KPV2 O8:一种具有非线性光学性质的钒磷酸盐
蒋中齐 符继红 陈兆慧
(新疆大学 化学与化工学院, 新疆 乌鲁木齐830046)
The diversities of crystal structures make them have different properties. According to the composition, there are inorganic, doping and organic⁃inorganic hybrid crystals,etc[1-4]. Nonlinear optical (NLO) crystals have received great attentions owing to their extensive technical applications[5-9]. Especially, the recent inspired fluoroxoborates have attracted wide attentions[10-14]. Many factors are involved in selecting outstanding NLO materials, such as wide transparent region, high laser damage threshold, large second⁃order nonlinear susceptibility and stable physical and chemical properties.In the past decades, some important NLO crystals have been discovered in the visible and UV regions, includingβ⁃BaB2O4(BBO)[15], KBe2BO3F2(KBBF)[16], LiB3O5(LBO)[17]and KTiOPO4(KTP)[18]. However,most of IR crystals suffer from relatively low laser⁃induced damage thresholds because of their relative small band gaps[19],thus limiting their high⁃power applications. Consequently,exploring new materials with better properties is still an important topic for IR NLO material.
Vanadium phosphates have been studied in the past decades due to their rich structural chemistry. Vn+has been found with oxidation states ranging from 2 to 5[20].The versatilities of the vanadium cations have led to the formation of diverse structures. Meanwhile, PO4tetrahedron of phosphates is rigid, thermally stable and resistant to chemical attack. In recent decades,researchers have discovered a series of vanadium phosphates such as Li3V2(PO4)3[21], NaVOPO4[22], Na2(VP2O8)[23], K2(VO)3(P2O7)2[24]and K2VO2PO4[25],which were known for their catalytic properties.
Our interest in the search for better functional NLO materials inspirits us to explore more vanadium phosphates. The crystal structure of KPV2O8was investigated by BERRAH et al. in 1999[26]. However,studies on additional functional properties were not presented. In this paper, the crystal structure, thermal behavior, UV⁃vis⁃IR transmittance spectrum, powder SHG measurements and the dipole moments calculation of KPV2O8were presented.
1 Experimental Section
1.1 Synthesis
Crystals of KPV2O8were synthesizedviahydrothermal method. The mixtures of KBr, KH2PO4,V2O5with the molar ratio 3 ∶ 1 ∶ 1, and 3 mL demonized H2O were sealed in an autoclave equipped with a Teflon liner (23 mL). Then the autoclave was heated to 220 ℃gradually, held for 3 d, followed by slow cooling to room temperature at a rate of 3 ℃/h.After that, the crystals were washed with deionized water and then were dried in air. Brown and transparent crystals were obtained in a yield ofca. 85%based on K.
1.2 Single⁃crystal XRD
A block crystal was mounted on a glass fiber with epoxy for structure determination. The data were collected by a Bruker SMART APEX II charge⁃coupled device(CCD) diffractometer using monochromatic Mo Kαradiation (λ=0.710 73 Å) at 296(2) K and integrated with the SAINT program. The numerical absorption corrections were carried out using the SADABS program[27]. The structure was solved by direct method and was refined by the full⁃matrix least⁃squares fitting onF2by SHELXL⁃97 system[28]. The structure was checked for missing symmetry elements with PLATON[29]. Details of crystal data and structure refinement information are summarized in Table 1.Atoms coordinates and isotropic or equivalent displacement parameters are listed in Table 2.

Table 1 Crystal data and structure refinement for KPV2O8

Table 2 Atomic coordinates, equivalent isotropic displacement parameters (Å2 ×103) of the KPV2O8.Ueq is defined as one⁃third of the trace of the orthogonalized Uij tensor
1.3 Powder XRD
Powder XRD data of polycrystalline material were obtained on a Bruker D2 PHASER diffractometer by using Cu Kαradiation (λ= 1.541 8 Å) at room temperature. The 2θrange was 10°-70° with a scan step width of 0.02° and a fixed time of 0.2 s and a fixed counting time of 1 s/step. The powder XRD patterns of the crystal of KPV2O8show good agreement with the calculated ones based on single⁃crystal model.Such an agreement suggests that the crystals are quite pure.
