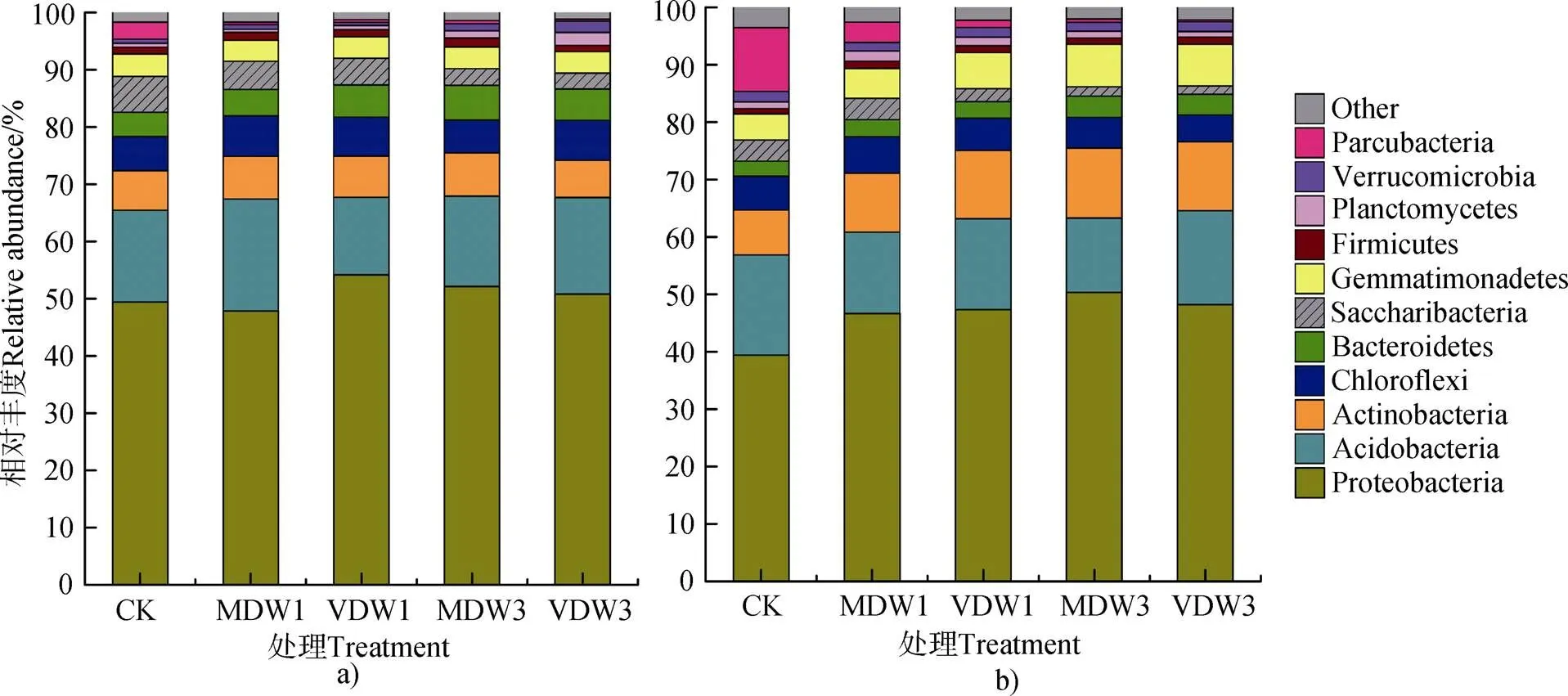
图1 不同干湿交替处理后免耕(a)和垄作(b)土壤微生物在门水平上的相对丰度

图2 不同干湿交替处理后免耕(a)和垄作(b)土壤微生物在属水平上的相对丰度
2.3 干湿交替后不同土壤微生物群落结构变化
对两种耕作方式下的土壤微生物群落进行主成分分析,发现第一主成分的变异主要是由耕作方式不同导致的。无论何种干湿交替处理,免耕和垄作土壤微生物群落总是分布在主成分分析图的左右两侧,说明不同耕作方式下土壤微生物群落结构差异极显著,这与我们前期的研究结果一致[21]。为深入考察不同干湿交替处理对每种耕作方式土壤微生物群落的影响,将两种耕作方式的主成分分析图独立呈现。
由图3可知,免耕土壤主成分分析结果显示其第一主成分(PC1)和第二主成分(PC2)的贡献率分别为48.8%和13.9%,垄作土壤PC1和PC2的贡献率分别为36.6%和18.6%。两种耕作方式下的土壤微生物群落对不同干湿交替处理的响应规律较为一致。与对照相比,干湿交替显著改变了两种土壤的微生物群落结构,同时微生物群落的变化受干湿交替的频率影响较大,而受干湿交替强度的影响较小。1次干旱—浇水循环和3次干旱—浇水循环处理的土壤微生物群落结构存在显著差异,然而在相同频率浇水循环下,不同强度的干旱处理对两种土壤微生物群落结构组成没有显著影响。

图3 干湿交替后免耕(a)和垄作(b)土壤微生物群落的主成分分析
2.4 土壤微生物群落结构与土壤理化性质的关系
选取土壤的有效磷、速效钾、全氮、全碳和pH作为环境因子,对免耕和垄作土壤的微生物11个门类做冗余分析。由图4a可知,5个理化因子解释了20.1%的总特征值,说明理化因子对免耕土壤的微生物的含量和分布有一定的影响。其中,速效钾是影响土壤微生物分布和数量的主要环境因素,对照处理(CK)和1次中等干旱—浇水循环(MDW1)的土壤微生物分布与速效钾呈正相关,与pH和全氮呈负相关;1次极干旱—浇水循环(VDW1)处理土壤微生物分布与有效磷呈正相关,与全碳呈负相关;3次中等干旱—浇水循环(MDW3)处理和3次极干旱—浇水循环(VDW3)处理的土壤微生物分布与pH和全氮呈正相关,与有效磷呈负相关。图4b表示5个理化因子对垄作土壤微生物含量和分布的影响,其解释了51.5%的总特征值,对垄作的微生物有一定的影响。进而说明,对照处理(CK)和1次中等干旱—浇水循环(MDW1)与速效钾呈正相关;3次中等干旱—浇水循环(MDW3)处理与有效磷、全氮和全碳呈正相关,与pH呈负相关。理化因子对垄作土壤的1次极干旱—浇水循环(VDW1)处理和3次极干旱—浇水循环(VDW3)处理的微生物分布影响较小。
3 讨 论
3.1 干湿交替对土壤微生物群落的影响
土壤微生物群落结构和组成特性在土壤有机质转化和养分循环过程中发挥着重要意义[24],而水分状况(如干湿交替)是影响土壤微生物群落数量、结构和多样性的重要作用因子[25-26]。本研究表明,干湿交替显著降低了免耕土壤中微生物群落的多样性,并且对免耕和垄作土壤微生物群落的相对丰度有着不同程度的影响。有研究表明,在干湿交替情况下土壤微生物量的降低主要表现在干旱后期,是由于干旱条件下有一部分微生物凋亡造成的[27]。在土壤中的某些微生物群落因为干旱的条件就表现出其群落的消失,在土壤湿润后,其群落也不会再次出现。另一方面,干湿交替导致土壤膨胀收缩,破坏了土壤中的菌丝体,从而切断了土壤与植物残体之间搭建起“养分运输的桥梁”,阻碍了微生物对土壤的碳氮等养分的截获[28-29],使得微生物可利用的养分减少,直接影响了微生物的自身生长代谢,导致微生物的生物量减少、多样性降低。

