层状氢氧化物的控制合成及应用进展
刘小鹤 易礼彬 陈发绅

摘 要:随着环境问题和全球能源危机的日益加剧,开发新型的效率高、成本低的材料用于污水降解,过滤净化以及能量储存和转化等变得更加迫切。层状氢氧化物由于种类丰富,具有独特的层状结构以及简易的合成方法而倍受青睐。本文综述了具有类水滑石结构的层状氢氧化物的晶体结构,合成制备的方法以及在光学、环境和能源领域的应用现状及进展。
关键词:层状氢氧化物;结构;制备;应用
中图分类号:TB34
文献标识码: A
1842年,天然水滑石矿在瑞典首次被发现。1915年,水滑石的组成被Manasse准确地确定下来,为[Mg6Al2(OH)16]CO3·4H2O。20世纪60年代,ALLMANN和TAYLOR通过单晶X射线衍射法测定了水滑石的结构[1-3]。层状双氢氧化物(LDH)由于结构与水滑石结构相似,因此被称为水滑类化合物或者阴离子黏土[4-5]。此外,一系列的具有类水滑石结构的层状氢氧化物均具有主板层阳离子类型可改变,层间阴离子可调控等特点,使得种类丰富,功能多样的层状氢氧化物广泛用于解决污染与能源危机等问题[6-10]。本文作者课题组长期以来一直从事层状氢氧化物的可控制备合成以及性能应用开发的研究。在此基础上结合近年来国内外研究的相关报道,本文对层状氢氧化物的晶体结构、合成手段以及在环境与能源等领域的应用做出分析、总结和展望。
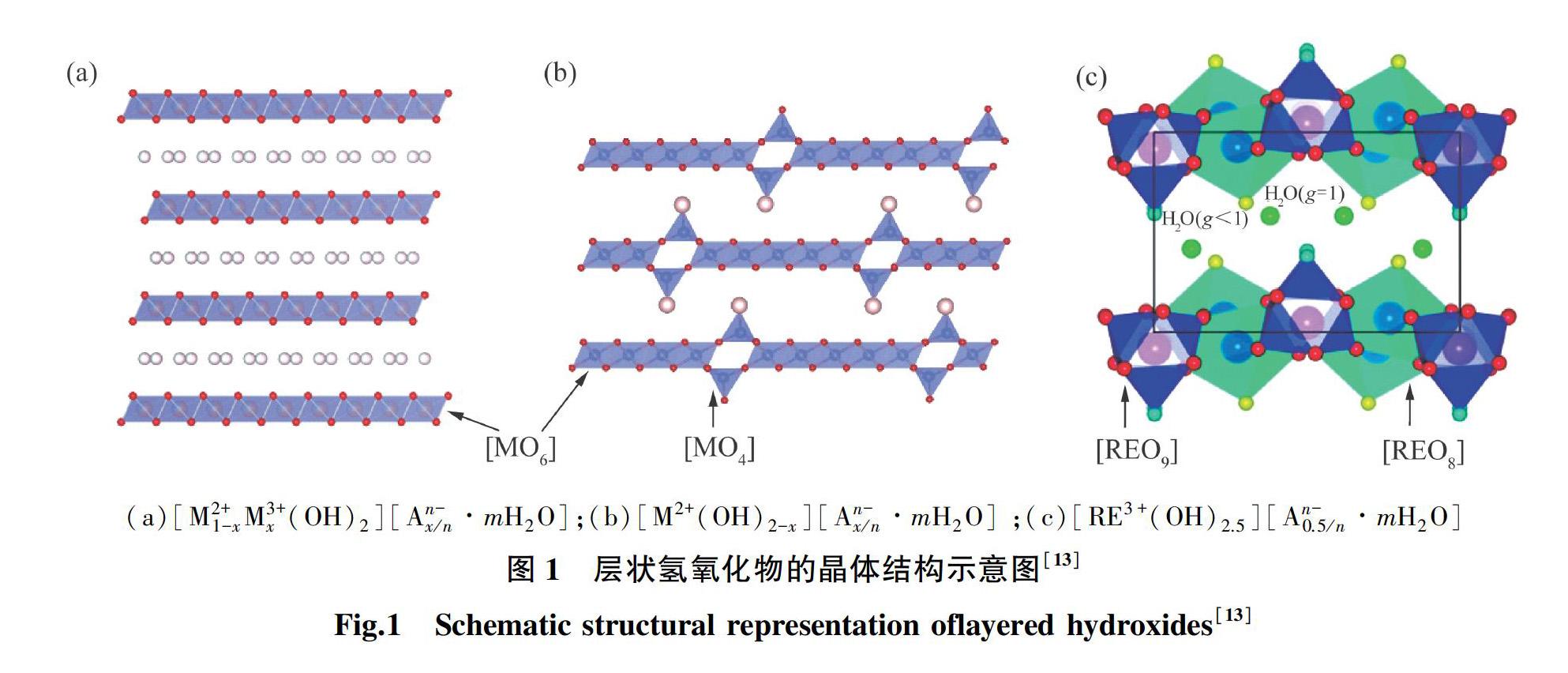
1 层状氢氧化物的组分与结构
2 层状氢氧化物的合成方法
2.1 水热法
水热法是19世纪中叶英国地质学家Roderick Murchison在模拟矿物质形成过程中提出的高温高压合成技术[15]。水热法的主要步骤是将预先配置好的原料溶液在水热釜中密封并加热至一定的温度。利用水热法制备的材料具有结晶性高,分散性良好和成本低等优点。IYI等[16]通过在AlCl3和MgCl2混合溶液中,加入六亚甲基四胺作为碱源,利用水热法成功制备了高结晶性,粒径为1~5 μm的层状Mg ̄Al氢氧化物。刘小鹤等[17-18]利用微波辅助加热的水热法制备了结晶性良好,形貌尺寸均匀,成分可控的层状氢氧化钴,氢氧化钴/镍纳米锥。
2.2 沉淀法
沉淀法主要包括共沉淀法和均相沉淀法。共沉淀法是最简单和最常用的制备层状双金属氢氧化物的方法。在含有二价和三价金属阳离子溶液中加入沉淀剂(氢氧化钠,氨水等),通过调控反应体系的pH,可获得不同形貌尺寸的层状双金属氢氧化物。由于反应体系是强碱性(pH为9~11),空气中的二氧化碳容易溶解到反应溶液中形成碳酸根,因此在制备非碳酸根插层的层状双金属氢氧化物时需要在保护性气氛(氮气或者氩气等)中进行反应[19]。利用共沉淀法制备的常见层状双金属氢氧化物有:层状Mg ̄Al[20],Zn ̄Al[21]氢氧化物等。通过共沉淀法制备得到的产物存在形貌不均匀,易团聚,粒径小(横向尺寸为几十纳米)等问题。利用均相沉淀法制备层状双金属氢氧化物的过程中通常使用尿素或者六亚甲基四胺等作为沉淀剂,在一定的温度下通过水解反应提供氢氧根离子,使在整个反应体系中缓慢地析出较均匀的,粒径较大的晶态沉淀。