1.4 Spectroscopy analysis
IR spectroscopy was carried out on the Shimadzu IRAffinity⁃1 FTIR spectrometer in the range of 400-4 000 cm-1with a resolution of 2 cm-1. The sample was pressed into discs with dried KBr (1 mg of the sample and 100 mg of KBr).
The diffuse reflectance data were collected with a Shimadza SolidSpec⁃3700DUV spectrophotometer in the wavelength range from 190 to 2 600 nm. Also,reflectance spectrum was converted to absorbance according to the Kubelka⁃Munk function[30].
1.5 TG/DSC analysis
The thermal analysis of KPV2O8was carried out on a simultaneous NETZSCH STA 449C thermal analyzer instrument, with a heating rate of 10 ℃ /min in N2from 30 to 1 000 ℃.
1.6 Second⁃order NLO measurements
The NCS structure of KPV2O8prompted us to measure its SHG properties. The test was performed on the microcrystalline samples of the KPV2O8by the Kurtz⁃Perry method[31]. A detailed description of the equipment and methodology has been published[32-33].Since the SHG efficiency shows strong dependency on particle sizes, polycrystalline samples were ground and sieved into distinct particle sizes:20-38,38-55,55-88, 88 - 105, 105 - 150 and 150 - 200 μm.Fundamental 1064 nm light is generated with a Q⁃switched Nd: YAG solid⁃state laser (1 064 nm,10 kHz, 10 ns). To make relevant comparison with known NLO material, crystalline KDP samples were ground and served as a reference and were sieved into the same particle sizes.
1.7 Structure⁃property relationships
To better understand the structure⁃property relationship of KPV2O8,we examined the direction and magnitude of the local dipole moments for the KO11,VO5, PO4groups using the bond⁃valence approach.The well⁃known Debye equation,μ=neR[34](μis the net dipole moment in Debye;nis the total number of electrons;eis the charge on an electron;Ris the difference), is used to calculate the local dipole moments of individual K-O, V-O and P-O bonds.
2 Results and Discussion
2.1 Crystal structure
KPV2O8crystallizes in the NCS orthorhombic space groupPna2(1) with lattice parametersa=13.914(4) Å,b=4.7441(12) Å,c=19.923(5)Å, andZ= 2. The crystal structure includes two unique potassium atoms, two unique phosphorus atoms, four unique vanadium atoms, and sixteen unique oxygen atoms. There are two kinds of [V2O8]∞chains (Fig.1) in KPV2O8crystal. In the first kind of[V2O8]∞chain, VO5pyramids are interconnected by sharing O atoms to form long chain along thebandaaxis, respectively, forming a regular latticed framework(Fig.1(a)). In the second kind of [V2O8]∞chain,VO5pyramids are connected by O atoms forming jagged chain viewed along thecaxis (Fig.1(b)). The two kinds of [V2O8]∞chains are arranged alternately each other and link with PO4tetrahedra via sharing O vertices, extending to a 3D [PV2O8]∞framework(Fig.2). P atom is surrounded by four O atoms and PO distances are in the range of 1.470(12)-1.582(13)Å. V atom is surrounded by five O atoms and V-O distances range from 1.562(12) to 2.44(3) Å. The K atoms are located in the large S⁃shaped tunnels(Fig.3). K atom is surrounded by eleven O atoms and K-O distances are in the range of 2.766(10)-3.392(9)Å.