图4 免耕(a)和垄作(b)土壤理化性质与土壤微生物门类冗余分析

3.2 耕作方式对土壤微生物多样性和结构的影响
有研究表明,耕作方式影响着土壤有机碳、土壤微生物量和酶活性[31-33],而土壤有机碳、土壤微生物量和酶活性影响着土壤微生物的群落组成。在本研究中垄作的土壤微生物量和微生物的多样性显著高于免耕土壤,这与陈娟等[34]研究相一致。其原因可能是垄作改变了田间地形,独特的垄沟结构,改善了土壤水、热、气和光等条件,为微生物的繁衍生息创造了良好的环境,此外有研究表明,酸性的土壤环境会降低土壤微生物多样性,抑制土壤微生物酶活性[35],并且垄作使土壤中的团聚体被破坏,释放出土壤中的生物碳和有机物质,有利于微生物的生长和繁殖。Xue等[36]研究也表明,垄作能为土壤微生物提供更多的有机物质,使微生物的数量增加。垄作提高了土壤微生物的多样性和丰富度,在一定程度上能够增加有机质的分解,有利于作物对营养物质吸收。在本实验中也出现,免耕土壤的3次中等干旱—浇水循环处理下微生物的丰富度显著高于1次中等干旱—浇水循环和1次极干旱—浇水循环,这可能是土壤干旱时微生物细胞萎蔫,细胞中能量转移受阻[37-38],养分吸收减少,微生物生长受阻。土壤中大量凋亡的微生物变成有机物质,再加之多次的干湿交替后土壤中团聚体裂解释放出有机质[6],在湿润后,微生物有了更好的生境,所以微生物的丰富度表现增加,而多样性表现降低。而垄作的土壤并没有出现这种状况,这可能是垄作土壤在耕作的时候已经破坏了土壤的团聚体,释放出有机质,在干湿交替实验后,也就没有微生物突增的基础,这也与上文关于垄作土壤的推测一致。
干湿交替的强度对两种耕作方式的土壤微生物群落结构影响均不大,而干湿交替的频率显著改变了免耕和垄作土壤的微生物群落结构。由此可见,两种耕作方式下土壤微生物群落对环境胁迫的响应是一致的。由RDA分析显示,免耕和垄作土壤微生物门类对环境因子的响应不相同,尤其在面对3次极干旱—浇水循环处理时,表现尤为明显。例如垄作和免耕土壤的对照(CK)处理对速效钾表现出相关性,但是相关程度不同,在3次极干旱—浇水循环的环境胁迫下,免耕土壤微生物门类与pH和全氮表现出相关,但是垄作土壤微生物与5个环境因子均表现出不相关。这就说明,两种土壤的微生物门类分布和数量存在差异。由相对丰度可以看出,免耕的土壤微生物群落在面对环境胁迫时,各个菌门之间的比例变化不大,菌属变化较大,而垄作的变化则与之相反,如Parcubacteria菌门变化了96.76%,芽单胞菌门(Gemmatimonadetes)变化了60.84%,在属水平上,垄作土壤微生物的结构变化小于免耕,例如免耕的鞘氨醇单胞菌属()和芽单胞菌属()变化了61.11%和68.71%,而垄作变化了50.01%和23.51%。在门水平上,免耕土壤的微生物的结构变化要小于垄作土壤的微生物,这有可能是免耕保护了土壤的团聚体,团聚体为土壤中微生物提供了庇护,保证了其微生物各个群落之间的结构稳定[39]。在环境胁迫解除后,由团聚体庇护的群落开始繁殖,保证了其群落结构的稳定。这在某种意义上说明,免耕对微生物的群落起到了保护作用,而在属水平上,这种保护作用并没有起效,更多反映出的是垄作提供了良好的土壤条件,减小菌属对环境变化时的冲击力。但在生产实践中对作物的影响,还需进一步实验证明。