刘兆平等[22]以尿素为碱源,通过油浴加热(约97 ℃),利用均相沉淀法制备了粒径为微米级,形貌为均匀六方片形的Co ̄Al,Fe ̄Al,Zn ̄Al和Ni ̄Al层状氢氧化物。此外,在均相沉淀法的基础上,刘小鹤等[23-27]通过加入结构导向剂(十二烷基硫酸钠),以尿素或六亚甲基四胺为碱源,以水浴或者油浴等方式进行加热,在氮气的保护下,可制备一系列微米级的层状氢氧化钴,氢氧化锌,氢氧化钇纳米锥。
2.3 拓扑化学氧化法
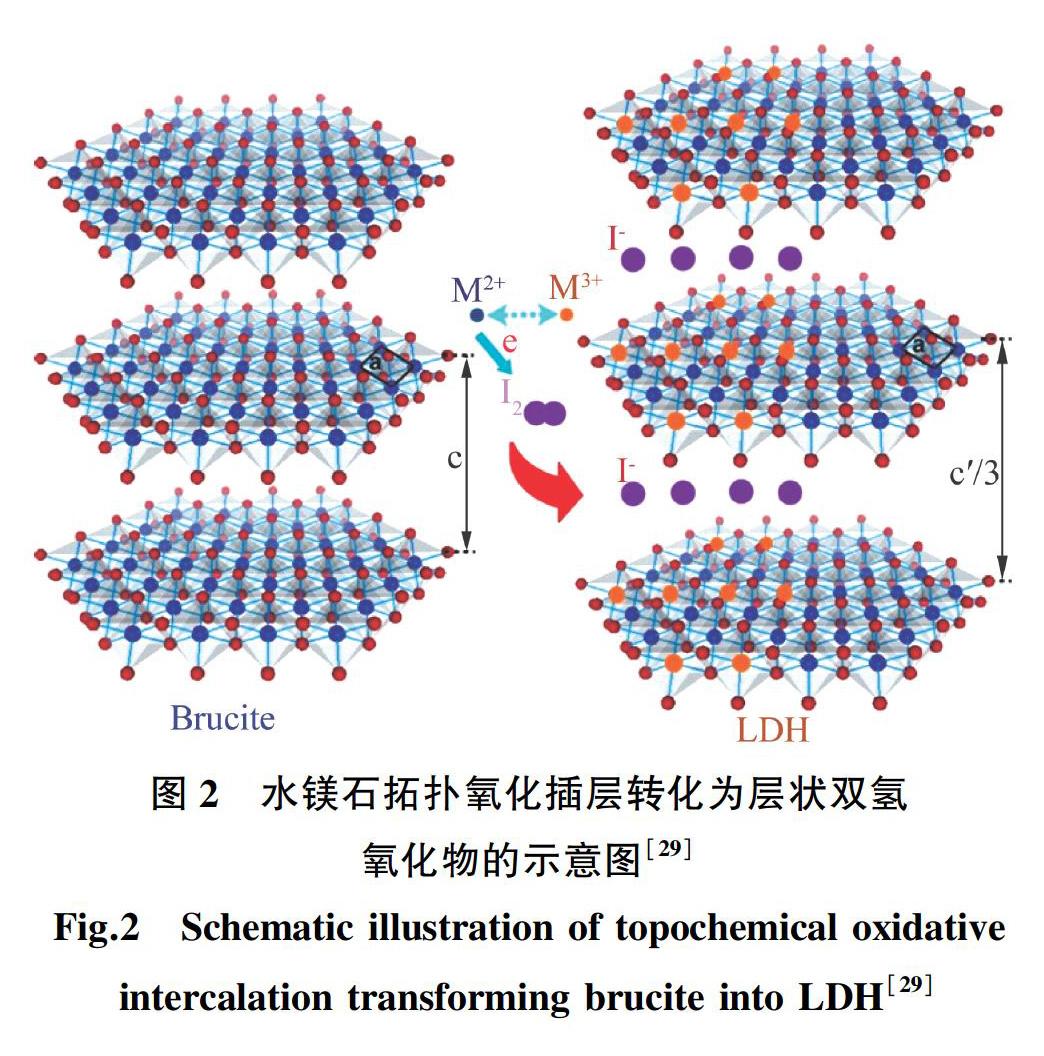
拓扑化学氧化法指的是原位部分氧化水镁石中氢氧化物板层中的二价阳离子,同时阴离子进入板层之间,平衡主板层的正电荷,最终形成层状双金属氢氧化物,如图2所示。此方法可用于合成高结晶性的非铝基层状双金属氢氧化物。利用拓扑化学氧化法,MA等[28-30]合成了结晶性良好,粒径为微米级的层状Co ̄Fe,Co ̄Co氢氧化物六方片,LIANG等[31]制备了不同Ni/Co比例的微米级层状氢氧化物六方片。利用拓扑化学氧化法所制备的样品具有结晶性好,形貌尺寸均匀,层间阴离子可交换,可化学剥离成单层氢氧化物纳米片等优点。
3 层状氢氧化物的应用
3.1 光致发光
层状稀土金属氢氧化物作为一种新型的发光材料主体,可通过掺杂Eu3+,Tb3+等稀土金属离子,发出常见的红光,绿光。胡林峰等[32]通过在正己烷/水界面上的自组装,制备了紧密填充的单层和多层的层状稀土氢氧化物Eu(OH)2.5Cl0.5·0.9H2O薄膜,该薄膜具有良好的光致发光和阴离子交换性能,并可能在光学器件等方面具有潜在的应用前景。但是,由于结构中与稀土金属离子发光中心直接配位的羟基或者水分子产生严重的荧光淬灭,导致层状稀土金属氢氧化物的发光强度远远达不到工业生产要求。因此,以层状稀土金属氢氧化物作为前驱物,通过加热煅烧去除羟基和水分子,能够有效地提高材料的发光强度。通過对层状稀土氢氧化物Gd(OH)2.5Cl0.5·0.9H2O∶0.05Eu薄膜进一步在空气中进行800 ℃高温煅烧2 h得到对应的氧化物Gd2O3∶0.05Eu薄膜,发现煅烧后氧化物产物的发光强度相对于层状稀土氢氧化物前驱体增强了527倍。高温煅烧后引起的肉眼可见的红光发射光增强以及结构变化如图3所示[33]。钟一顺等[26]同样发现,相比Yb和Er共掺杂的层状氢氧化钇纳米锥,煅烧后得到的氧化物Y2O3∶Yb,Er纳米锥具有更加优异的上转换发光性能。此外,他们还发现,通过对层状氢氧化钇纳米锥进行阴离子交换,能够有效地降低得到高纯氧化物的煅烧温度(煅烧温度:十二烷基硫酸根插层氢氧化物为1000 ℃,硝酸根插层氢氧化物为600 ℃)以及更好地保持锥状形貌。
3.2 光催化降解
具有特定形貌和组成的层状氢氧化物,亦可用作制备高性能光催化降解水中有机污染物催化剂的前驱体。马炜等[24]通过在油浴合成过程中加入具有结构导向作用的十二烷基硫酸钠,制备出纳米锥形的十二烷基硫酸根插层的层状氢氧化锌,并将上述纳米锥静置在浓度为1 mol/L氯化钠溶液中,得到长方片形的层状氢氧化锌。以纳米锥形和长方片形得层状氢氧化锌作为前驱物,经过层间阴离子交换,高温煅烧后,可获得氧化锌纳米锥和纳米片。如图4所示,在紫外光的照射下,100 mg上述样品均能在60min内将50mL浓度为15mg/L的有插图为不同紫外线照射时间对应得到亚甲基蓝悬浮液照片。