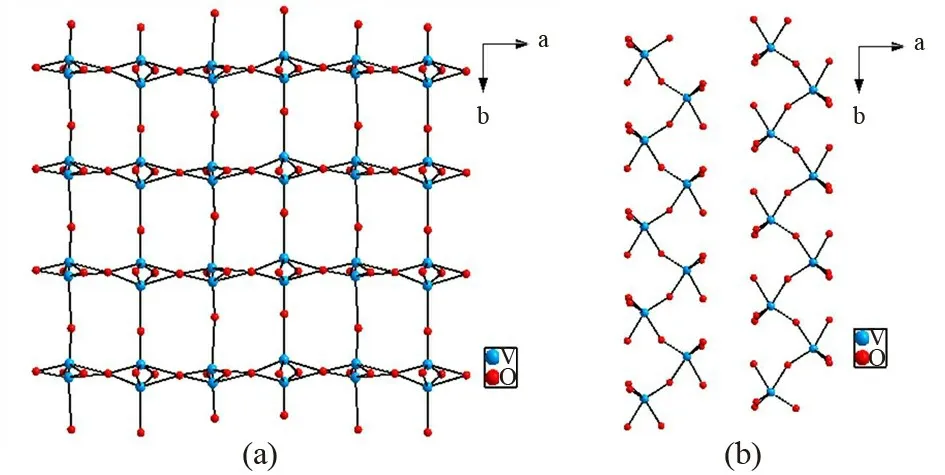
Fig.1 (a) The first kind of [V2O8] chains viewed down c axis. (b) The second kind of[V2O8] chains viewed down c axis
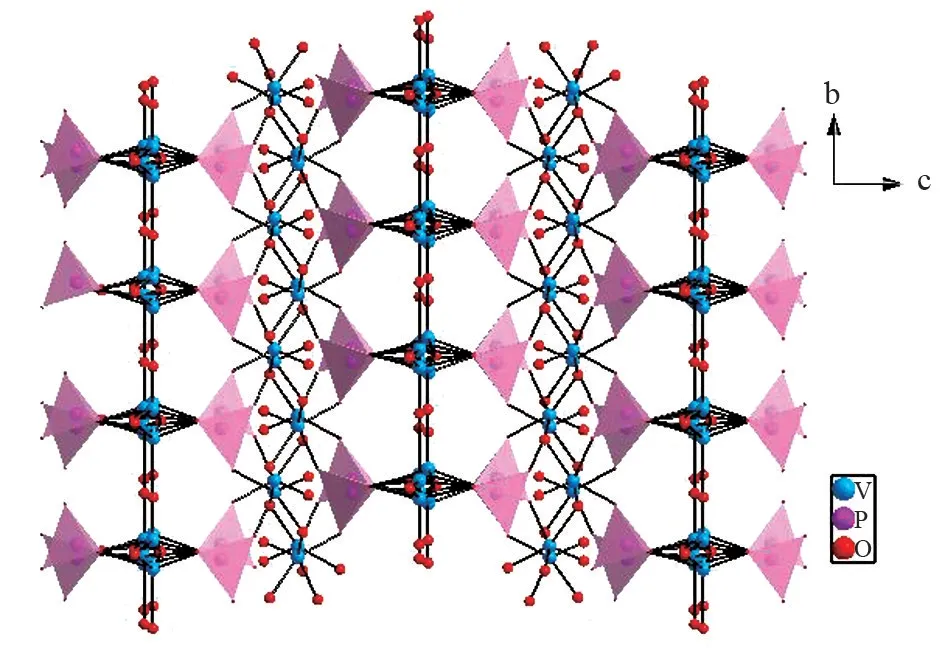
Fig.2 Connection framework of two kinds of [V2O8]chainsviewed down a axis
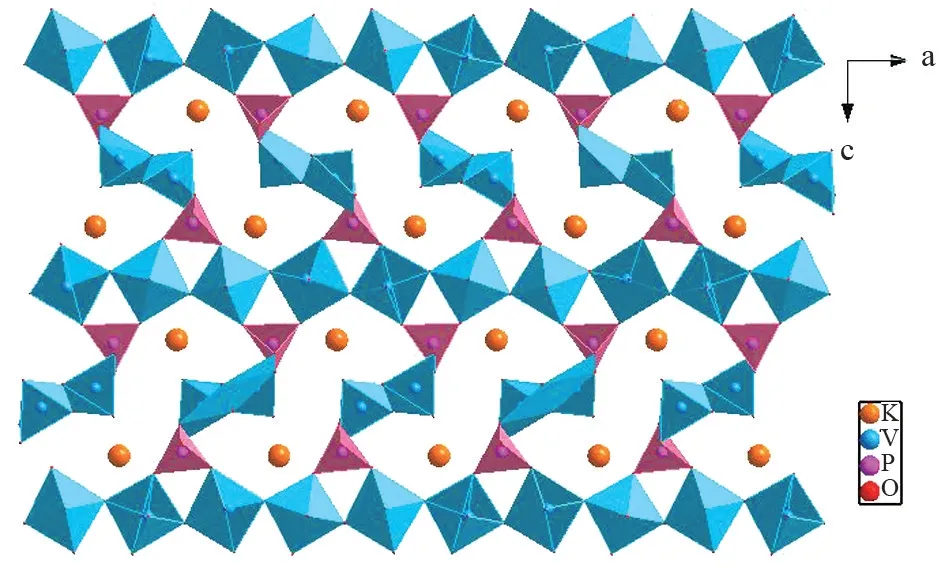
Fig.3 Polyherda representation of KPV2O8 along the b axis
2.2 Spectroscopy analysis
The IR spectrum of KPV2O8was measured at room temperature. The band at 1 067 cm-1could be attributed to the stretching vibration of P-O in the PO4tetrahedron. The absorption band at 1 000 cm-1is the V =O stretche of the free apex in the VO5pyramid. The bands at 865 and 748 cm-1may be attributed to V-O vibration. The absorption bands at 613 and 576 cm-1are due to bending vibration in O-P-O. The stretches from 500-550 cm-1can be attributed to V-O-V vibrations.