4 结 论
干湿交替的频率越高干旱强度越大,免耕土壤微生物的多样性越低;而干湿交替对垄作土壤微生物多样性的影响不显著。两种耕作方式下,干湿交替频率的不同导致土壤微生物群落结构发生明显改变,而干湿交替的强度对土壤微生物群落结构没有显著影响。
[1] Copley J. Ecology goes underground. Nature,2000,406(6795):452—454.
[2] Harris J A. Measurements of the soil microbial community for estimating the success of restoration. European Journal of Soil Science,2003,54(4):801—808.
[3] Lei W,Charles A L. Global climate change and its impacts. Advances in Water Science,2003,14(5):667—674.
[4] Huntington T G. Evidence for intensification of the global water cycle:Review and synthesis. Journal of Hydrology,2006,319(1):83—95.
[5] Bao L J,Jia Z J. Effect of simulation of dry-wet alternation on the community structure of archaea in paddy soil. Acta Pedologica Sinica,2017,54(1):191—202.[包丽君,贾仲君. 模拟干湿交替对水稻土古菌群落结构的影响. 土壤学报,2017,54(1):191—202.]
[6] Wang Y,Song X S,Wang J,et al. Effects of alternating wet and dry on soil carbon pool and organic carbon mineralization. Acta Pedologica Sinica,2014,51(2):342—350. [王苑,宋新山,王君,等. 干湿交替对土壤碳库和有机碳矿化的影响. 土壤学报,2014,51(2):342—350.]
[7] Xia W W,Jia Z J. Technical evaluation of soil microbial communities by high-throughput sequenceng and DGGE analysis. Acta Microbiologica Sinica,2014,54(12):1489—1499. [夏围围,贾仲君. 高通量测序和DGGE分析土壤微生物群落的技术评价.微生物学报,2014,54(12):1489—1499.]