机污染物亚甲基蓝完全降解。而且相比纳米片形貌,氧化锌纳米锥催化活性更高,降解速度更快。张丹等[25]同样报道了通过以锥形的层状氢氧化锌作为前驱体合成的具有高比表面积的氧化锌纳米锥,相比氧化锌纳米棒具有更优异的光催化降解效率。
3.3 选择性过滤膜
全球环境污染越来越严重,导致淡水短缺危机深化,加速了对低成本、低能耗、易操作的过滤与分离薄膜的研究。孙鹏展等[34]对层状Mg ̄Al和Co ̄Al氢氧化物六方片在甲酰胺中进行机械震荡剥离,分别获得带正电荷单层的Mg ̄Al和Co ̄Al氢氧化物纳米片,通过与带负电荷的氧化石墨烯组成超晶格单元,进一步真空抽滤成新型复合膜。所制备的复合膜具有面积可控、厚度可控、半透明、可弯曲、机械强度高等特点。如图5所示,该类复合膜用于过滤分离废液时,对一价(例如Na+,K+)和三价阳离子(例如Al3+)的相对选择性高达30。同为一价的钾盐和钠盐的渗透,只与阳离子的价态相关,几乎与阴离子类型(例如Cl-,NO-3和CO2-3)无关。无论是氧化石墨烯/Co ̄Al氢氧化物纳米片复合膜还是氧化石墨烯/Mg ̄Al氢氧化物纳米片复合膜均具有高选择性电荷导向离子过滤和分离的特点,在废水处理和再利用、化学精制、生物仿生选择性离子传输等方面具有广阔的应用前景。
3.4 超级电容器
超级电容器中能量的储存和释放可归纳成两类电化学过程:离子吸附/解吸附过程和氧化还原法拉第反应。具有高表面积的碳基材料(例如活性炭,碳纳米管和石墨烯等)具有离子在材料表面快速吸附/解吸附的能力,一般用于构建大功率的双电层电容器。而具有强氧化还原特性的过渡金属(Fe,Co,Ni,Mn,Ru等)氧化物/氢氧化物常被用作构建高比容量的法拉第准电容器[35-42]。
如图6所示,刘小鹤等[18,23]通过调节层状氢氧化钴的层间插层阴离子,发现相比层间阴离子为十二烷基硫酸根和硝酸根的层状氢氧化钴,氯离子插层的层状氢氧化钴用作超级电容器电极材料具有更高的比容量。此外,通过调控层状氢氧化钴中主板层的金属阳离子(Co/Ni)比例发现,在电流密度为10 A/g时,Co0.5Ni0.5(OH)2具有最高的比容量(1580 F/g),甚至可媲美贵金属氧化物RuO2的性能。相比导电性良好的贵金属RuO2,非贵金属的
过渡金属(Fe,Co,Ni,Mn)氧化物/氢氧化物导电性很差,电导率仅为10-6~10-5 S/cm。为了进一步提高层状氢氧化物在超级电容器应用中的电化学性能,可以通过将层状氢氧化物与导电性良好的石墨烯[43-45],碳纳米管[46],石墨纸[47],碳布[48-49],碳素纤维[50],泡沫镍[51-53]等进行复合或原位生长,提高氧化还原反应中的电子转移速率,进而提升复合电极材料的比容量、倍率性以及稳定性。
3.5 电催化水分解