UV⁃vis⁃IR diffuse reflectance spectrum was collected for the reported crystal KPV2O8(Fig.4). The results indicate that the cutoff edge of the crystal is about 510 nm and the experimental band gap value of the crystal is 2.43 eV.
2.3 TG/DSC analysis
TG and DSC curves in Fig.5 show the thermal properties of KPV2O8. As shown in the TG curve, the compound KPV2O8is stable up to 1000 ℃nearly with no weight loss. Only one endothermic peak was observed at 543 ℃in the DSC diagram, which may be the melting point of KPV2O8crystal.
2.4 SHG measurements
Owing to acentric, it is worthy to study the SHG property of KPV2O8.The crystals were ground into powder with different sizes and were loaded into a quartz cell.Powder SHG measurements revealed that the SHG response of KPV2O8is about 0.5 × KDP. (Fig.6).
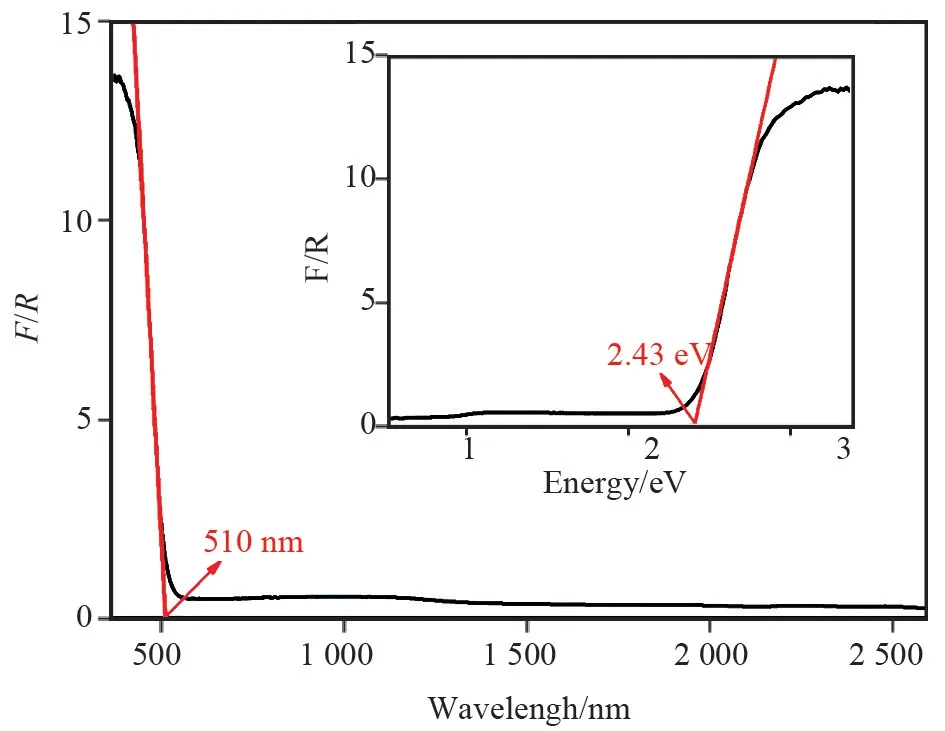
Fig.4 UV⁃Vis⁃IR diffusereflectance spectra of KPV2O8

Fig.5 TG and DSC cruves for KPV2O8

Fig.6 Powder SHG curve of KPV2O8 crystal
2.5 Structure⁃property relationships
In order to explain the observed moderate SHG efficiency, we examined the local dipole moments for the KO11, PO4, VO5polyhedra using bond valence approach. A complete calculation of dipole moments for the constituted polyhedra is listed in Table 3. It is clear that the magnitude of dipole moments along theaandbaxes are canceled