[8] Han X Z,Wang S Y,Song C Y,et al. Impacts of land use/cover change on black soil ecological environment. Scientia Geographica Sinica,2005,25(2):203—208. [韩晓增,王守宇,宋春雨,等. 土地利用/覆盖变化对黑土生态环境的影响. 地理科学,2005,25(2):203—208.]
[9] Wang J K,Li S Y,Zhang X D,et al. Changes in soil fertility quality in the typical black soil regions of Northeast China in the past 20 years. Chinese Journal of Eco-Agriculture,2007,15(1):19—24. [汪景宽,李双异,张旭东,等. 20年来东北典型黑土地区土壤肥力质量变化. 中国生态农业学报,2007,15(1):19—24.]
[10] Zhang X Y,Song Y Y,Song C Y. Degeneration process of farmland black soil. Soil and Crop,2013,2(1):1—6. [张兴义,隋跃宇,宋春雨. 农田黑土退化过程. 土壤与作物,2013,2(1):1—6.]
[11] Yang D,Liu Q. Spatial variability of soil total nitrogen and organic matter in Hexi area:A case study of Ganzhou District in Zhangye City. Agricultural Research in the Arid Areas,2010,28(4):183—187. [杨东,刘强. 河西地区土壤全氮及有机质空间变异特征分析——以张掖市甘州区为例. 干旱地区农业研究,2010,28(4):183—187.]
[12] Chiu C Y,Chen T H,Imberger K,et al. Particle size fractionation of fungal and bacterial biomass in subalpine grassland and forest soils. Geoderma,2006,130(3):265—271.
[13] West T O,Post W M. Soil organic carbon sequestration rates by tillage and crop rotation:A global data analysis. Soil Science Society of America Journal,2002,66(6):13—16.
[14] Du Z L,Ren T S,Hu C S. Tillage and residue removal effects on soil carbon and nitrogen storage in the North China Plain. Soil Science Society of America Journal,2010,74(1):196—202.
[15] Sun J,Liu M,Li L J,et al. Effects of different tillage methods on soil water and heat in dry farmland in Inner Mongolia.Acta Ecologica Sinica,2010,30(6):1539—1547. [孙建,刘苗,李立军,等. 不同耕作方式对内蒙古旱作农田土壤水热状况的影响. 生态学报,2010,30(6):1539—1547.]
[16] Zhang R Z,Huang G B,Cai L Q,et al. Practice of several conservation tillage practices in dryland farming on the Loess Plateau. Chinese Journal of Eco-Agriculture,2013,21(1):61—69. [张仁陟,黄高宝,蔡立群,等. 几种保护性耕作措施在黄土高原旱作农田的实践. 中国生态农业学报,2013,21(1):61—69.]
[17] Zhong W H,Cai Z C. Research progress in the effect of soil management measures and environmental factors on soil microbial diversity. Biodiversity Science,2004,12(4):456—465. [钟文辉,蔡祖聪. 土壤管理措施及环境因素对土壤微生物多样性影响研究进展. 生物多样性,2004,12(4):456—465.]
[18] Wang G H,Jin J,Xu M N,et al. Effects of plant,soil and soil management on soil microbial community structure. Chinese Journal of Ecology,2006,25(5):550—556. [王光华,金剑,徐美娜,等. 植物、土壤及土壤管理对土壤微生物群落结构的影响. 生态学杂志,2006,25(5):550—556.]
[19] Huang M,Jiang L G,Zou Y B,et al. Changes in soil microbial properties with no-tillage in Chinese cropping systems. Biology and Fertility of Soils,2013,49(4):373–377.
[20] Lu Y Q,Zhu A N,Zhang J B,et al. Effects of no-tillage and straw return on soil enzyme activity and microbial community. Chinese Journal of Soil Science,2014,45(1):85—90. [路怡青,朱安宁,张佳宝,等. 免耕和秸秆还田对土壤酶活性和微生物群落的影响. 土壤通报,2014,45(1):85—90.]
[21] Zhang B,He H B,Ding X L. et al. Soil microbial community dynamics over a maize(L.)growing season under conventional-tillage and no-tillage practices in a rainfed agroecosystem. Soil & Tillage Research,2012,124(4):153—160.