水分解可以将太阳能和风能以化学燃料的形式储存起来,即氢能。一直以来析氧反应(OER)被视为电催化水分解的瓶颈。即使是商用的贵金属催化剂RuO2和IrO2,在电催化析氧反应中依然需要比较高的过电势。开发高效、廉价的析氧反应催化剂是当前可再生能源研究的主要课题之一。层状过渡金属氧化物及其衍生物因其多样性和稳定性,而成为有吸引力的催化剂候选材料[54-58]。
SONG等[59]研究了三种层状双金属氢氧化物的电催化析氧活性,如图7(a)所示。研究发现,无论是氢氧化物块体还是剥离后的纳米片,催化活性的顺序为:NiFe>NiCo>CoCo。剥离后的NiFe和NiCo氢氧化物纳米片即使在相对较低的负载量(007 mg/cm2)时,仍然呈现出比负载量较高(0.21 mg/cm2)的商用IrO2纳米颗粒更低的过电势以及更小的塔菲尔斜率。
马炜等[60-61]通过将导电性较差的带负电荷的Ni ̄Fe或Ni ̄Mn纳米片与导电性较好的氧化石墨烯(GO)和还原氧化石墨烯(rGO)复合形成超晶格结构复合材料,从而提高层状双金属氧化物的电催化活性。在碱性电解液中,相比未复合前的NiFe纳米片的电催化析氧活性(过电势:310 mV,塔菲尔斜率:76 mV/decade),复合后的Ni2/3Fe1/3 ̄GO(过电势:230 mV,塔菲尔斜率:42 mV/decade)和Ni2/3Fe1/3 ̄rGO(过电势:210 mV,塔菲尔斜率:40 mV/decade)超晶格结构复合材料的催化性能均有很大的提升,如图7(b)所示。当以Ni2/3Fe1/3 ̄rGO复合材料作为电催化水分解的催化剂,仅用一节1.5 V电池作为电源即可将水分解产生氢气和氧气。
3.6 氢氧根离子传导
目前燃料电池主要基于酸性体系,以贵金属作为电催化剂,以质子导体为隔膜。贵金属催化性能优异,但是价格昂贵而且储量少。开发新型氢氧根离子传导膜有利于实现燃料电池向碱性体系转变,以相比廉价且性能良好的过渡金属氢氧化物或氧化物替代贵金属作为电催化剂,从而降低成本。近年来,研究者发现层状双氢氧化物具有氢氧根离子传导特性,并测量了块状氢氧化物的氢氧根离子传导率(<10-2 S/cm),但远低于商用质子传导Nafion膜的传导率(约10-1 S/cm)[62-65]。孙鹏展等[66]报道了对层状双氢氧化物块体晶体进行化学剥离形成纳米单片后,发现相比未剥离的块状前驱体,剥离后得到的纳米片氢氧根离子的传导率提高一到三个数量级,而且纳米片面内的氢氧根离子传导率比垂直于面内的氢氧根离子传导率高四到五个数量级,在一定的温度湿度下能够达到10-1 S/cm,如圖8所示。因此,以层状氢氧化物纳米片为基本构建单元,制备碱性燃料电池中高性能氢氧根离子传导膜或导体具有良好的发展前景。
4 结论与展望
层状氢氧化物由于结构的独特性,具有主板层阳离子种类/价态可调,层间阴离子可控的特点。通过水热法,沉淀法,拓扑氧化法等制备手段,可实现对层状氢氧化物的结晶性,形貌(六方片,纳米锥),尺寸(纳米,微米),组分(Mg,Ca,Mn,Fe,Co,Ni,Cu和Zn等),价态(M2+,M3+),配位(MO6,MO4)等的调控。基于层状氢氧化物及其衍生物(煅烧后得到的氧化物,剥离后的纳米片,复合形成超晶格材料)的多樣性和稳定性,可广泛应用于环境(光催化降解,选择性过滤膜)和能源(超级电容器,水分解,燃料电池)等领域。