and the direction of the sum vectors are along the polarcaxis (Fig.7). We find that the total dipole moments of the K(1)O11and K(2)O11polyhedra are almost canceled along thecaxis. The direction of dipole moments of V(1)O5and V(4)O5pyramids are along the positivecaxis, whereas V(2)O5,V(3)O5and P(2) O4polyhedra are along the negativecaxis. So the weak SHG efficiency of KPV2O8ascribed to the partially offsetting dipole moments of these polyhedral along the polarcaxis.

Table 3 Direction and magnitude of KO11, PO4and VO5polyhedra in KPV2O8
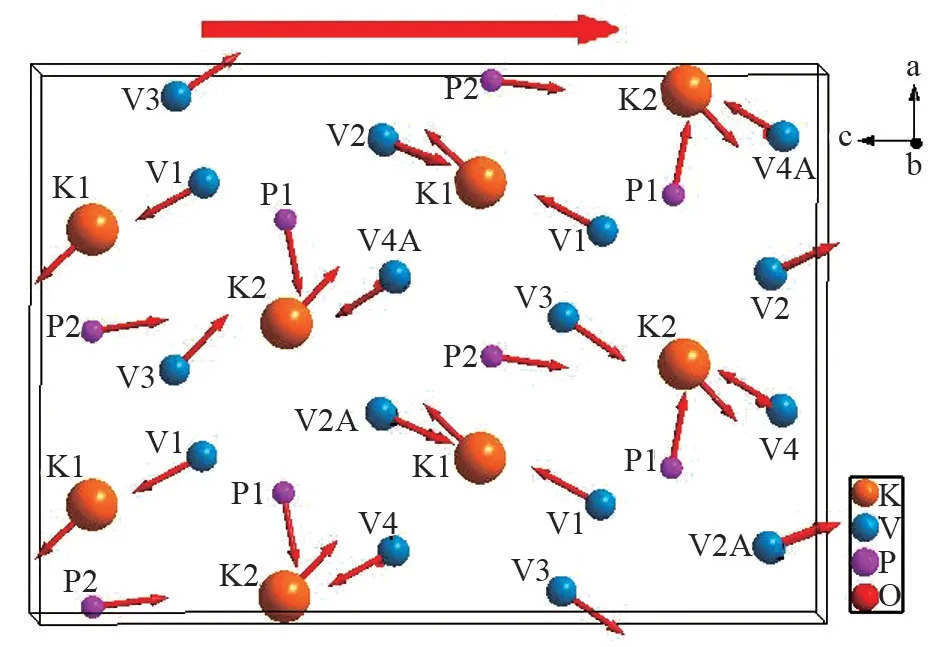
Fig.7 Direction of the local dipole moments for KO11, PO4and VO5polyhedra in KPV2O8
3 Conclusions
In summary, the crystal of KPV2O8has been grown by hydrothermal method. KPV2O8crystallizes in the NCS space groupPna2 (1) and features a complicated 3D framework [PV2O8]∞composed of two different [V2O8]∞chains and PO4tetrahedra with large S⁃shaped tunnels. The SHG response of KPV2O8is about 0.5 times that of KDP. By the calculation of dipole moments, we know that the cooperative effect of VO5and PO4units along thecaxis give significant contributions to the SHG efficiency of the NLO material. Continuing efforts are underway to find new NLO materials in vanadium phosphates and to understand the structural nature and their functional properties.