[22] Ding X L,Zhang B,Zhang X D,et al. Effects of tillage and crop rotation on soil microbial residues in a rainfed agroecology system of northeast China. Soil & Tillage Research,2011,114(1):43–49.
[23] Bao S D. Soil and agricultural chemistry analysis. Beijing:China Agriculture Press,2000:103—101,79—87. [鲍士旦. 土壤农化分析. 北京:中国农业出版社,2000:101—103,79—87.]
[24] Bell T,Newman J A,Silverman B W,et al. The contribution of species richness and composition to bacterial services. Nature,2005,436(7054):1157—1160.
[25] Wu J,Brookes P C. The proportional mineralisation of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biology & Biochemistry,2005,37(3):507—515.
[26] Zhang B,Yao S H,Hu F. Microbial biomass dynamics and soil wettability as affected by the intensity and frequency of wetting and drying during straw decomposition. European Journal of Soil Science,2010,58(6):1482—1492.
[27] Hamer U,Unger M,Makeschin F. Impact of air-drying and rewet-ting on PLFA profiles of soil microbial communities. Journal of Plant Nutrition and Soil Science,2007,170(2):259—264.
[28] van der Heijden M G A,Martin F M,Selosse M A,et al. Mycorrhizal ecology and evolution:The past,the present and the future. New Phytologist,2015,205(4):1406—1423.
[29] van der Heijden M G A,Klironomos J N,Ursic M,et al. Mycorrhizal fungal diversity determines plant biodiversity,ecosystem variability and productivity. Nature,1998,396(6706):69—72.
[30] Helen G,Philip M,Richard D. Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biology & Biochemistry,2008,40(2):302—311.
[31] Wang X J,Zhang R Z,Bi D M,et al. Effects of conservation tillage on soil organic carbon components. Journal of Soil and Water Conservation,2009,23(2):115—121. [王新建,张仁陟,毕冬梅,等. 保护性耕作对土壤有机碳组分的影响. 水土保持学报,2009,23(2):115—121.]
[32] Cambardella C A,Elliott E T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Science Society of America Journal,1992,56(3):777—783.
[33] Zhou L K. Soil enzymology. Beijing:Science Press,1987:263—278. [周礼恺. 土壤酶学. 北京:科学出版社,1987:263—278.]
[34] Chen J,Ma Z M,Liu L L,et al. Effects of different tillage methods on soil organic carbon,microbial biomass and enzyme activity. Journal of Plant Nutrition and Fertilizers,2016,22(3):667—675. [陈娟,马忠明,刘莉莉,等. 不同耕作方式对土壤有机碳、微生物量及酶活性的影响. 植物营养与肥料学报,2016,22(3):667—675.]
[35] Sinsabaugh R L,Lauber C L,Weintraub M N,et al. Stoichiometry of soil enzyme activity at global scale. Ecology Letters,2008,11(11):1252—1264.
[36] Xue L Z,Li M,Frank S G,et al. Effects of raised-bed planting for enhanced summer maize yield on rhizosphere soil microbial functional groups and enzyme activity in Henan Province,China. Field Crops Research,2012,13(2):28—37.
[37] Rosacker L L,Kieff T L. Biomass and adenylate energy charge of a grassland soil during drying. Soil Biology & Biochemistry,1990,22(8):1121—1127.
[38] Fierer N,Schimel J P. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid re-wetting of a dry soil. Soil Science Society of America Journal,2003,67(3):798—805.
[39] Lupwayi N Z,Arshad M A,Rice W A,et al. Bacterial diversity in water-stable aggregates of soils under conventional and zero tillage management. Applied Soil Ecology,2001,16(3):251—261.