相对块状层状氢氧化物晶体,经过剥离后得到的氢氧化物纳米片具有更高的比表面积,更多暴露的活性位点。一方面,通过在分子尺度自组装,与导电石墨烯形成超晶格的微观结构,进一步增强不同层状结构材料功能间的协同耦合作用。另一方面,可基于对单个的纳米片进行测试及性能表征,同时利用分子动力学模拟,能够更好地分析并获得材料内在的本征性能,进而指导并推进层状结构材料在能源和环境技术的突破。
参考文献:
[1]ALLMANN R. The crystal structure of pyroaurite[J]. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1968, 24(7): 972-977.
[2]TAYLOR H F W. Segregation and cation ̄ordering in sjgrenite and pyroaurite[J]. Mineralogical Magazine, 1969, 37: 338-342.
[3]TAYLOR H F W. Crystal structures of some double hydroxide minerals[J]. Mineralogical Magazine, 1973, 39: 377-389.
[4]RIVES V. Layered double hydroxides: present and future[M]. New York: Nova Science Publishers, Inc., 2001.
[5]KHAN A I, O′HARE D. Intercalation chemistry of layered double hydroxides: recent developments and applications[J]. Journal of Materials Chemistry, 2002, 12(11): 3191-3198.
[6]GOH K H, LIM TT, DONG Z. Application of layered double hydroxides for removal of oxyanions: a review[J]. Water research, 2008, 42(6/7): 1343-1368.
[7]NALAWADE P, AWARE B, KADAM V J, et al. Layered double hydroxides: A review[J].Journal of scientific and industrial research, 2009, 68(4):267-272.
[8]MOHAPATRA L, PARIDA K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts[J]. Journal of Materials Chemistry A, 2016, 4(28): 10744-10766.
[9]TICHIT D, LAYRAC G, GéRARDIN C. Synthesis of layered double hydroxides through continuous flow processes: A review[J]. Chemical Engineering Journal, 2019,369:302-332.
[10]QU J, SHA L, WU C, et al. Applications of Mechanochemically Prepared Layered Double Hydroxides as Adsorbents and Catalysts: A Mini ̄Review[J]. Nanomaterials, 2019, 9(1): 80.
[11]EVANS D G, SLADE R C T. Structural aspects of layered double hydroxides[M]//Layered double hydroxides. Berlin, Heidelberg:Springer,2006: 1-87.
[12]MA R Z, LIU Z P, TAKADA K, et al. Tetrahedral Co (II) coordination in α ̄type cobalt hydroxide: rietveld refinement and X ̄ray absorption spectroscopy[J]. Inorganic chemistry, 2006, 45(10): 3964-3969.
[13]GENG F X, MA R Z, SASAKI T. Anion ̄exchangeable layered materials based on rare ̄earth phosphors: unique combination of rare ̄earth host and exchangeable anions[J]. Accounts of chemical research, 2010, 43(9): 1177-1185.
[14]GENG F X, MATSUSHITA Y, MA R Z, et al. General Synthesis and Structural Evolution of a Layered Family of Ln8 (OH) 20Cl4· n H2O (Ln= Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Y)[J]. Journal of the American Chemical Society, 2008, 130(48): 16344-16350.
[15] SMIYA S. Hydrothermal Reactions for Materials Science and Engineering: An Overview of Research in Japan[M]. Netherlands:Springer, 1989.
[16]IYI N, MATSUMOTO T, KANEKO Y, et al. A novel synthetic route to layered double hydroxides using hexamethylenetetramine[J]. Chemistry letters, 2004, 33(9): 1122-1123.
[17]LIU X H, MA R Z, BANDO Y, et al. Layered Cobalt Hydroxide Nanocones: Microwave ̄Assisted Synthesis, Exfoliation, and Structural Modification[J]. Angewandte Chemie International Edition, 2010, 49(44): 8253-8256.
[18]LIU X H, MA R Z, BANDO Y, et al. A general strategy to layered transition ̄metal hydroxide nanocones: tuning the composition for high electrochemical performance[J]. Advanced Materials, 2012, 24(16): 2148-2153.
[19]THEISS F L, AYOKO G A, FROST R L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co ̄precipitation methods ̄A review[J]. Applied Surface Science, 2016, 383: 200-213.
[20]PANDA H S, SRIVASTAVA R, BAHADUR D. Synthesis and in situ mechanism of nuclei growth of layered double hydroxides[J]. Bulletin of Materials Science, 2011, 34(7): 1599-1604.
[21]SEFTEL E M, POPOVICI E, MERTENSM, et al. Zn ̄Al layered double hydroxides: synthesis, characterization and photocatalytic application[J]. Microporous and Mesoporous Materials, 2008, 113(1/3): 296-304.
[22]LIU Z P, MA R Z, OSADA M, et al. Synthesis, anion exchange, and delamination of Co ̄Al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto ̄optical studies[J]. Journal of the American Chemical Society, 2006, 128(14): 4872-4880.
[23]LIU X H, MA R Z, BANDO Y, et al. High ̄yield Preparation, Versatile Structural Modification, and Properties of Layered Cobalt Hydroxide Nanocones[J]. Advanced Functional Materials, 2014, 24(27): 4292-4302.
[24]MA W, MA R Z, LIANG J B, et al. Layered zinc hydroxide nanocones: synthesis, facile morphological and structural modification, and properties[J]. Nanoscale, 2014, 6(22): 13870-13875.
[25]ZHANG D, LIU X H, WAN H, et al. Large ̄Scale Preparation, Chemical Exfoliation, and Structural Modification of Layered Zinc Hydroxide Nanocones: Transformation into Zinc Oxide Nanocones for Enhanced Photocatalytic Properties[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5869-5879.
[26]ZHONG Y S, CHEN G, LIU X H, et al. Layered rare ̄earth hydroxide nanocones with facile host composition modification and anion ̄exchange feature: topotactic transformation into oxide nanocones for upconversion[J]. Nanoscale, 2017, 9(24): 8185-8191.
[27]XIAO Y, ZHONG Y S, LIU X H, et al. Terbium ̄Doped Layered Yttrium Hydroxide Nanocone: Controlled Synthesis, Structure Variations, Phase Conversion to Oxide/Oxysulfate Nanocone and Their Luminescence Properties[J]. Particle & Particle Systems Characterization, 2018, 35(7): 1800075.1-1800075.10.