Responses of Soil Microbial Community to Drying-Wetting Alternation Relative to Tillage Mode
LIU Kui1, GE Zhuang1, XU Yingde1, LIU Lei1, YE Chao1, LI Ming1, ZHAO Bo1, LIANG Aizhen2, ZHANG Bin1†, WANG Jingkuan1
(1.College of Land and Environment, Shenyang Agricultural University, Shenyang 110866, China; 2.Northeast Institute of Geography and Agricultural Ecology, Chinese Academy of Sciences, Changchun 130102, China; 3. School of Applied Meteorology, Nanjing University of Information Science & Technology, Nanjing 210044, China)
In this study, effects of dry-wet alternation on diversity, abundance and structure of the soil microbial community in the black soil of a long-term stationary field experiment in Dehui of Jilin on tillage modes, no-tillage or ridge tillage, were investigated. So far few reports were detected in the literature about responses of the soil microbial community in the soil subjected to drying-wetting alternation under no-tillage or ridge tillage. In this paper, soil samples were collected for analysis by means of high-throughput sequencing in laboratory. This research was expected to be of great significance to prediction of impacts of drought on soil functions under different tillage practices.No-tillage and ridge tillage plots in the field experiment had been cultivated with maize (L.) for 13 years. Soil samples were collected with a T sampler, ground to pass a 4-mm sieve, and then packed separately into PVC pipes with a sealing film at the bottom of each pipe to make the soil in the pipe the same in bulk density as that in the plot under no-tillage or ridge tillage. The amount of soil packed into the pipe for no-tillage was 115.75 g and for ridge tillage, 113.04 g. Five dry-wet treatments different in frequency and intensity were designed and implemented: (1) CK as control; (2) MDW1, one round of moderate dry-wet alternation; (3) VDW1, one round of very dry-wet alternation; (4) MDW3, three rounds of moderate dry-wet alternation; and (5) VDW3, three rounds of very dry-wet alternation. Soil microbial communities were investigated by means of Illumina Miseq sequencing. Soil available phosphorus and available potassium were determined with conventional analysis methods. Soil pH was determined with a composite electrode. And soil water ratio was set as 1: 5. For measuring carbon and nitrogen, a part of each treated soil sample was ground to pass a 0.85 mm sieve. Total carbon and total nitrogen of the soils were determined with the Vario Max produced by the German Elementar Company.Results show that dry-wet alternation significantly reduced microbial diversity in the soils under no-tillage, and the effect was amplified with rising frequency and intensity of the dry-wet alternation. However, dry-wet alternation did not affect microbial diversity in the soils under ridge tillage. Compared to the control, the treatments under dry-wet alternation significantly increased the relative abundances of Planctomycetes and Verrucomicrobia in the soils under no-tillage and significantly reduced the relative abundances of Saccharibacteria and Parcubacteria in the soils under either no-tillage or ridge tillage, and increased the relative abundance of Gemmatimonadetes in the soils under ridge-tillage. Relative abundances of Proteobacteria and Acidobacteria were significantly lower in the soils under ridge tillage than in the soil under no-tillage, while that of Actinobacteria was significantly higher in the soils under ridge tillage. Relative abundances of Firmicutes and phyla in the“Others”did not vary much between the two tillage modes. Frequency of dry-wet alternation did affect structure of the soil microbial community. However, intensity of drought in the treatments did not have much effect on structure of the soil microbial community. So soil microbial community structure is significantly altered by frequency of the alternation, but not by intensity of the drought in the dry-wet alternation. Redundancy analysis was conducted with available phosphorus, readily available potassium, total N, total C and pH of soil as explanatory variables and 11 phyla of microbes in the soils under no-tillage and ridge tillage as response variables. Readily available potassium was the main environmental factor affecting the distribution and quantity of soil microorganisms.Dry-wet alternation has certain significant effects on soil microbial communities, but such effects are dependent on tillage practices and frequency of the dry-wet alternation. This study is expected to provide a theoretical basis for predicting effects of arid climate on soil ecological functions.
Tillage practice; Dry-wet alternation; Soil microbial community; Black soil; Illumina Miseq sequencing
S154
A
10.11766/trxb201808230253
刘奎,葛壮,徐英德,刘磊,叶超,李明,赵搏,梁爱珍,张彬,汪景宽. 不同耕作方式下黑土微生物群落对干湿交替的响应[J].土壤学报,2020,57(1):206–216.
LIU Kui,GE Zhuang,XU Yingde,LIU Lei,YE Chao,LI Ming,ZHAO Bo,LIANG Aizhen,ZHANG Bin,WANG Jingkuan. Responses of Soil Microbial Community to Drying-Wetting Alternation Relative to Tillage Mode[J]. Acta Pedologica Sinica,2020,57(1):206–216.
* 国家自然科学基金项目(41401332)和辽宁省自然科学基金计划重点项目(20170540794)资助Supported by the National Natural Science Foundation of China(No. 41401332)and the Natural Science Foundation of Liaoning(No. 20170540794)
,E-mail:zhangbin84@yeah.net
刘 奎(1993—),男,辽宁盘锦人,硕士研究生,主要从事土壤肥力与土壤生态研究。E-mail:505062917@qq.com
2018–08–23;
2018–11–08;
2018–11–26
(责任编辑:卢 萍)