[28]MA R Z, LIU Z P, TAKADA K, et al. Synthesis and exfoliation of Co2+-Fe3+ layered double hydroxides: An innovative topochemical approach[J]. Journal of the American Chemical Society, 2007, 129(16): 5257-5263.
[29]MA R Z, LIANG J B, TAKADA K, et al. Topochemical synthesis of Co ̄Fe layered double hydroxides at varied Fe/Co ratios: unique intercalation of triiodide and its profound effect[J]. Journal of the American chemical society, 2010, 133(3): 613-620.
[30]MA R Z, LIANG J B, LIU X H, et al. General insights into structural evolution of layered double hydroxide: underlying aspects in topochemical transformation from brucite to layered double hydroxide[J]. Journal of the American Chemical Society, 2012, 134(48): 19915-19921.
[31]LIANG J B, MA R Z, IYI N, et al. Topochemical synthesis, anion exchange, and exfoliation of Co ̄Ni layered double hydroxides: A route to positively charged Co ̄Ni hydroxide nanosheets with tunable composition[J]. Chemistry of Materials, 2009, 22(2): 371-378.
[32]HU L F, MA R Z, OZAWA T C, et al. Oriented films of layered rare ̄earth hydroxide crystallites self ̄assembled at the hexane/water interface[J]. Chemical Communications, 2008 (40): 4897-4899.
[33]HU L F, MA R Z, OZAWA T C, et al. Oriented Monolayer Film of Gd2O3: 0.05 Eu Crystallites: Quasi ̄Topotactic Transformation of the Hydroxide Film and Drastic Enhancement of Photoluminescence Properties[J]. Angewandte Chemie International Edition, 2009, 48(21): 3846-3849.
[34]SUN P Z, MA R Z, MA W, et al. Highly selective charge ̄guided ion transport through a hybrid membrane consisting of anionic graphene oxide and cationic hydroxide nanosheet superlattice units[J]. NPG Asia Materials, 2016, 8(4): e259.1-e259.10.
[35]PORTET C, TABERNA P L, SIMON P, et al. High power density electrodes for carbon supercapacitor applications[J]. Electrochimica Acta, 2005, 50(20): 4174-4181.
[36]MALAK ̄POLACZYK A, VIX ̄GUTERL C, FRACKOWIAK E. Carbon/layered double hydroxide (LDH) composites for supercapacitor application[J]. Energy & Fuels, 2010, 24(6): 3346-3351.
[37]WANG G P, ZHANG L, ZHANG J J. A review of electrode materials for electrochemical supercapacitors[J]. Chemical Society Reviews, 2012, 41(2): 797-828.
[38]ZHI M J, XIANG C C, LI J T, et al. Nanostructured carbon ̄metal oxide composite electrodes for supercapacitors: a review[J]. Nanoscale, 2013, 5(1): 72-88.
[39]CHEN H, HU L F, CHEN M, et al. Nickel ̄cobalt layered double hydroxide nanosheets for high ̄performance supercapacitor electrode materials[J]. Advanced Functional Materials, 2014, 24(7): 934-942.
[40]YAN A L, WANG X C, CHENG J P. Research progress of NiMn layered double hydroxides for supercapacitors: a review[J]. Nanomaterials, 2018, 8(10): 747.
[41]WANG S B, LIU H J, LI Z, et al. Facile preparation of Ni ̄Mn layered double hydroxide nanosheets/carbon for supercapacitor[J]. Journal of Materials Science: Materials in Electronics, 2019, 30(8): 7524-7533.
[42]ZHANG Y Q, WEI S. Mg ̄Co ̄Al ̄LDH nanoparticles with attractive electrochemical performance for supercapacitor[J]. Journal of Nanoparticle Research, 2019, 21(1): 14.
[43]WANG L, WANG D, DONG X Y, et al. Layered assembly of graphene oxide and Co ̄Al layered double hydroxide nanosheets as electrode materials for supercapacitors[J]. Chemical Communications, 2011, 47(12): 3556-3558.
[44]MA R Z, LIU X H, LIANG J B, et al. Molecular ̄Scale Heteroassembly of Redoxable Hydroxide Nanosheets and Conductive Graphene into Superlattice Composites for High ̄Performance Supercapacitors[J]. Advanced Materials, 2014, 26(24): 4173-4178.
[45]LI S S, CHENG P P, LUO J X, et al. High ̄performance flexible asymmetric supercapacitor based on CoAl ̄LDH and rGO electrodes[J]. Nano ̄micro letters, 2017, 9(3): 31.
[46]YU L, SHI N N, LIU Q, et al. Facile synthesis of exfoliated Co ̄Al LDH ̄carbon nanotube composites with high performance as supercapacitor electrodes[J]. Physical Chemistry Chemical Physics, 2014, 16(33): 17936-17942.
[47]YANG J, YU C, FAN X M, et al. 3D Architecture Materials Made of NiCoAl ̄LDH Nanoplates Coupled with Ni ̄CoCarbonate Hydroxide Nanowires Grown on Flexible Graphite Paper for Asymmetric Supercapacitors[J]. Advanced Energy Materials, 2014, 4(18): 1400761.1-1400761.7.
[48]SEKHAR S C, NAGARAJU G, YU J S. Conductive silver nanowires ̄fenced carbon cloth fibers ̄supported layered double hydroxide nanosheets as a flexible and binder ̄free electrode for high ̄performance asymmetric supercapacitors[J]. Nano Energy, 2017, 36: 58-67.
[49]GE X J, HE Y, PLACHY T, et al. Hierarchical PANI/NiCo ̄LDH Core ̄Shell Composite Networks on Carbon Cloth for High Performance Asymmetric Supercapacitor[J]. Nanomaterials, 2019, 9(4): 527.
[50]FANG K L, CHEN M F, CHEN J Z, et al. Cotton stalk ̄derived carbon fiber@ Ni ̄Al layered double hydroxide nanosheets with improved performances for supercapacitors[J]. Applied Surface Science, 2019, 475: 372-379.
[51]WANG B, LIU Q, QIAN Z Y, et al. Two steps in situ structure fabrication of Ni ̄Al layered double hydroxide on Ni foam and its electrochemical performance for supercapacitors[J]. Journal of Power Sources, 2014, 246: 747-753.
[52]CHEN H, HU L F, CHEN M, et al. Nickel ̄cobalt layered double hydroxide nanosheets for high ̄performance supercapacitor electrode materials[J]. Advanced Functional Materials, 2014, 24(7): 934-942.
[53]SU D Q, TANG Z H, XIE J F, et al. Co, Mn ̄LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors[J]. Applied Surface Science, 2019, 469: 487-494.
[54]GONG M, DAI H J. A mini review of NiFe ̄based materials as highly active oxygen evolution reaction electrocatalysts[J]. Nano Research, 2015, 8(1): 23-39.
[55]YAN Y, XIA B Y, ZHAO B, et al. A review on noble ̄metal ̄free bifunctional heterogeneous catalysts for overall electrochemical water splitting[J]. Journal of Materials Chemistry A, 2016, 4(45): 17587-17603.
[56]ANANTHARAJ S, KARTHICK K, KUNDU S. Evolution of layered double hydroxides (LDH) as high performance water oxidation electrocatalysts: A review with insights on structure, activity and mechanism[J]. Materials today energy, 2017, 6: 1-26.
[57]DENG X L, HUANG J Z, WAN H, et al. Recent progress in functionalized layered double hydroxides and their application in efficient electrocatalytic water oxidation[J]. Journal of Energy Chemistry,2019,32(5):93-104.
[58]WANG Y Y, YAN D F, EL HANKARI S, et al. Recent progress on layered double hydroxides and their derivatives for electrocatalytic water splitting[J]. Advanced Science, 2018, 5(8): 1800064.
[59]SONG F, HU X L. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis[J]. Nature communications, 2014, 5: 4477.
[60]MA W, MA R Z, WANG C X, et al. A superlattice of alternately stacked Ni ̄Fe hydroxide nanosheets and graphene for efficient splitting of water[J]. ACS nano, 2015, 9(2): 1977-1984.
[61]MA W, MA R Z, WU J H, et al. Development of efficient electrocatalysts via molecular hybridization of NiMn layered double hydroxide nanosheets and graphene[J]. Nanoscale, 2016, 8(19): 10425-10432.
[62]TADANAGA K, FURUKAWA Y, HAYASHI A, et al. Direct ethanol fuel cell using hydrotalcite clay as a hydroxide ion conductive electrolyte[J]. Advanced materials, 2010, 22(39): 4401-4404.
[63]KUBO D, TADANAGA K, HAYASHI A, et al. Improvement of electrochemical performance in alkaline fuel cell by hydroxide ion conducting Ni ̄Al layered double hydroxide[J]. Journal of Power Sources, 2013, 222: 493-497.
[64]MIYAZAKI K, ASADA Y, FUKUTSUKA T, et al. Structural insights into ion conduction of layered double hydroxides with various proportions of trivalent cations[J]. Journal of Materials Chemistry A, 2013, 1(46): 14569-14576.
[65]TAMAKI T, NAKANISHI N, OHASHIH, et al. The effect of particle size and surface area on the ion conductivity of layered double hydroxide[J]. Electrochemistry Communications, 2012, 25: 50-53.
[66]SUN P Z, MA R Z, BAI X Y, et al. Single ̄layer nanosheets with exceptionally high and anisotropic hydroxyl ion conductivity[J]. Science advances, 2017, 3(4): e1602629.1-e1602629.8.
(責任编辑:周晓南)

